Investigating Influential Factors of the Gas Absorption Capacity in Shale Reservoirs Using Integrated Petrophysical, Mineralogical and Geochemical Experiments: A Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Petrophysical Measurements
2.3. Low Pressure N2 Adsorption and MIP Experiments
2.4. Geochemical and Mineralogical Examinations
3. Results and Discussions
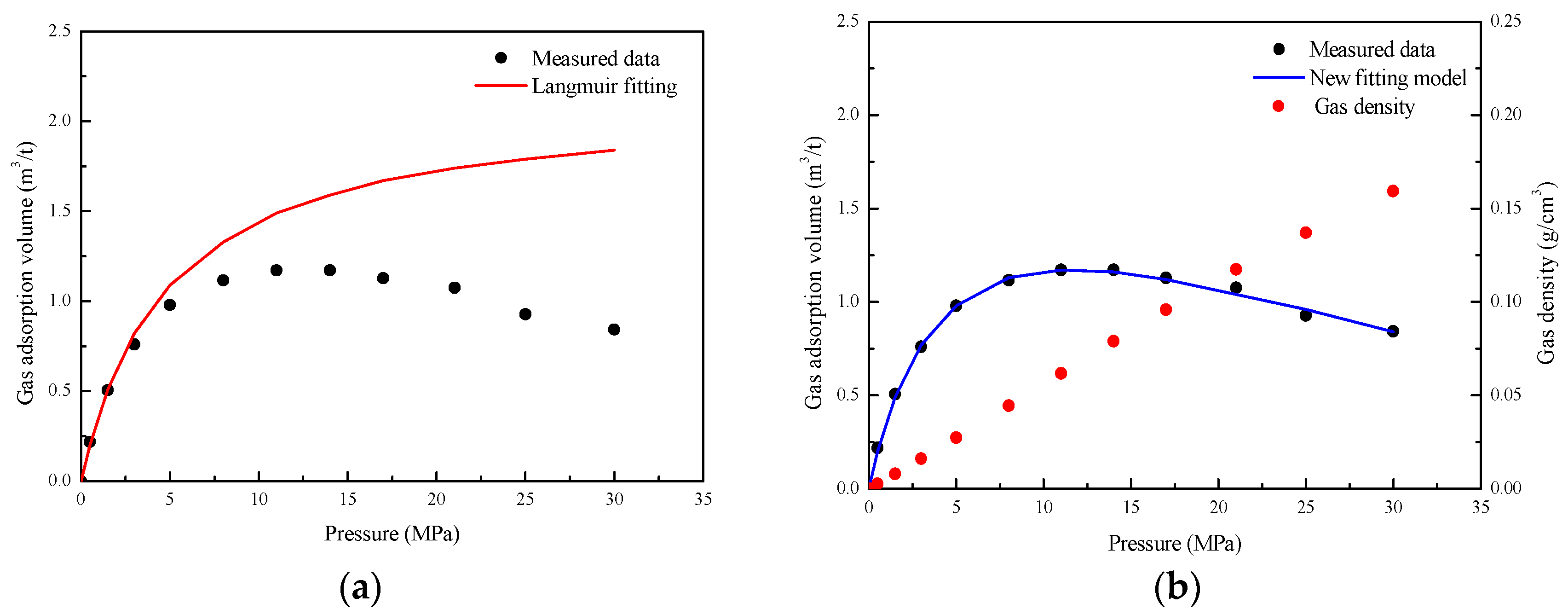
3.1. Gas Adsorption Volume Correction
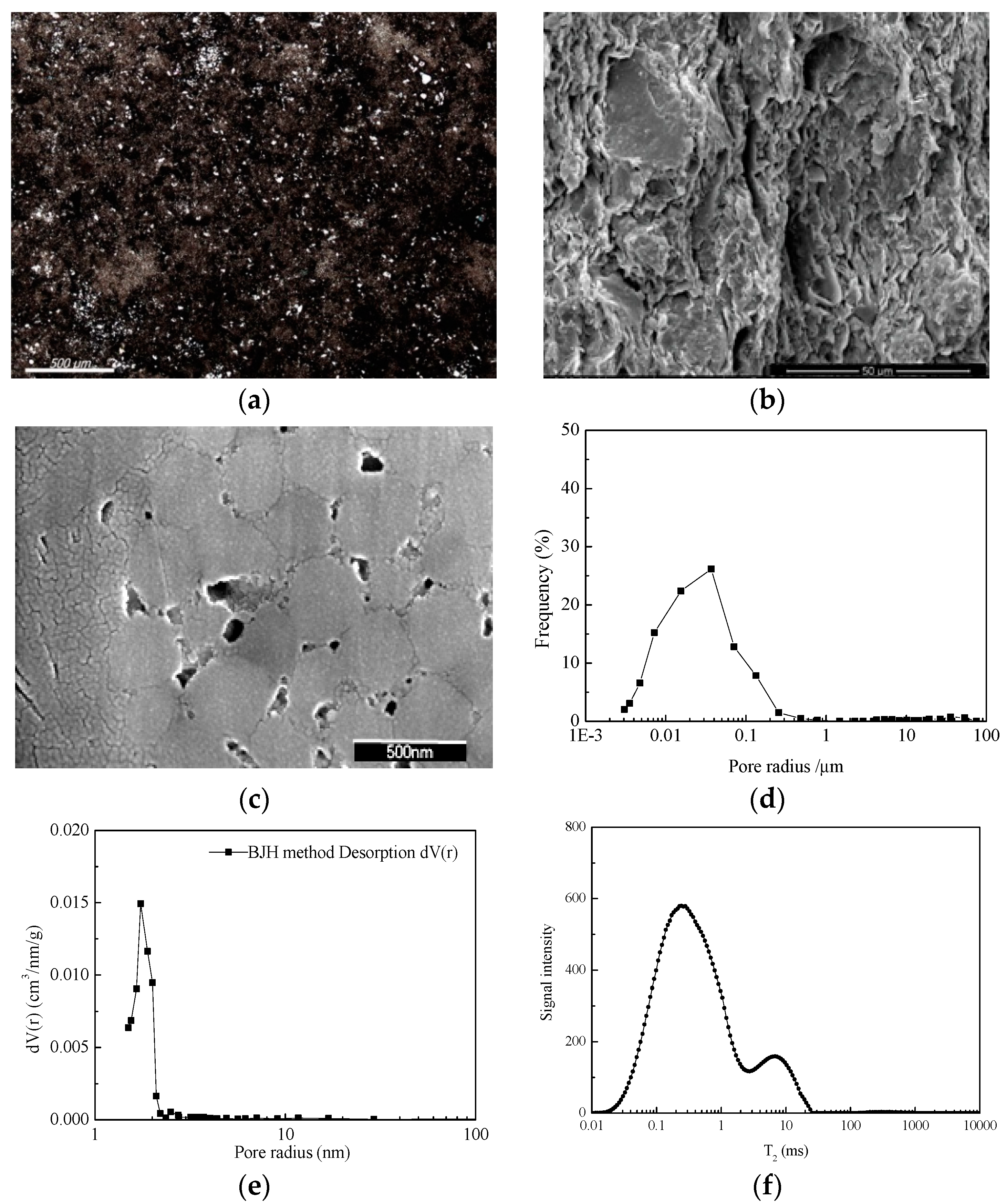
3.2. Pore Structure Characterization
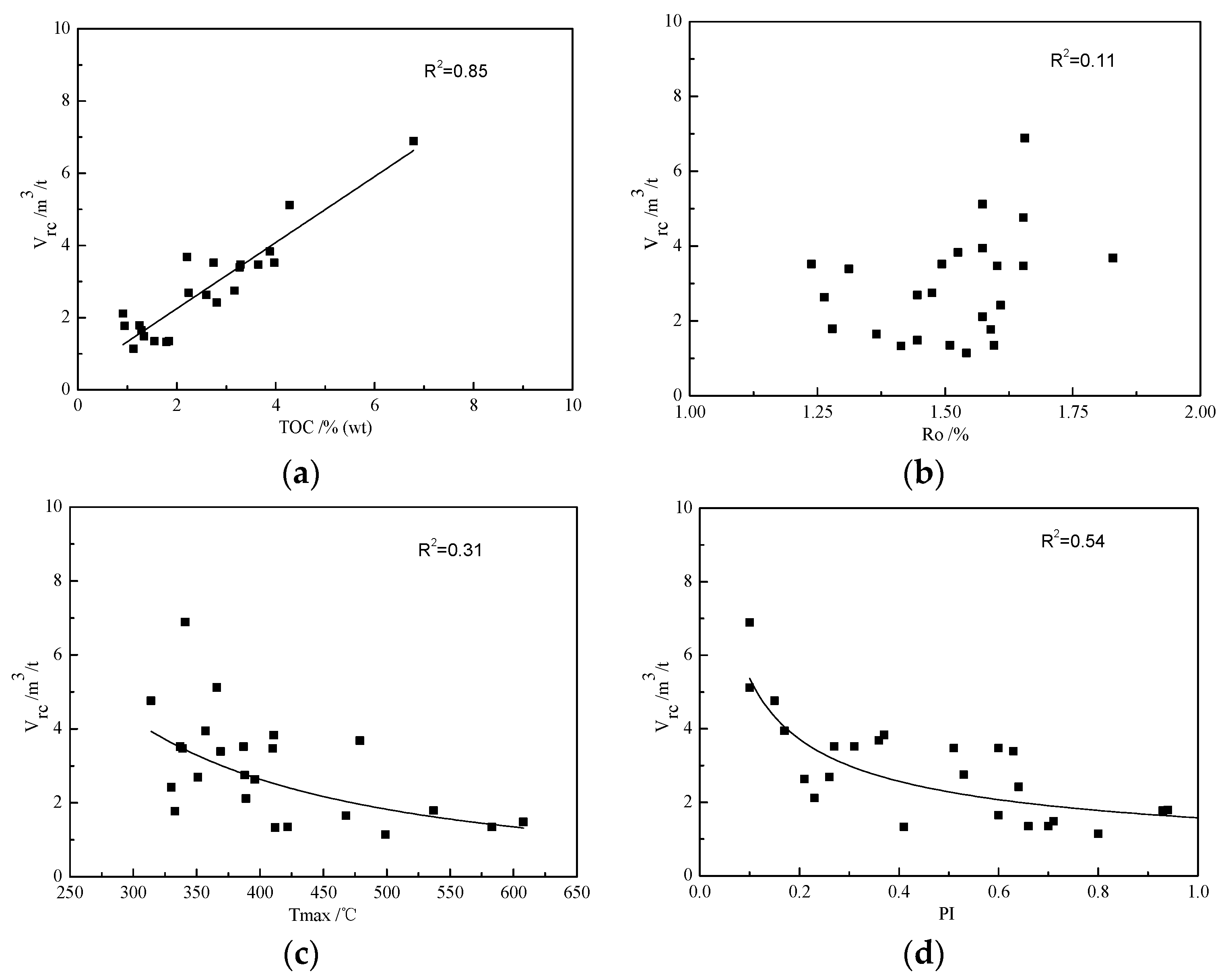
3.3. Effects of Geochemical Properties
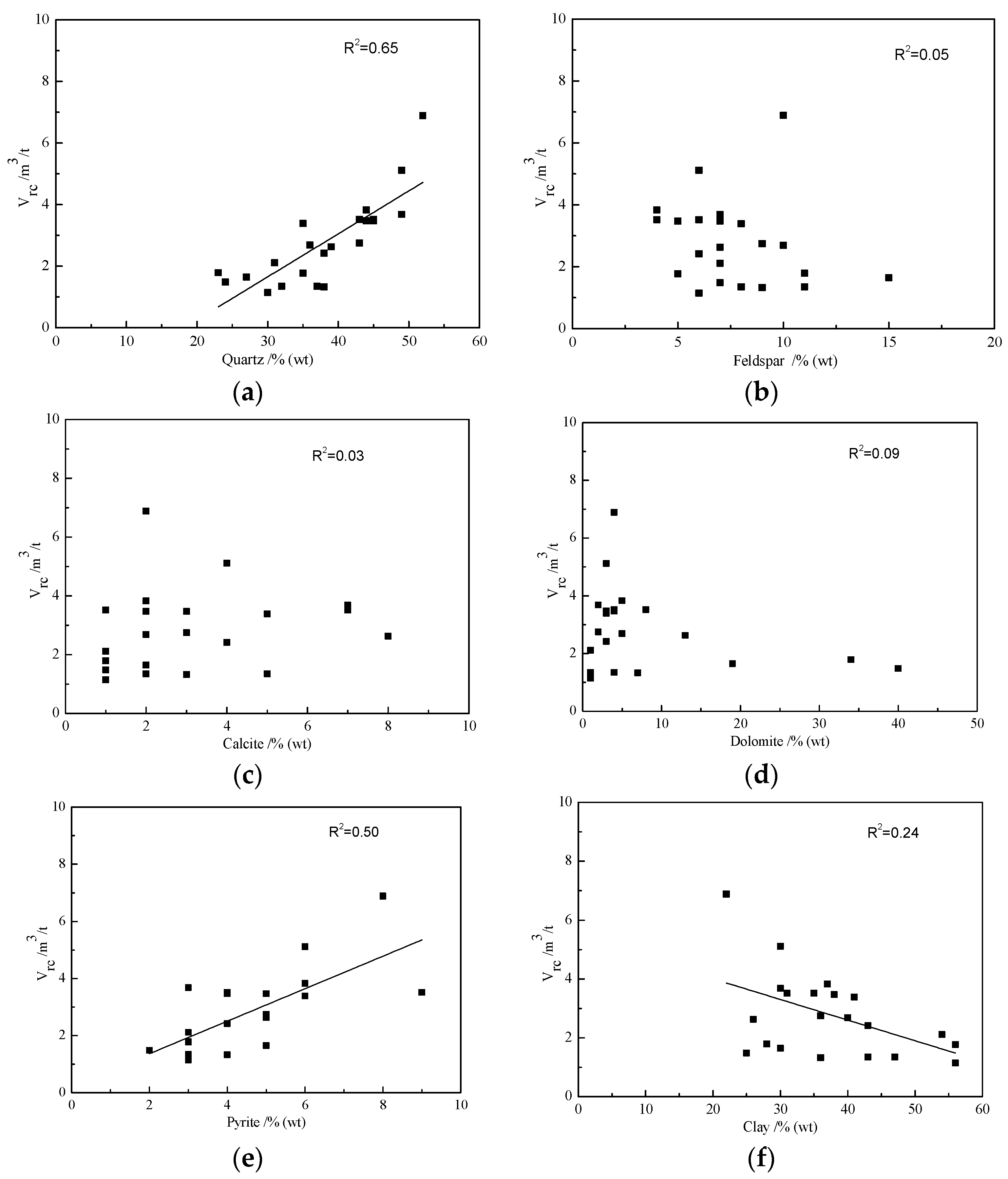
3.4. Effects of Mineralogical Compositions
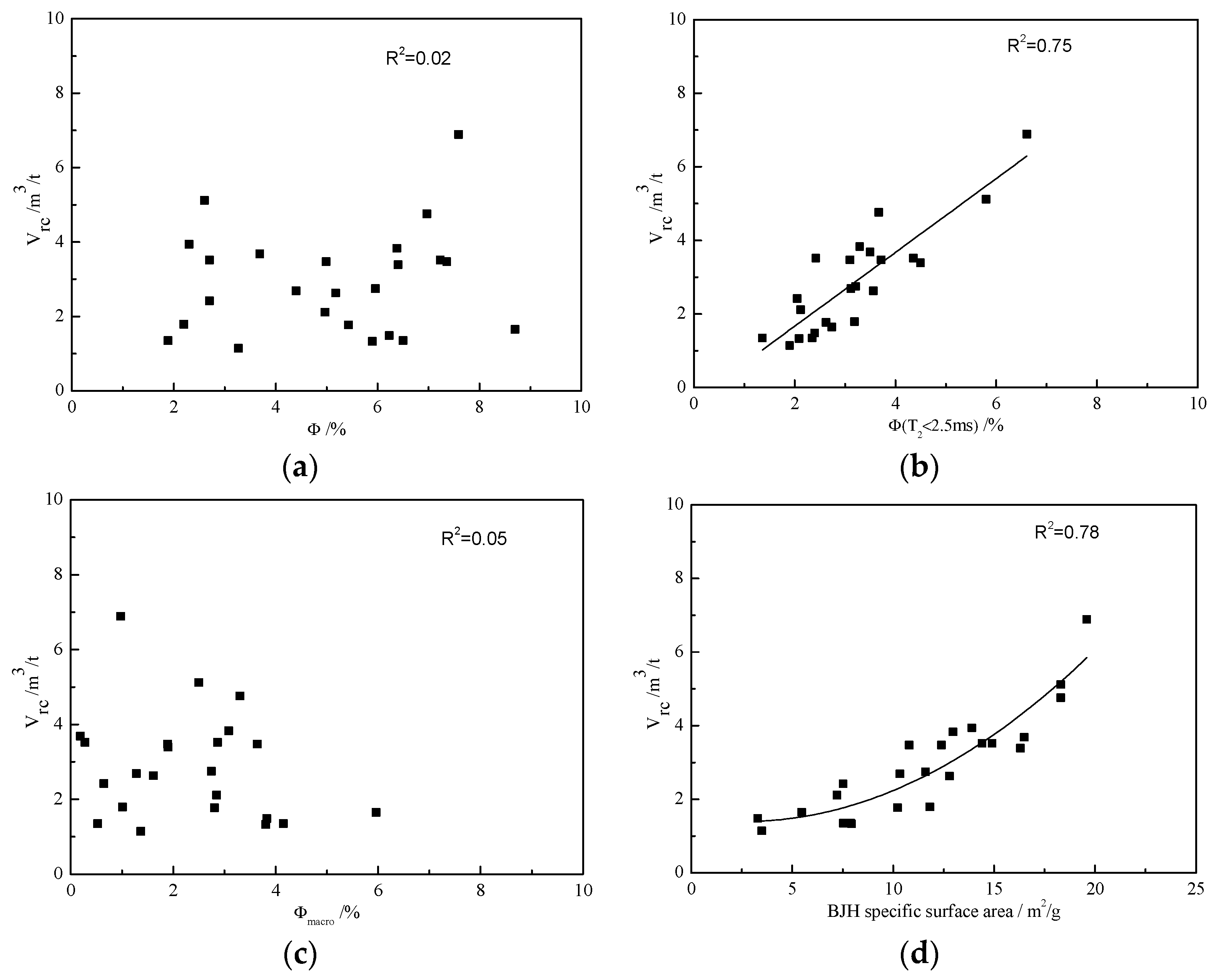
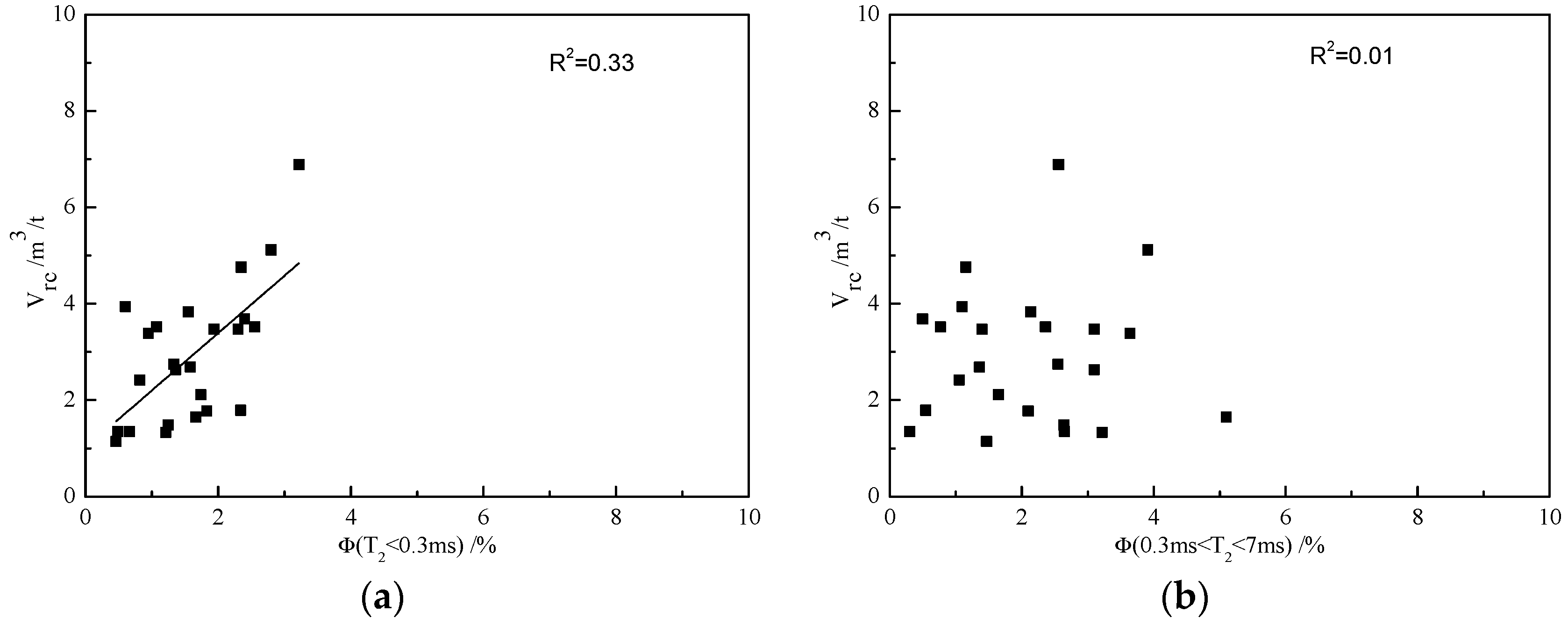
3.5. Effects of Pore Size Distribution
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ross, D.J.K.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Petrol. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Hu, H. Methane adsorption comparison of different thermal maturity kerogens in shale gas system. Chin. J. Geochem. 2014, 33, 425–430. [Google Scholar] [CrossRef]
