Hydrothermal Carbonization of Peat Moss and Herbaceous Biomass (Miscanthus): A Potential Route for Bioenergy
Abstract
1. Introduction
2. Materials and Methods
2.1. Carbonization
2.2. Proximate Analysis
2.3. Ultimate Analysis
2.4. Heating Value
2.5. Mass and Energy Yield
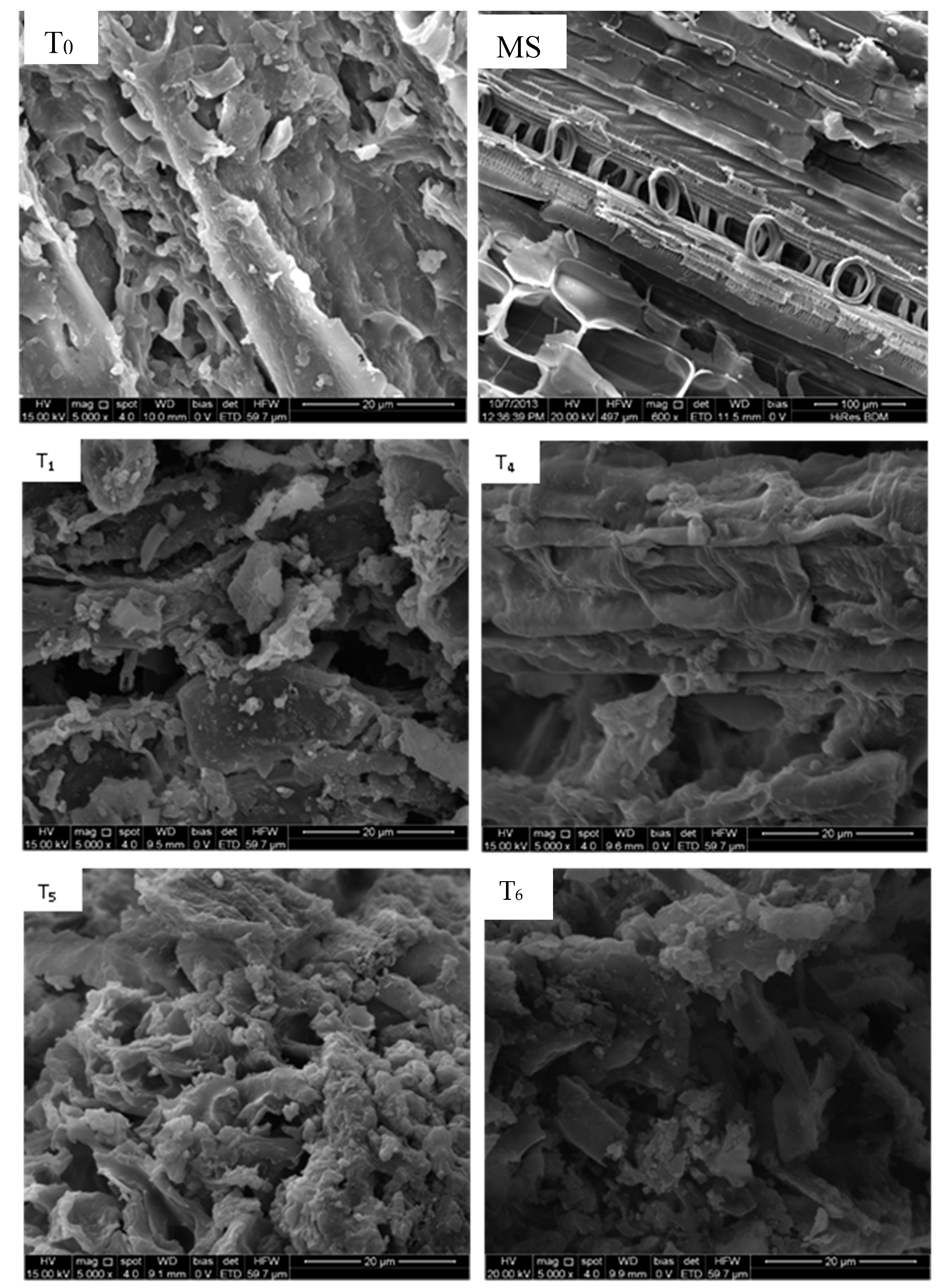
2.6. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX)
2.7. Combustion Indices of Hydrochar
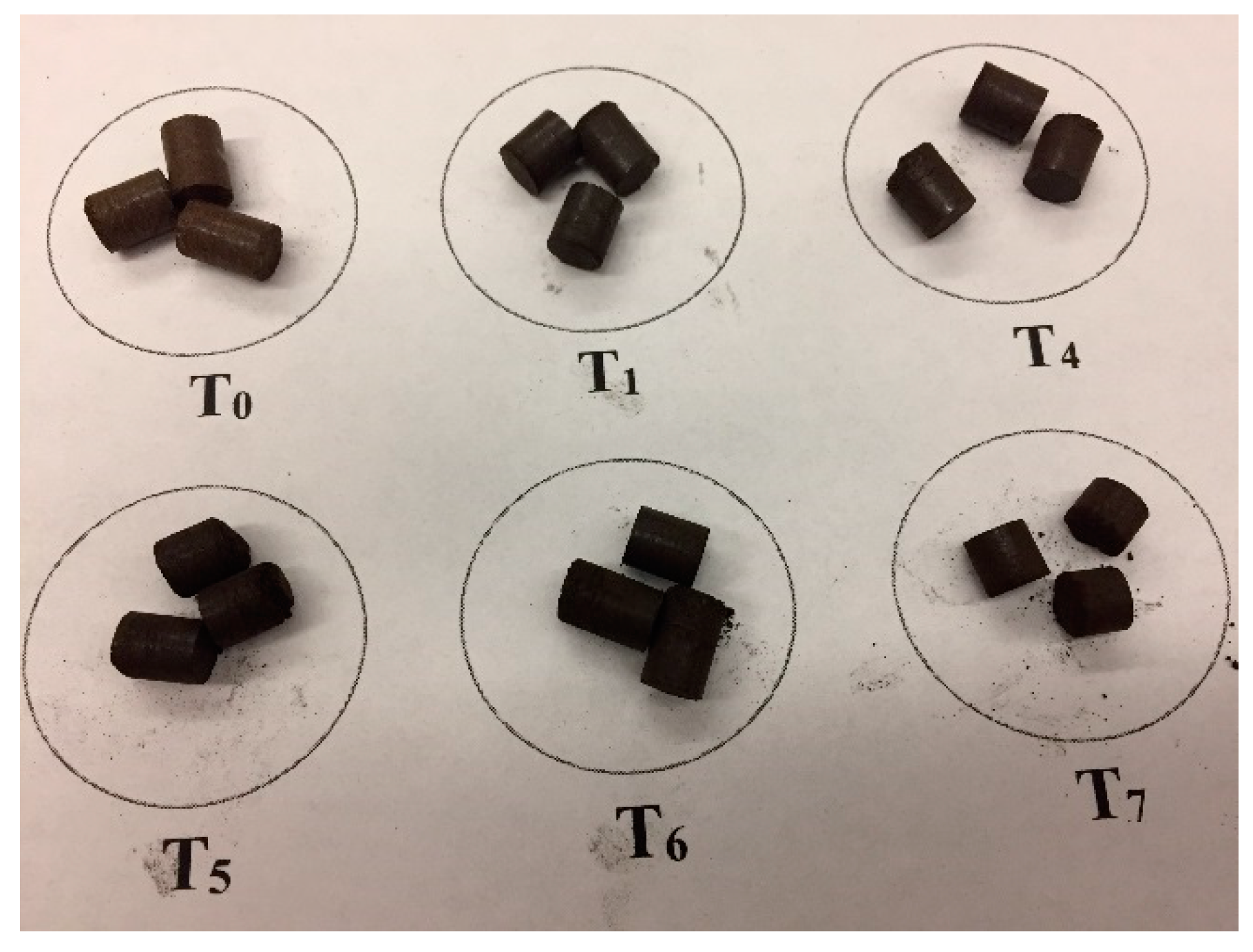
2.8. Pelletization
2.9. Equilibrium Moisture Content (EMC)
- MC = Moisture content of the sample
- Mi = Initial mass of the sample
- Md = Mass of the oven dried sample
2.10. Hardness of Pellets
2.11. Durability
3. Results and Discussion
3.1. Effect of Processing Conditions on the Compositions of Hydrochar
3.2. Effect of Processing on the Mass Yield, Energy Yield, and HHV
3.3. Effect of Hydrothermal Carbonization on the Equilibrium Moisture Content (EMC)
3.4. Hardness and Durability of Pellets
3.5. Effect of HTC on the Combustion Indices of Hydrochar
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Acknowledgments
Conflicts of Interest
References
- Towards Climate-responsible Peatlands Management. Available online: http://www.fao.org/3/a-i4029e.pdf (accessed on 27 February 2018).
