Structural and Electrochemical Properties of Dense Yttria-Doped Barium Zirconate Prepared by Solid-State Reactive Sintering
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Dense BaZr0.8Y0.2O3−δ
2.2. Measurement of Electrochemical Properties of the Dense BaZr0.8Y0.2O3−δ
3. Results and Discussion
3.1. Sintering Behavior and Structure of BaZr0.8Y0.2O3−δ
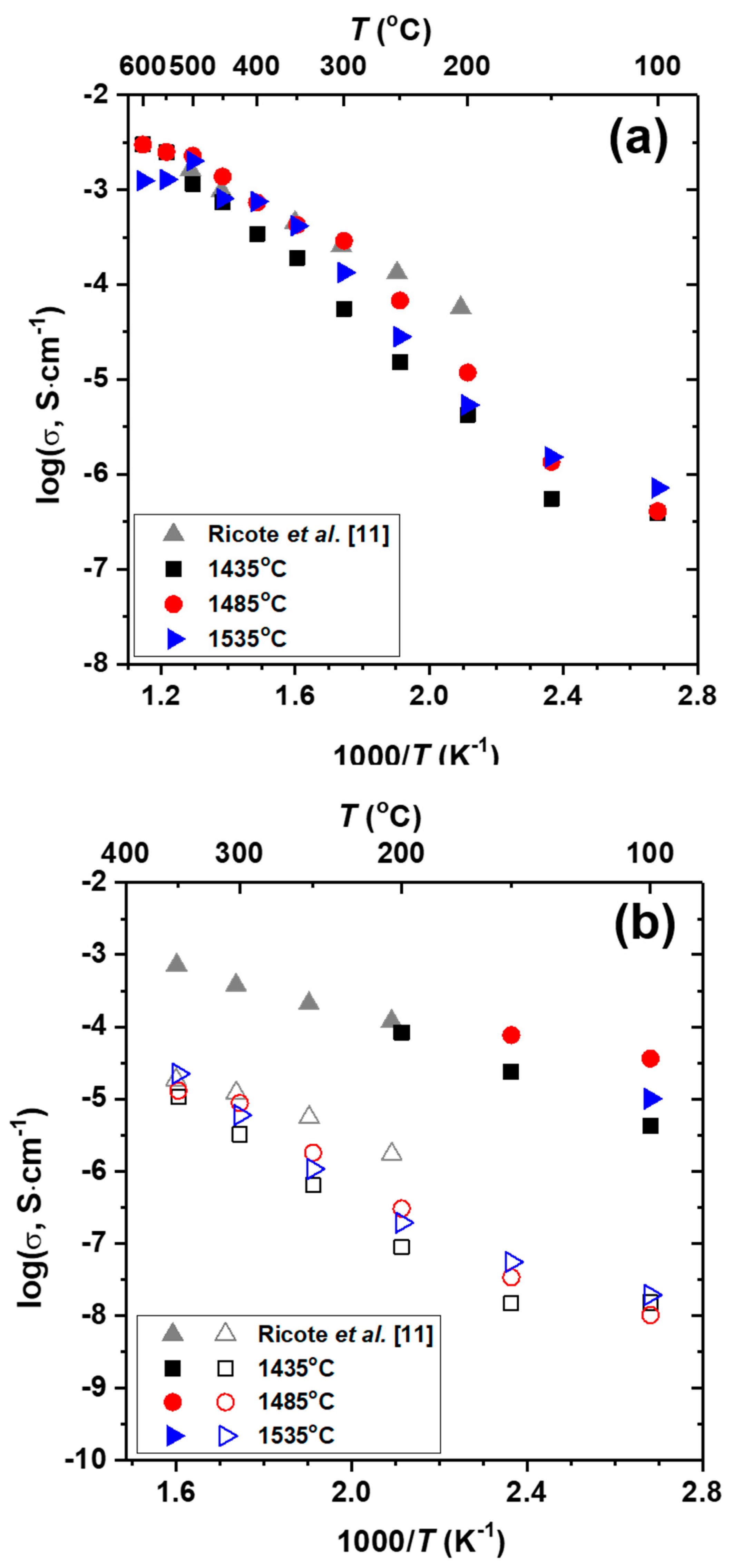
3.2. Electrochemical Properties of BaZr0.8Y0.2O3−δ
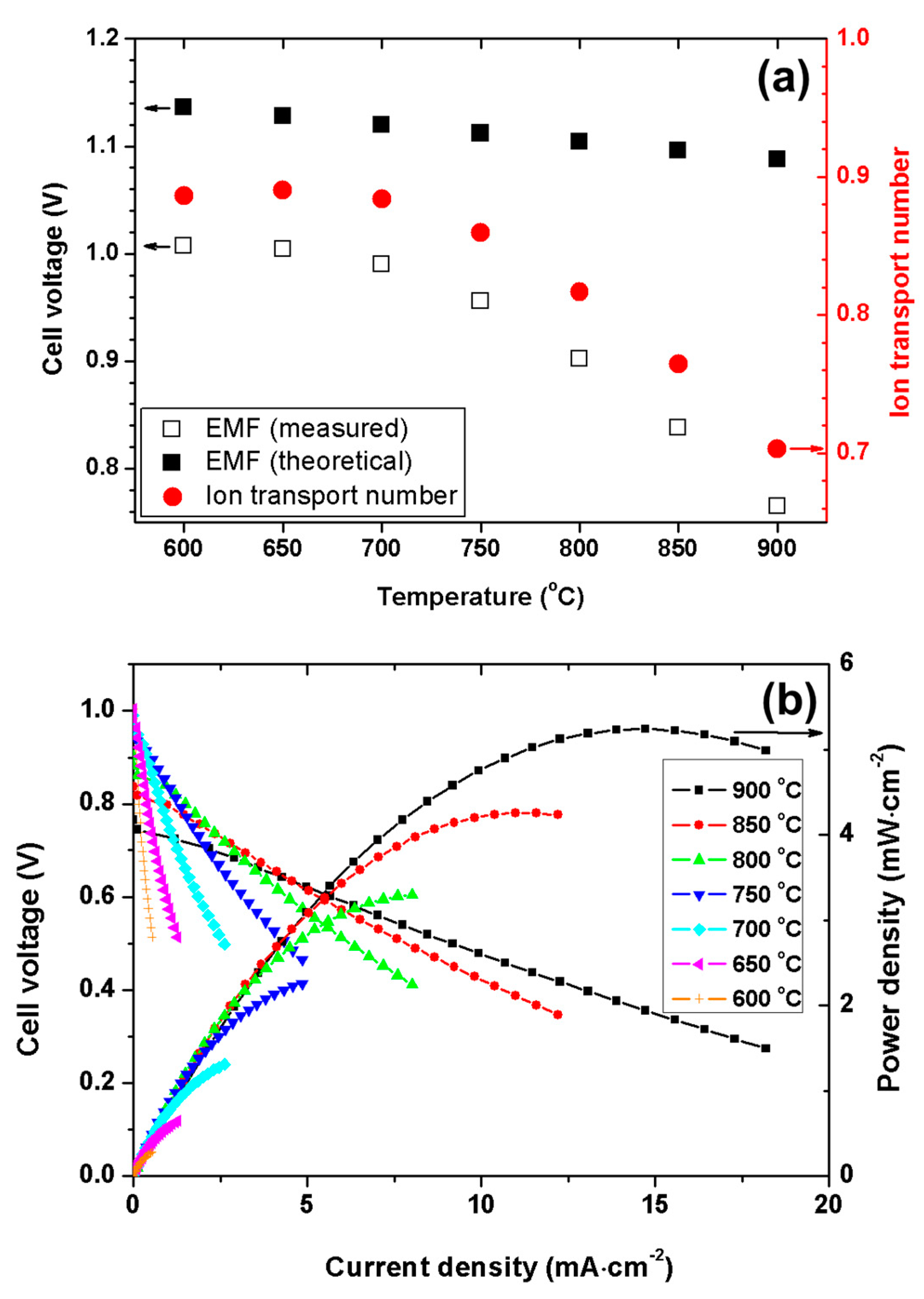
3.3. Electromotive Force Characteristics and Fuel Cell Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iwahara, H. Technological challenges in the application of proton conducting ceramics. Solid State Ion. 1995, 77, 289–298. [Google Scholar] [CrossRef]
- Bi, L.; Traversa, E. Synthesis strategies for improving the performance of doped-BaZrO3 materials in solid oxide fuel cell applications. J. Mater. Res. 2013, 29, 1–15. [Google Scholar] [CrossRef]
- Bi, L.; Boulfrad, S.; Traversa, E. Steam electrolysis by solid oxide electrolysis cells (SOECs) with proton-conducting oxides. Chem. Soc. Rev. 2014, 43, 8255–8270. [Google Scholar] [CrossRef] [PubMed]
- Babilo, P.; Uda, T.; Haile, S.M. Processing of yttrium-doped barium zirconate for high proton conductivity. J. Mater. Res. 2007, 22, 1322–1330. [Google Scholar] [CrossRef]
- Fabbri, E.; Pergolesi, D.; Licoccia, S.; Traversa, E. Does the increase in Y-dopant concentration improve the proton conductivity of BaZr1−xYxO3−δ fuel cell electrolytes? Solid State Ion. 2010, 181, 1043–1051. [Google Scholar] [CrossRef]