- Li, J.; Yan, X.; Wang, W.; Zhan, Y.; Yin, J.; Lu, S.; Chen, F.; Meng, Y.; Zhang, X.; Chen, X.; et al. Key factors controlling the gas adsorption capacity of shale: A study based on parallel experiments. Appl. Geochem. 2015, 58, 88–96. [Google Scholar] [CrossRef]
- Montgomery, S.L.; Jarvie, D.M.; Bowker, K.A.; Pallastro, R.M. Mississippian Barnett Shale, Fort Worth basin, north-central Texas: Gas-shale play with multi-trillion cubic foot potential. AAPG Bull. 2005, 89, 155–175. [Google Scholar] [CrossRef]
- Zhang, T.; Ellis, G.; Ruppel, S.; Milliken, K.; Yang, R. Effect of organic-matter type and thermal maturity on methane adsorption in shale-gas systems. Org. Geochem. 2012, 47, 120–131. [Google Scholar] [CrossRef]
- Topóra, T.L.; Derkowski, A.; Ziemiański, P.; Szczurowski, J.; McCarty, D.K. The effect of organic matter maturation and porosity evolution on methane storage potential in the Baltic Basin (Poland) shale-gas reservoir. Int. J. Coal Geol. 2017, 180, 46–56. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Liu, S.; Zhang, R. Pore characterization and its impact on methane adsorption capacity for organic-rich marine shales. Fuel 2016, 181, 227–237. [Google Scholar] [CrossRef]