- Sphagnum Farming for Replacing Peat in Horticultural Substrates. Available online: http://www.fao.org/3/a-i4417e.pdf (accessed on 5 February 2018).
- Environment Canada. North American Waterfowl Management Plan. Available online: https://www.fws.gov/birds/management/bird-management-plans/north-american-waterfowl-management-plan.php (accessed on 27 February 2018).
- Brown, D.; Rowe, A.; Wild, P. A techno-economic analysis of using mobile distributed pyrolysis facilities to deliver a forest residue resource. Bioresour. Technol. 2013, 150, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Hitzl, M.; Corma, A.; Pomares, F.; Renz, M. The hydrothermal carbonization (HTC) plant as a decentral biorefinery for wet biomass. Catal. Today 2015, 257, 154–159. [Google Scholar] [CrossRef]
- Tang, Y.; Cong, W.; Xu, J.; Zhang, P.; Liu, D. Ultrasonic vibration-assisted pelleting for cellulosic biofuels manufacturing: A study on in-pellet temperatures. Renew. Energy 2015, 76, 296–302. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, S.; Wang, C.; Mu, C.; Huang, X. High-strength charcoal briquette preparation from hydrothermal pretreated biomass wastes. Fuel Process. Technol. 2018, 171, 293–300. [Google Scholar] [CrossRef]
- Bragazza, L.; Buttler, A.; Siegenthaler, A.; Mitchell, E.A.D. Plant Litter Decomposition and Nutrient Release in Peatlands. Carbon Cycl. North Peatl. 2008, 184, 99–110. [Google Scholar]
- Davis, R.C. Peat respiration and decomposition in Antarctic terrestrial moss communities. Biol. J. Linn. Soc. 1980, 14, 39–49. [Google Scholar] [CrossRef]
- Frolking, S.; Talbot, J.; Jones, M.C.; Treat, C.C.; Kauffman, J.B.; Tuittila, E.-S.; Roulet, N. Peatlands in the Earth’s 21st century climate system. Environ. Rev. 2011, 19, 371–396. [Google Scholar] [CrossRef]
- Yu, Z.C.; Beilman, D.W.; Frolking, S.; MacDonald, G.M.; Roulet, N.T.; Camill, P.; Charman, D.J. Peatlands and their role in the global carbon cycle. Eos. Trans. Am. Geophys Union 2011, 92, 97–98. [Google Scholar] [CrossRef]
- Association of Canadian Sphagnum Peat Moss. SAFA Case Study: A first Industry Social Responsibility Report for the Canadian Sphagnum Peat Moss Association using SAFA Guidelines. Available online: http://tourbehorticole.com/wp-content/uploads/2015/07/CSPMA_ISR_Report_2014_web_LW.pdf (accessed on 25 February 2018).
- Environment Canada. National Inventory Report 1990–2011: Greenhouse Gas Sources and Sinks in Canada. Available online: http://publications.gc.ca/collections/collection_2013/ec/En81-4-2011-1-eng.pdf (accessed on 25 February 2018).
- Rubec CDA. Canadian Wetland Inventory: Hard Issues and Realities. Available online: http://nawcc.wetlandnetwork.ca/Can%20Peat%20Harvesting%202001-1.pdf (accessed on 15 February 2018).
- Moss CSP, Association. Compost Council of Canada Peat Industry Sustainability Presentation. 2013. Available online: http://www.compost.org/conf2013/FC3_Partners_in_the_field/2_Sustaining_the_Peat_Industry_CSPMA.pdf (accessed on 25 February 2018).
- Sustainability Canadian Horticultural Peat Industry Position Paper. Available online: http://tourbehorticole.com/wp-content/uploads/2015/09/CSPMA-Sustainability-Position-Paper.pdf (accessed on 12 March 2018).