- Babilo, P.; Haile, S.M. Enhanced Sintering of Yttrium-Doped Barium Zirconate by Addition of ZnO. J. Am. Ceram. Soc. 2005, 88, 2362–2368. [Google Scholar] [CrossRef]
- Han, D.; Hatada, N.; Uda, T. Chemical Expansion of Yttrium-Doped Barium Zirconate and Correlation with Proton Concentration and Conductivity. J. Am. Ceram. Soc. 2016, 99, 3745–3753. [Google Scholar] [CrossRef]
- Shirpour, M.; Rahmati, B.; Sigle, W.; van Aken, P.A.; Merkle, R.; Maier, J. Dopant Segregation and space charge effects in proton-conducting BaZrO3 perovskites. J. Phys. Chem. C 2012, 116, 2453–2461. [Google Scholar] [CrossRef]
- Shirpour, M.; Merkle, R.; Maier, J. Space charge depletion in grain boundaries of BaZrO3 proton conductors. Solid State Ion. 2012, 225, 304–307. [Google Scholar] [CrossRef]
- Sun, Z.; Fabbri, E.; Bi, L.; Traversa, E. Electrochemical Properties and Intermediate-Temperature Fuel Cell Performance of Dense Yttrium-Doped Barium Zirconate with Calcium Addition. J. Am. Ceram. Soc. 2012, 95, 627–635. [Google Scholar] [CrossRef]
- Cervera, R.; Oyama, Y.; MIyoshi, S.; Kobayashi, K.; Yagi, T.; Yamaguchi, S. Structural study and proton transport of bulk nanograined Y-doped BaZrO3 oxide protonics materials. Solid State Ion. 2008, 179, 236–242. [Google Scholar] [CrossRef]
- Sun, Z.; Fabbri, E.; Bi, L.; Traversa, E. Lowering grain boundary resistance of BaZr0.8Y0.2O3−δ with LiNO3 sintering-aid improves proton conductivity for fuel cell operation. Phys. Chem. Chem. Phys. 2011, 13, 7692–7700. [Google Scholar] [CrossRef] [PubMed]