- Aringhieri, R. Nanoporosity characteristics of some natural clay minerals and soils. Clays Clay Miner. 2004, 52, 700–704. [Google Scholar] [CrossRef]
- Ji, W.; Song, Y.; Jiang, Z.; Wang, X.; Bai, X.; Xing, J. Geological controls and estimation algorithms of lacustrine shale gas adsorption capacity: A case study of the Triassic strata in the southeastern Ordos Basin, China. Int. J. Coal Geol. 2014, 134, 134–135. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, G.; Lv, C.; Yang, W.; Xu, Y.; Yang, S. Experimental study of the impact on methane adsorption capacity of continental shales with thermal evolution. J. Nat. Gas Geol. 2016, 1, 165–172. [Google Scholar] [CrossRef]
- Gasparik, M.; Bertier, P.; Gensterblum, Y.; Ghanizadeh, A.; Krooss, B.M.; Littke, R. Geological controls on the methane storage capacity in organic-rich shales. Int. J. Coal Geol. 2014, 123, 34–51. [Google Scholar] [CrossRef]
- Xiong, F.; Wang, X.; Amooiea, M.A.; Soltanian, M.R.; Jiang, Z.; Moortgat, J. RETRACTED: The shale gas sorption capacity of transitional shales in the Ordos Basin, NW China. Fuel 2017, 208, 236–246. [Google Scholar] [CrossRef]