- Carlson, M.; Chen, J.; Elgie, S.; Henschel, C.; Montenegro, Á.; Roulet, N.; Scott, N.; Tarnocai, C.; Wells, J. Maintaining the role of Canada’s forests and peatlands in climate regulation. For. Chron. 2010, 86, 434–443. [Google Scholar] [CrossRef]
- Belyea, L.R. Separating the effects of litter quality and microenvironment on decomposition rates in a patterned Peatland. OIKOS 1996, 77, 529–539. [Google Scholar] [CrossRef]
- Mathur, S.P.; Levesque, M.P. Relationship between acid phosphatase activities and decomposition rates of twenty-two virgin peat materials. Commun. Soil Sci. Plant Anal. 1980, 11, 155–162. [Google Scholar] [CrossRef]
- Ok, Y.S.; Uchimiya, S.M.; Chang, S.X.; Bolan, N. Biochar: Production, Characterization, and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Rudz, P. Carbon and Nutrient Dynamics of Downed Woody Debris in a Northern Hardwood Forest. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2013. [Google Scholar]
- Cleary, J.; Roulet, N.T.; Moore, T.R. Greenhouse Gas emissions from canadian peat extraction, 1990–2000: A life-cycle analysis. AMBIO J. Hum. Environ. 2005, 34, 456–461. [Google Scholar] [CrossRef]
- Frolking, S.; Roulet, N.T.; Moore, T.R.; Richard, P.J.H.; Lavoie, M.; Muller, S.D. Modeling northern peatland decomposition and peat accumulation. Ecosystems 2001, 4, 479–498. [Google Scholar] [CrossRef]
- Moore, T.R. Growth and net production of Sphagnum at five fen sites, subarctic eastern Canada. Can. J. Bot. 1989, 67, 1203–1207. [Google Scholar] [CrossRef]
- Authority OP. Achieving Balance: Ontario’s Long-term Energy Plan. Available online: http://www.ontariopetroleuminstitute.com/wp-content/uploads/2014/06/Achieving-Balance-Ontarios-Long-Term-Energy-Plan.pdf (accessed on 5 March 2018).
- Canada CE. Ontario’s Coal Phaseout in Perspective. Available online: http://cleanenergycanada.org/ontarios-coal-phaseout-perspective/ (accessed on 5 March 2018).
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Felix, L.; Farthing, W. Hydrothermal carbonization (HTC) of loblolly pine using a continuous, reactive twin-screw extruder. Energy Convers. Manag. 2017, 134, 247–259. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, L.; Sik, Y.; Gao, B. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Takaya, C.A.; Fletcher, L.A.; Singh, S.; Anyikude, K.U.; Ross, A.B. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Kambo, H.S.; Dutta, A. Comparative evaluation of torrefaction and hydrothermal carbonization of lignocellulosic biomass for the production of solid biofuel. Energy Convers. Manag. 2015, 105, 746–755. [Google Scholar] [CrossRef]
- Kludze, H.; Deen, B.; Dutta, A. Impact of agronomic treatments on fuel characteristics of herbaceous biomass for combustion. Fuel Process. Technol. 2013, 109, 96–102. [Google Scholar] [CrossRef]
- Vamvuka, D.; Zografos, D. Predicting the behaviour of ash from agricultural wastes during combustion. Fuel 2004, 83, 2051–2057. [Google Scholar] [CrossRef]
- Skrifvars, B.J.; Backman, R.; Hupa, M.; Sfiris, G.; Abyhammar, T.; Lyngfelt, A. Ash behaviour in a CFB boiler during combustion of coal; peat or wood. Fuel 1998, 77, 65–70. [Google Scholar] [CrossRef]
- Narayanasamy, L.; Murugesan, T. Degradation of Alizarin Yellow R using UV/H2O2 advanced oxidation process. Environ. Sci. Technol. 2014, 33, 482–489. [Google Scholar]
- Kambo, H.S.; Dutta, A. Strength, storage, and combustion characteristics of densified lignocellulosic biomass produced via torrefaction and hydrothermal carbonization. Appl. Energy 2014, 135, 182–191. [Google Scholar] [CrossRef]
- Stelte, W.; Holm, J.K.; Sanadi, A.R.; Barsberg, S.; Ahrenfeldt, J.; Henriksen, U.B. Fuel pellets from biomass: The importance of the pelletizing pressure and its dependency on the processing conditions. Fuel 2011, 90, 3285–3290. [Google Scholar] [CrossRef]
- Roy, P.; Shimizu, N.; Kimura, T. Effect of temperature distribution on the quality of parboiled rice produced by traditional parboiling process. Food Sci. Technol. Res. 2007, 10, 254–260. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R.V. Factors affecting strength and durability of densified biomass products. Biomass Bioenergy. 2009, 33, 337–359. [Google Scholar] [CrossRef]
- Khan, A.A.; de Jong, W.; Jansens, P.J.; Spliethoff, H. Biomass combustion in fluidized bed boilers: Potential problems and remedies. Fuel Process. Technol. 2009, 90, 21–50. [Google Scholar] [CrossRef]
- Roy, P.; Dutta, A.; Acharya, B.; Deen, B. An investigation of raw and torrefied lignocellulosic biomasses with CaO during combustion. J. Energy Inst. 2018, 91, 584–594. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). The Main Characteristics of Tropical Peats-FAO (Food and Agriculture Organization of the United Nations). Available online: http://www.fao.org/docrep/x5872e/x5872e06.htm (accessed on 5 February 2018).