- Ricote, S.; Bonanos, N.; Manerbino, A.; Sullivan, N.P.; Coors, W.G. Effects of the fabrication process on the grain-boundary resistance in BaZr0.9Y0.1O3−δ. J. Mater. Chem. A 2014, 2, 16107–16115. [Google Scholar] [CrossRef]
- Duval, S.B.C.; Holtappels, P.; Stimming, U.; Graule, T. Effect of minor element addition on the electrical properties of BaZr0.9Y0.1O3−δ. Solid State Ion. 2008, 179, 1112–1115. [Google Scholar] [CrossRef]
- Peng, C.; Melnik, J.; Li, J.; Luo, J.; Sanger, A.R.; Chuang, K.T. ZnO-doped BaZr0.85Y0.15O3−δ proton-conducting electrolytes: Characterization and fabrication of thin films. J. Power Sources 2009, 190, 447–452. [Google Scholar] [CrossRef]
- Gao, D.; Guo, R. Structural and electrochemical properties of yttrium-doped barium zirconate by addition of CuO. J. Alloys Compd. 2010, 493, 288–293. [Google Scholar] [CrossRef]
- Tong, J.; Clark, D.; Hoban, M.; O’Hayre, R. Cost-effective solid-state reactive sintering method for high conductivity proton conducting yttrium-doped barium zirconium ceramics. Solid State Ion. 2010, 181, 496–503. [Google Scholar] [CrossRef]
- Tong, J.; Clark, D.; Bernau, L.; Subramaniyan, A.; O’Hayre, R. Proton-conducting yttrium-doped barium cerate ceramics synthesized by a cost-effective solid-state reactive sintering method. Solid State Ion. 2010, 181, 1486–1498. [Google Scholar] [CrossRef]
- Nikodemski, S.; Tong, J.; O’Hayre, R. Solid-state reactive sintering mechanism for proton conducting ceramics. Solid State Ion. 2013, 253, 201–210. [Google Scholar] [CrossRef]