- Guo, S.; Lü, X.; Song, X.; Liu, Y. Methane adsorption characteristics and influence factors of Mesozoic shales in the Kuqa Depression, Tarim Basin, China. J. Pet. Sci. Eng. 2017, 157, 187–195. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, H.; Ning, Y.; Guo, W.; Zhang, Q. Experimental study of supercritical methane adsorption in Longmaxi shale: Insights into the density of adsorbed methane. Fuel 2018, 211, 140–148. [Google Scholar] [CrossRef]
- Ji, W.; Song, Y.; Jiang, Z.; Chen, L.; Li, Z.; Yang, X.; Meng, M. Estimation of marine shale methane adsorption capacity based on experimental investigations of Lower Silurian Longmaxi formation in the Upper Yangtze Platform, south China. Mar. Petrol. Geol. 2015, 68, 94–106. [Google Scholar] [CrossRef]
- Ma, X.; Song, Y.; Liu, S.; Jiang, L.; Hong, F. Experimental study on history of methane adsorption capacity of Carboniferous-Permian coal in Ordos Basin, China. Fuel 2016, 184, 10–17. [Google Scholar] [CrossRef]
- Pang, Y.; Soliman, M.Y.; Deng, H.; Xie, X. Experimental and analytical investigation of adsorption effects on shale gas transport in organic nanopores. Fuel 2017, 199, 272–288. [Google Scholar] [CrossRef]