- Farmer, V.C.; Morrison, R.I. Lignin in sphagnum and phragmites and in peats derived from these plants. Geochim. Cosmochim. Acta 1964, 28, 1537–1546. [Google Scholar] [CrossRef]
- Plank, N. The nature of cellulose in sphagnum. Am. J. Bot. 1946, 33, 335–337. [Google Scholar] [CrossRef]
- Sermyagina, E.; Saari, J.; Kaikko, J.; Vakkilainen, E. Hydrothermal carbonization of coniferous biomass: Effect of process parameters on mass and energy yields. J. Anal. Appl. Pyrolysis 2015, 113, 551–556. [Google Scholar] [CrossRef]
- Cao, L.; Yuan, X.Z.; Li, H.; Li, C.Z.; Xiao, Z.H.; Jiang, L.B.; Hunag, B.H.; Xiao, Z.H.; Chen, X.H.; Wang, H.; et al. Complementary effects of torrefaction and co-pelletization: Energy consumption and characteristics of pellets. Bioresour. Technol. 2015, 185, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Puig-Arnavat, M.; Ahrenfeldt, J.; Henriksen, U.B. Validation of a Multiparameter Model to Investigate Torrefied Biomass Pelletization Behavior. Energy Fuels 2017, 31, 1644–1649. [Google Scholar] [CrossRef]
- Yan, W.; Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Thermal pretreatment of lignocellulosic biomass. Environ. Prog. Sustain. Energy 2009, 28, 435–440. [Google Scholar] [CrossRef]
- Ahn, B.J.; Chang, H.-S.; Lee, S.M.; Choi, D.H.; Cho, S.T.; Han, G.-S.; Yang, I. Effect of binders on the durability of wood pellets fabricated from Larix kaemferi C. and Liriodendron tulipifera L. sawdust. Renew. Energy 2014, 62, 18–23. [Google Scholar] [CrossRef]
- Carone, M.T.; Pantaleo, A.; Pellerano, A. Influence of process parameters and biomass characteristics on the durability of pellets from the pruning residues of Olea europaea L. Biomass Bioenergy 2011, 35, 402–410. [Google Scholar] [CrossRef]
- Jiang, L.B.; Liang, J.; Yuan, X.Z.; Li, H.; Li, C.Z.; Xiao, Z.H.; Huang, H.Z.; Wang, H.; Zeng, G.M. Co-pelletization of sewage sludge and biomass: The density and hardness of pellet. Bioresour. Technol. 2014, 166, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Larsson, S.H.; Rudolfsson, M.; Nordwaeger, M.; Olofsson, I.; Samuelsson, R. Effects of moisture content; torrefaction temperature; and die temperature in pilot scale pelletizing of torrefied Norway spruce. Appl. Energy 2013, 102, 827–832. [Google Scholar] [CrossRef]
- Hu, Q.; Yang, H.P.; Yao, D.D.; Zhu, D.C.; Wang, X.H.; Shao, J.G.; Chen, H.P. The densification of bio-char: Effect of pyrolysis temperature on the qualities of pellets. Bioresour. Technol. 2016, 200, 521–752. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yoshikawa, K.; Fukuhara, M.; Xin, D.; Muhan, L. Development of an ultra-small biomass gasification and power generation system: Part 1. A novel carbonization process and optimization of pelletization of carbonized wood char. Fuel 2017, 210, 674–683. [Google Scholar] [CrossRef]
- Reza, M.T.; Uddin, M.H.; Lynam, J.G.; Coronella, C.J. Engineered pellets from dry torrefied and HTC biochar blends. Biomass Bioenergy 2014, 63, 229–238. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.H.; Legros, R.; Bi, X.T.T.; Jim Lim, C.; Sokhansanj, S. Pelletization of torrefied sawdust and properties of torrefied pellets. Appl. Energy 2012, 93, 680–685. [Google Scholar] [CrossRef]