- Yoo, C.-Y.; Yun, D.S.; Joo, J.H.; Yu, J.H. The effects of NiO addition on the structure and transport properties of proton conducting BaZr0.8Y0.2O3−δ. J. Alloys Compd. 2015, 621, 263–267. [Google Scholar] [CrossRef]
- Han, D.; Iihara, J.; Uemura, S.; Kazumi, K.; Hiraiwa, C.; Majima, M.; Uda, T. A high temperature reduction cleaning (HTRC) process: A novel method for conductivity recovery of yttrium-doped barium zirconate electrolytes. J. Mater. Chem. A 2016, 4, 10601–10608. [Google Scholar] [CrossRef]
- Polfus, J.M.; Fontaine, M.-L.; Thøgersen, A.; Riktor, M.; Norby, T.; Bredesen, R. Solubility of transition metal interstitials in proton conducting BaZrO3 and similar perovskite oxides. J. Mater. Chem. A 2016, 4, 8105–8112. [Google Scholar] [CrossRef]
- Nasani, N.; Pukazhselvan, D.; Kovalevsky, A.V.; Shaula, A.L.; Fagg, D.P. Conductivity recovery by redox cycling of yttrium doped barium zirconate proton conductors and exsolution of Ni-based sintering additives. J. Power Sources 2017, 339, 93–102. [Google Scholar] [CrossRef]
- Ciria, D.; Ben Hassine, M.; Jiménez-Melendo, M.; Iakovleva, A.; Haghi-Ashtiani, P.; Aubin, V.; Dezanneau, G. Mechanical degradation under hydrogen of yttrium doped barium zirconate electrolyte material prepared with NiO additive. J. Power Sources 2016, 321, 226–232. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Fang, S.; Wang, S.; Brinkman, K.S.; Chen, F. A sinteractive Ni–BaZr0.8Y0.2O3−δ composite membrane for hydrogen separation. J. Mater. Chem. A 2014, 2, 5825–5833. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hernandez-Sanchez, R.; Haile, S.M. Cation non-stoichiometry in yttrium-doped barium zirconate: Phase behavior, microstructure, and proton conductivity. J. Mater. Chem. 2010, 20, 8158–8166. [Google Scholar] [CrossRef]