- Han, H.; Zhong, N.; Ma, Y.; Huang, C.; Wang, Q.; Chen, S.; Lu, J. Gas storage and controlling factors in an over-mature marine shale: Acase study of the Lower Cambrian Lujiaping shale in the Dabashan arclike thrustefold belt, southwestern China. J. Pet. Sci. Eng. 2016, 33, 839–853. [Google Scholar] [CrossRef]
- Xing, J.; Hu, S.; Jiang, Z.; Wang, X.; Wang, J.; Sun, L.; Bai, Y.; Chen, L. Classification of controlling factors and determination of a prediction model for shale gas adsorption capacity: A case study of Chang 7 shale in the Ordos Basin. J. Pet. Sci. Eng. 2018, 49, 260–274. [Google Scholar] [CrossRef]
- Ge, X.; Liu, J.; Fan, Y.; Xing, D.; Deng, S.; Cai, J. Laboratory investigation into the formation and dissociation process of gas hydrate by low field NMR technique. J. Geophys. Res.-Sol. Earth 2018, 123, 3339–3346. [Google Scholar] [CrossRef]
- Ge, X.; Fan, Y.; Cao, Y.; Li, J.; Cai, J.; Liu, J.; Wei, S. Investigation of organic related pores in unconventional reservoir and its quantitative evaluation. Energy Fuel 2016, 30, 4699–4709. [Google Scholar] [CrossRef]