- Azad, A.K.; Savaniu, C.; Tao, S.; Duval, S.; Holtappels, P.; Ibberson, R.M.; Irvine, J.T.S. Structural origins of the differing grain conductivity values in BaZr0.9Y0.1O2.95 and indication of novel approach to counter defect association. J. Mater. Chem. 2008, 18, 3414–3418. [Google Scholar] [CrossRef]
- Nomura, K.; Kageyama, H. Transport properties of BaZr0.8Y0.2O3−δ perovskite. Solid State Ion. 2007, 178, 661–665. [Google Scholar] [CrossRef]
- Nikodemski, S.; Tong, J.; Duan, C.; O’Hayre, R. Ionic transport modification in proton conducting BaCe0.6Zr0.3Y0.1O3−δ with transition metal oxide dopants. Solid State Ion. 2016, 294, 37–42. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hernandez-Sanchez, R.; Haile, S.M. High Total Proton Conductivity in Large-Grained Yttrium-Doped Barium Zirconate. Chem. Mater. 2009, 21, 2755–2762. [Google Scholar] [CrossRef]
- Tong, J.; Clark, D.; Bernau, L.; Sanders, M.; O’Hayre, R. Solid-state reactive sintering mechanism for large-grained yttrium-doped barium zirconate proton conducting ceramics. J. Mater. Chem. 2010, 20, 6333–6341. [Google Scholar] [CrossRef]
- Kjølseth, C.; Fjeld, H.; Prytz, Ø.; Dahl, P.I.; Estournès, C.; Haugsrud, R. Space–charge theory applied to the grain boundary impedance of proton conducting BaZr0.9Y0.1O3−δ. Solid State Ion. 2010, 181, 268–275. [Google Scholar] [CrossRef]
- Iguchi, F.; Sata, N.; Yugami, H. Proton transport properties at the grain boundary of barium zirconate based proton conductors for intermediate temperature operating SOFC. J. Mater. Chem. 2010, 20, 6265–6270. [Google Scholar] [CrossRef]
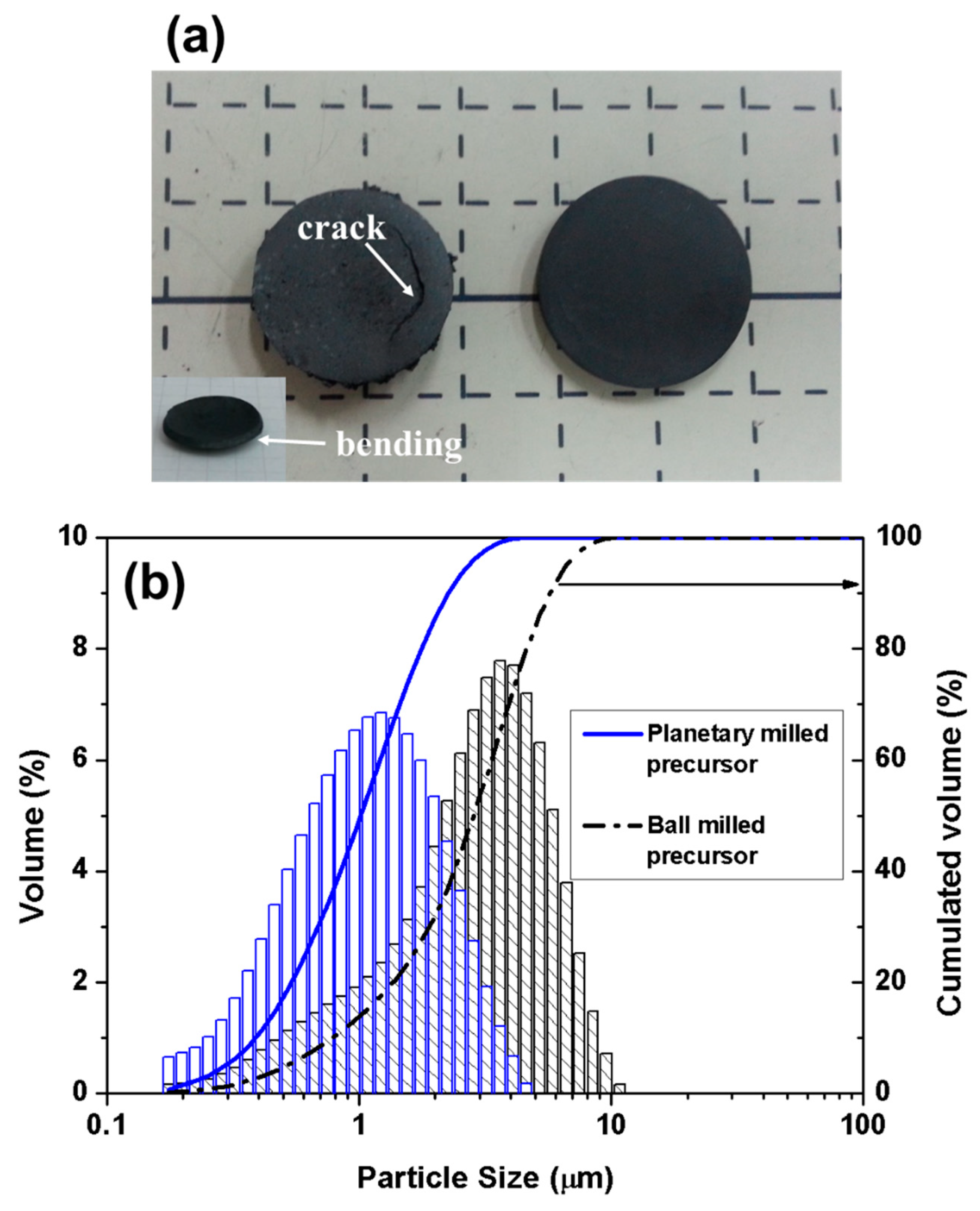


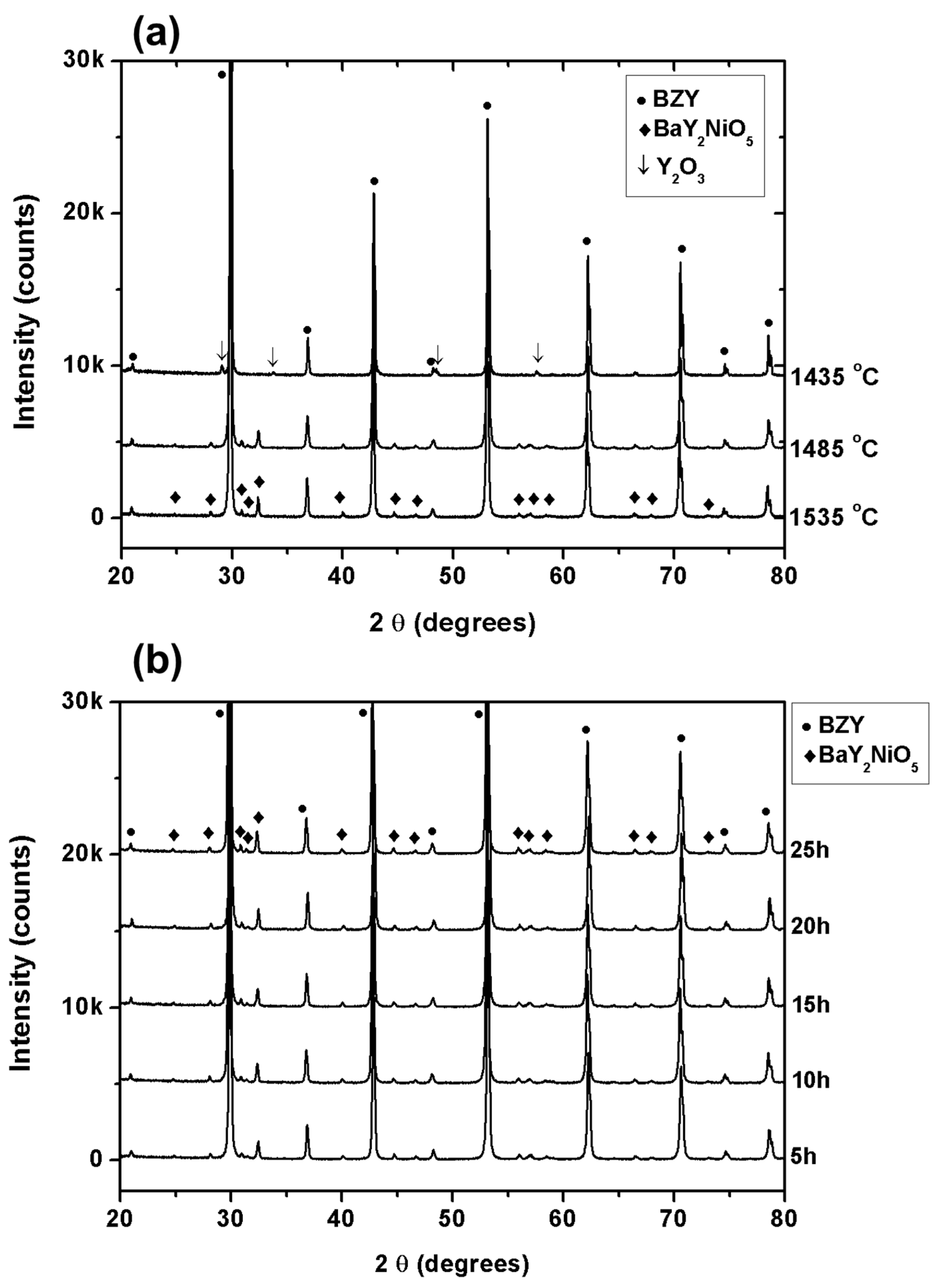
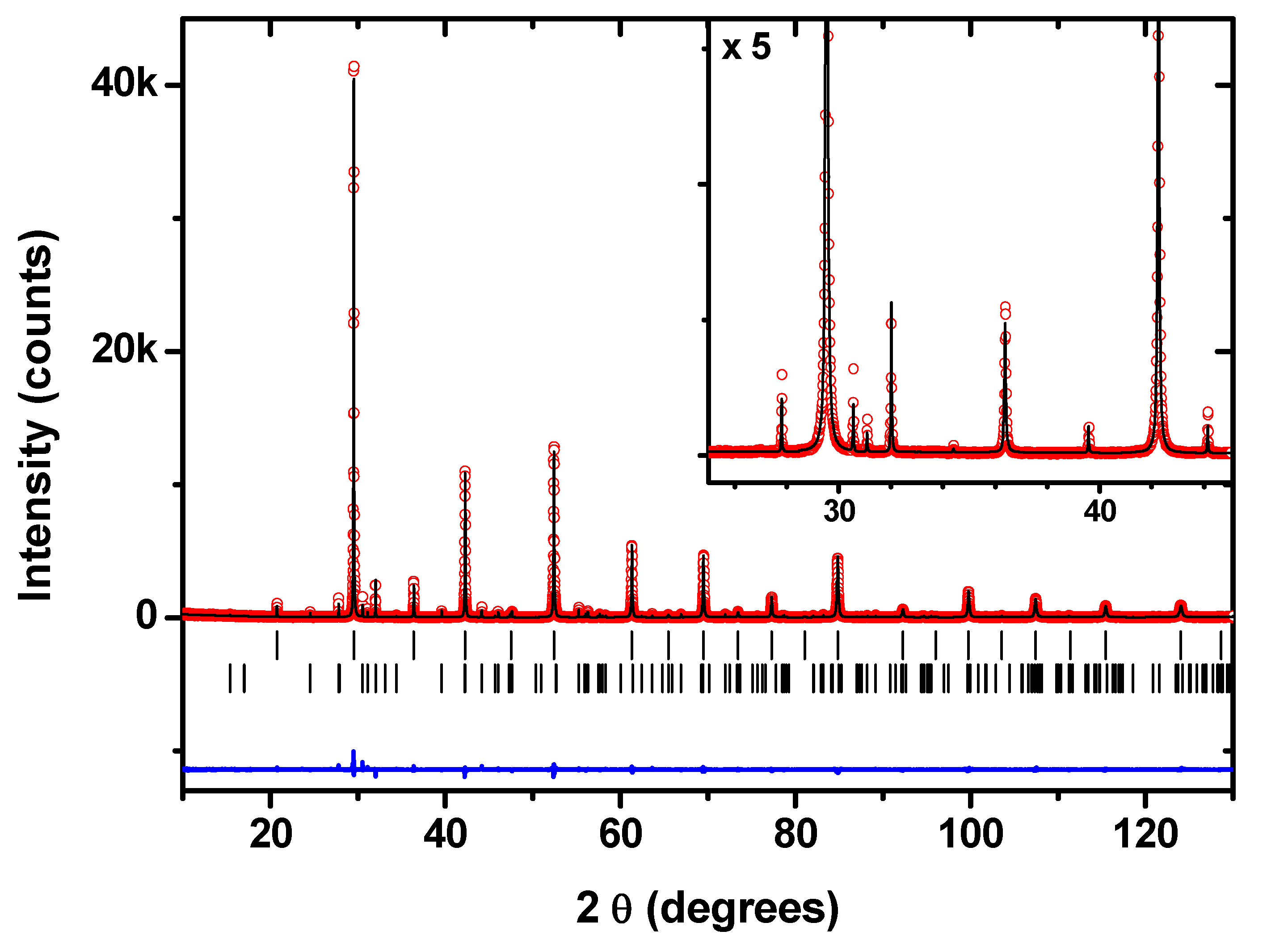
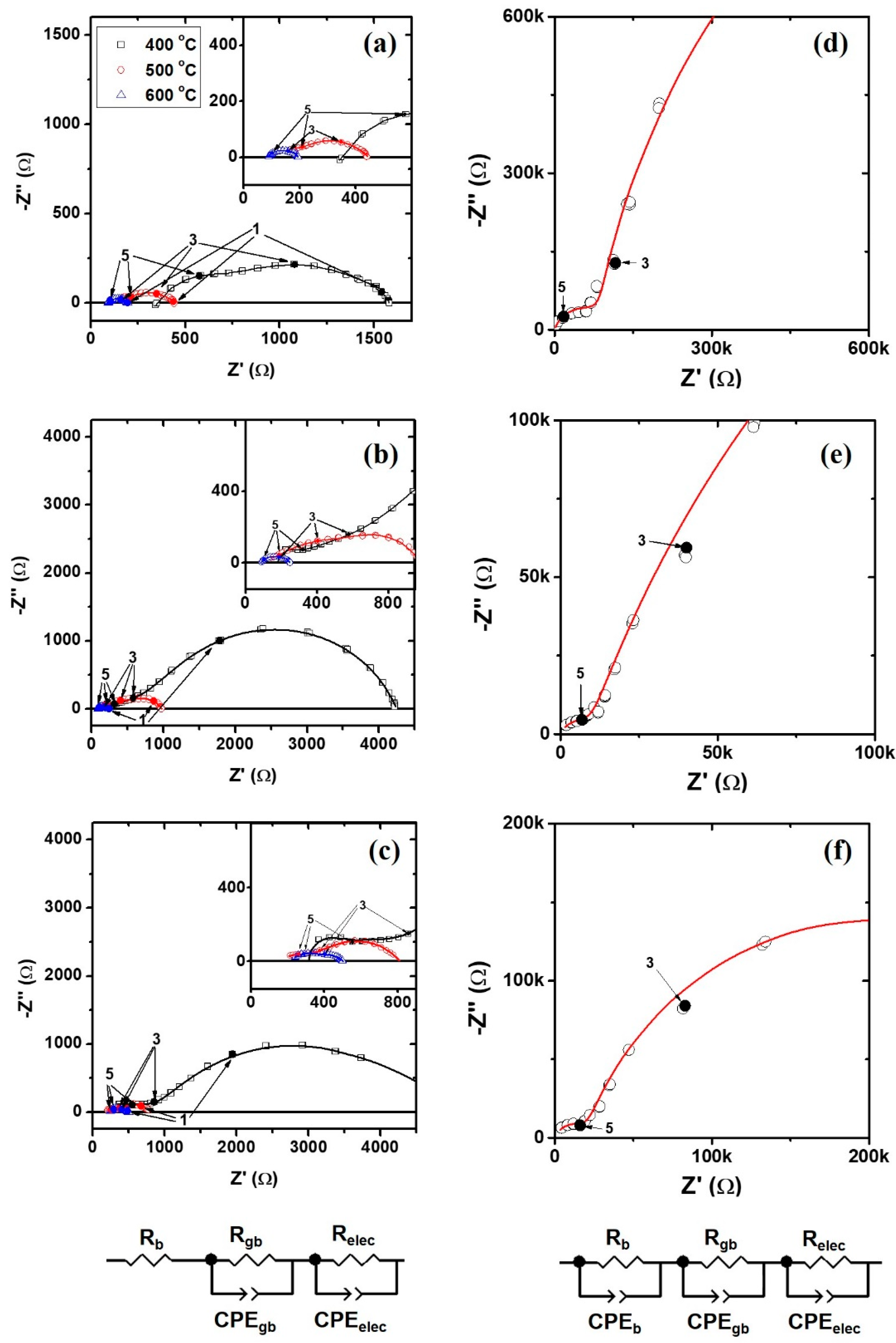
| Relative Density (%) | Ball-Milled Precursor | Planetary Ball-Milled Precursor | |||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Temperature (°C) | ||||||
| 1435 | 1485 | 1535 | 1435 | 1485 | 1535 | ||
| Time (h) | 5 | 56 | - | 87 | 94 | 94 | 94 |
| 10 | - | 82 | - | - | 94 | - | |
| 15 | 70 | - | 94 | 94 | 95 | 95 | |
| 20 | - | - | - | - | 95 | - | |
| 25 | - | - | - | - | 95 | - | |
| Grain Size (μm) | Ball-Milled Precursor | Planetary Ball-Milled Precursor | |||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Temperature (°C) | ||||||
| 1435 | 1485 | 1535 | 1435 | 1485 | 1535 | ||
| Time (h) | 5 | 0.51 | - | 1.02 | 0.61 | 0.83 | 1.06 |
| 10 | - | 0.88 | - | - | 0.93 | - | |
| 15 | 0.72 | - | 1.19 | 0.82 | 0.96 | 1.39 | |
| 20 | - | - | - | - | 1.04 | - | |
| 25 | - | - | - | - | 1.16 | - | |
| Lattice Parameter (Å) | Planetary Ball-Milled Precursor | |||
|---|---|---|---|---|
| Temperature (°C) | ||||
| 1435 | 1485 | 1535 | ||
| Time (h) | 5 | 4.213 | 4.211 | 4.208 |
| 10 | - | 4.211 | - | |
| 15 | 4.211 | 4.212 | 4.213 | |
| 20 | - | 4.209 | - | |
| 25 | - | 4.206 | - | |
| (a) BaZr0.8Y0.2O3−δ, space group:, a = 4.21163 (1) Å | |||||