- Heller, R.; Zoback, M. Adsorption of methane and carbon dioxide on gas shale and pure mineral samples. J. Uncon. Oil Gas Resour. 2014, 8, 14–24. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, H.; Xue, H.; Guo, W.; Lu, B. Difference between excess and absolute adsorption capacity of shale and a new shale gas reserve calculation method. Nat. Gas Ind. 2016, 36, 12–20. [Google Scholar]
- Gasparik, G.; Ghanizadeh, A.; Bertier, P.; Gensterblum, Y.; Bouw, S.; Krooss, B.M. High-pressure methane sorption isotherms of black shales from the Netherlands. Energy Fuel 2012, 26, 4995–5004. [Google Scholar] [CrossRef]
- Rexer, T.F.T.; Benham, M.J.; Aplin, A.C.; Thomas, K.M. Methane adsorption on shale under simulated geological temperature and pressure conditions. Energy Fuel 2013, 27, 3099–3109. [Google Scholar] [CrossRef] [Green Version]
- Singer, P.M.; Rylander, E.; Jiang, T.; McLin, R.; Lewis, R.E.; Sinclair, S.M. 1D and 2D NMR core-log intergration in organic shale. In Proceedings of the International Symposium of the Society of Core Analysts, Napa Valley, CA, USA, 16–19 September 2013. [Google Scholar]