| Atom | Site | x | y | z | Uiso (100 *Å2) |
| Ba | 1b | 0.5 | 0.5 | 0.5 | 1.30 (1) |
| Zr/Y | 1a | 0.0 | 0.0 | 0.0 | 0.46 (1) |
| O1 | 3d | 0.5 | 0.0 | 0.0 | 1.80 (5) |
| (b) BaY2NiO5, space group: Immm, a = 3.76162 (1) Å, b = 5.76193 (2) Å, c = 11.33135 (5) Å | |||||
| Atom | Site | x | y | z | Uiso (100 *Å2) |
| Ba | 2a | 0.5 | 0.5 | 0.0 | 1.1 (1) |
| Ni | 4j | 0.0 | 0.0 | 0.0 | 1.9 (2) |
| Y | 2c | 0.5 | 0.5 | 0.2018 (3) | 0.75 (9) |
| O1 | 2d | 0.0 | 0.211 (3) | 0.152 (1) | 1.1 (3) |
| O2 | 8l | 0.5 | 0.0 | 0.0 | 1.1 (3) |
| BaZr0.8Y0.2O3−δ Powder Synthesis Method | Sintering T and Time (°C/h) | Conductivity (mS∙cm−1, 600 °C) | Conductivity (mS∙cm−1, 500 °C) | Atmosphere (gas/atm with H2O) | Reference |
|---|---|---|---|---|---|
| Glycine-nitrate combustion | 1600/10 | 6.91 | 5.2 | N2/0.03 | [4] |
| Sol-gel combustion | 1500/6 | 2.14 | 1.12 | Ar/0.03 | [8] |
| Sol-gel combustion | 1600/6 | 4.45 | 2.95 | Ar/0.03 | [10] |
| Solid-state reactive sintering | 1500/24 | 33.1 | 16.9 | Ar/0.03 | [15] |
| Solid-state reaction | 1450/5 | 3.09 | 1.26 | Ar/0.03 | [18] |
| Solid-state reaction | 1675/10 | 3.62 | 2.03 | 40% H2/0.04 | [27] |
| EDTA-citric acid precipitation | 1600/24 | 22.9 | 14.8 | N2,/0.03 | [29] |
| Solid-state reactive sintering | 1435/15 | 3.00 | 1.15 | 3% H2/0.03 | This study |
| Solid-state reactive sintering | 1485/15 | 3.00 | 2.28 | 3% H2/0.03 | This study |
| Solid-state reactive sintering | 1535/15 | 1.24 | 2.01 | 3% H2/0.03 | This study |
| Sintering Temperature (°C) | 1435 | 1485 | 1535 |
|---|---|---|---|
| Sintering Time (h) | 15 | 15 | 15 |
| Cbulk (F) | 6.64 × 10–11 | 9.60 × 10–11 | 6.64 × 10–11 |
| CGB (F) | 1.88 × 10–9 | 3.88 × 10–9 | 1.88 × 10–9 |
| εr | 266 | 384 | 266 |
| λ (nm) | 0.35 | 0.42 | 0.60 |
| Δϕ(0) | 0.18 | 0.29 | 0.09 |
| λ* (nm) | 1.47 | 2.22 | 1.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, D.S.; Kim, J.; Kim, S.-J.; Lee, J.-H.; Kim, J.-N.; Yoon, H.C.; Yu, J.H.; Kwak, M.; Yoon, H.; Cho, Y.; et al. Structural and Electrochemical Properties of Dense Yttria-Doped Barium Zirconate Prepared by Solid-State Reactive Sintering. Energies 2018, 11, 3083. https://doi.org/10.3390/en11113083
Yun DS, Kim J, Kim S-J, Lee J-H, Kim J-N, Yoon HC, Yu JH, Kwak M, Yoon H, Cho Y, et al. Structural and Electrochemical Properties of Dense Yttria-Doped Barium Zirconate Prepared by Solid-State Reactive Sintering. Energies. 2018; 11(11):3083. https://doi.org/10.3390/en11113083
Chicago/Turabian StyleYun, Dae Sik, Jaegyeom Kim, Seung-Joo Kim, Jong-Heun Lee, Jong-Nam Kim, Hyung Chul Yoon, Ji Haeng Yu, Minseok Kwak, Hana Yoon, Younghyun Cho, and et al. 2018. "Structural and Electrochemical Properties of Dense Yttria-Doped Barium Zirconate Prepared by Solid-State Reactive Sintering" Energies 11, no. 11: 3083. https://doi.org/10.3390/en11113083
APA StyleYun, D. S., Kim, J., Kim, S.-J., Lee, J.-H., Kim, J.-N., Yoon, H. C., Yu, J. H., Kwak, M., Yoon, H., Cho, Y., & Yoo, C.-Y. (2018). Structural and Electrochemical Properties of Dense Yttria-Doped Barium Zirconate Prepared by Solid-State Reactive Sintering. Energies, 11(11), 3083. https://doi.org/10.3390/en11113083