- Kuila, U.; Prasad, M. Specific surface area and pore-size distribution in clays and shales. Geophys. Prospect. 2013, 61, 341–362. [Google Scholar] [CrossRef]
- Ge, X.; Fan, Y.; Cao, Y.; Xu, Y.; Liu, X.; Chen, Y. Reservoir pore structure classification technology of carbonate rock based on NMR T2 spectrum decomposition. Appl. Magn. Reson. 2014, 45, 155–167. [Google Scholar] [CrossRef]
- Jarvie, D.M.; Hill, R.J.; Ruble, T.E.; Pollastro, R.M. Unconventional shale-gas systems: The Mississippian Barnett Shale of north-central Texas as one model for thermogenic shale-gas assessment. AAPG Bull. 2007, 91, 475–499. [Google Scholar] [CrossRef]
- Chalmers, G.R.; Bustin, R. Lower Cretaceous Gas Shales of Northeastern British Columbia: Geological Controls on Gas Capacity and Regional Evaluation of a Potential Resource. Bull. Can. Pet. Geol. 2008, 56, 1–21. [Google Scholar] [CrossRef]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Jarvie, D.M. Morphology, genesis, and distribution of nanometer-scale pores in siliceous mudstones of the Mississippian Barnett Shale. J. Sediment. Res. 2009, 79, 848–861. [Google Scholar] [CrossRef]
- Milliken, K.L.; Rudnicki, M.; Awwiller, D.N.; Zhang, T. Organic matter-hosted pore system, Marcellus Formation (Devonian), Pennsylvania. AAPG Bull. 2013, 97, 177–200. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
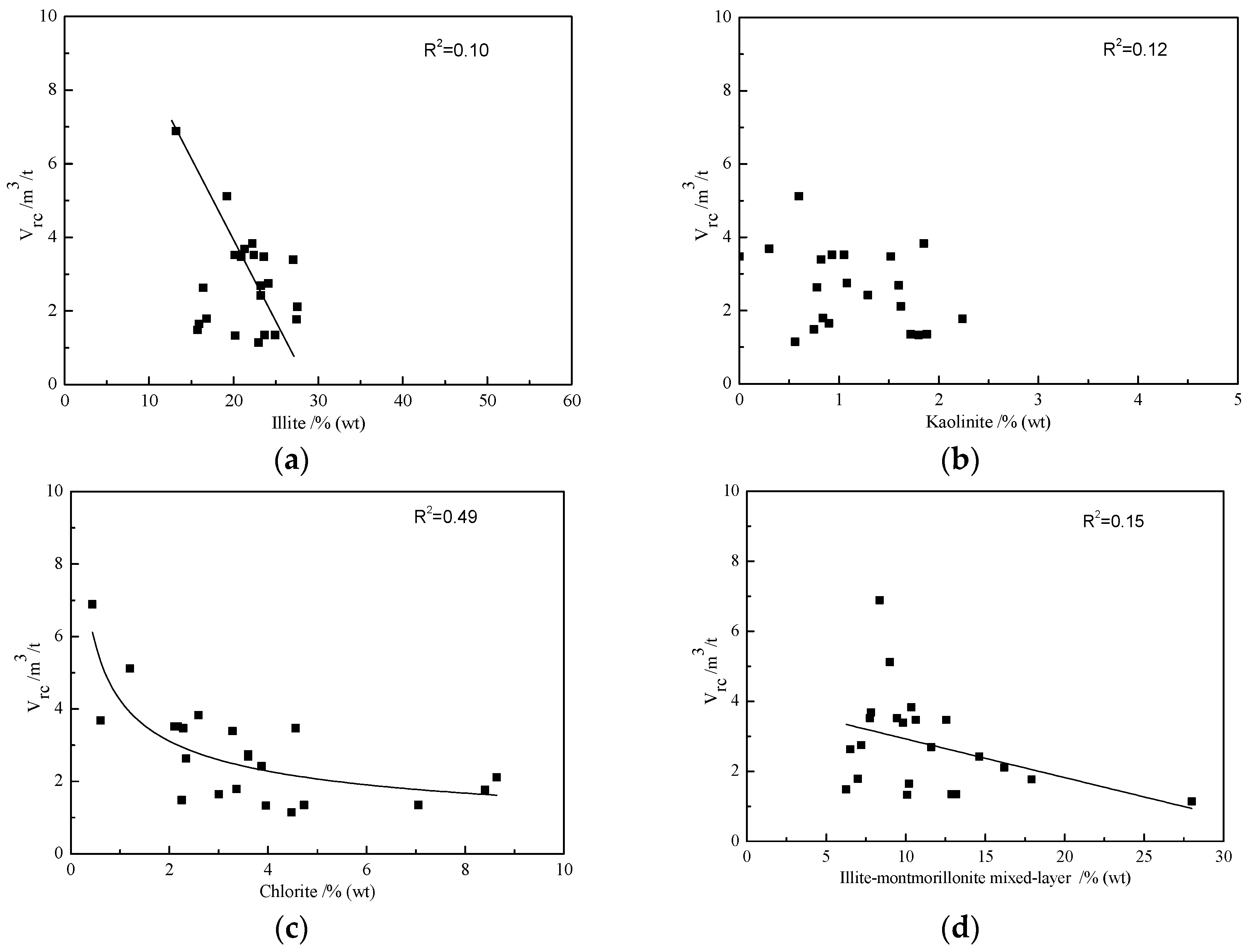
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.; Hou, J.; Ge, X.; Zhao, P.; Liu, J. Investigating Influential Factors of the Gas Absorption Capacity in Shale Reservoirs Using Integrated Petrophysical, Mineralogical and Geochemical Experiments: A Case Study. Energies 2018, 11, 3078. https://doi.org/10.3390/en11113078
Fan Z, Hou J, Ge X, Zhao P, Liu J. Investigating Influential Factors of the Gas Absorption Capacity in Shale Reservoirs Using Integrated Petrophysical, Mineralogical and Geochemical Experiments: A Case Study. Energies. 2018; 11(11):3078. https://doi.org/10.3390/en11113078
Chicago/Turabian StyleFan, Zhuoying, Jiagen Hou, Xinmin Ge, Peiqiang Zhao, and Jianyu Liu. 2018. "Investigating Influential Factors of the Gas Absorption Capacity in Shale Reservoirs Using Integrated Petrophysical, Mineralogical and Geochemical Experiments: A Case Study" Energies 11, no. 11: 3078. https://doi.org/10.3390/en11113078