- Van Dam, J.E.; van den Oever, M.J.; Teunissen, W.; Keijsers, E.R.; Peralta, A.G. Process for production of high density/high performance binderless boards from whole coconut husk: Part 1: Lignin as intrinsic thermosetting binder resin. Ind. Crops Prod. 2004, 19, 207–216. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.G.; Vasquez, V.R.; Coronella, C.J. Pelletization of biochar from hydrothermally carbonized wood. Environ. Prog. Sustain. Energy 2012, 31, 225–234. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R.V. Natural binders and solid bridge type binding mechanisms in briquettes and pellets made from corn stover and switchgrass. Bioresour. Technol. 2010, 101, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Garcia Moscoso, J.L.; Kumar, S.; Cao, X.; Mao, J.; Schafran, G. Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J. Environ. Manag. 2012, 109, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.M.; White, R.J.; Falco, C.; Sevilla, M. Black perspectives for a green future: hydrothermal carbons for environment protection and energy storage. Energy Environ. Sci. 2012, 5, 6796–6822. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.G.; Uddin, M.H.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Perspective: Jatropha cultivation in southern India: Assessing farmers’ experiences. Biofuels Bioprod. Biorefin. 2012, 6, 246–256. [Google Scholar]
- Dayton, D.C.; Jenkins, B.M.; Turn, S.Q.; Bakker, R.R.; Williams, R.B.; Belle-Oudry, D.; Hill, L.M. Release of inorganic constituents from leached biomass during thermal conversion. Energy Fuel. 1999, 13, 860–870. [Google Scholar] [CrossRef]

| Feedstock | Composition, % | Reference | ||
|---|---|---|---|---|
| Hemicellulose | Cellulose | Lignin | ||
| Peat moss | 24.1 | 17.1 (44.2 *) | 18.0 (25–40 ^) | [42,43,44] |
| Miscanthus | 36.3 | 38.6 | 11.5 | [18] |
| Sample | Proximate Analysis (%) | Ultimate Analysis (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| VM | Ash | FC | N | C | H | S | O | |
| T0 | 65.96 | 6.56 | 27.47 | 1.17 | 51.09 | 5.46 | 0.23 | 42.05 |
| T1 | 52.44 | 7.08 | 40.48 | 1.32 | 59.55 | 4.80 | 0.21 | 34.12 |
| T2 | 51.43 | 6.18 | 42.39 | 1.40 | 61.55 | 5.07 | 0.21 | 31.78 |
| T3 | 51.55 | 6.67 | 41.78 | 1.40 | 62.13 | 4.98 | 0.19 | 31.30 |
| T4 | 60.27 | 5.38 | 34.35 | 1.03 | 60.32 | 5.07 | 0.15 | 33.43 |
| T5 | 61.74 | 5.67 | 32.59 | 0.72 | 55.66 | 4.67 | 0.10 | 38.85 |
| T6 | 63.89 | 2.22 | 33.90 | 0.61 | 66.60 | 5.29 | 0.07 | 27.43 |
| T7 | 74.58 | 0.87 | 24.55 | 0.29 | 68.67 | 5.12 | 0.00 | 25.92 |
| UMS | 87.50 | 1.57 | 10.93 | 0.21 | 46.66 | 6.00 | 0 | 45.34 |
| Feedstock | HHV, MJ/kg | EDR | Mass Yield, % | Energy Yield, % |
|---|---|---|---|---|
| T0 | 21.29 * | 1.00 | 100 | 100 |
| T1 | 25.21 | 1.20 | 73.75 | 88.69 |
| T2 | 25.37 | 1.21 | 72.60 | 87.86 |
| T3 | 25.79 | 1.23 | 70.34 | 86.54 |
| T4 | 25.16 | 1.23 | 70.06 | 86.51 |
| T5 | 25.07 | 1.27 | 62.95 | 79.74 |
| T6 | 27.80 | 1.45 | 47.74 | 69.07 |
| T7 | 27.80 | 1.49 | 45.87 | 68.45 |
| Samples | Time, s | Extension, mm | Hardness, N | Durability, % |
|---|---|---|---|---|
| T0 | 11.1 | 1.1 | 53.9 | 89.9 |
| T1 | 10.6 | 1.1 | 46.7 | 80.0 |
| T2 | 10.3 | 1.0 | 29.3 | 60.4 |
| T3 | 9.8 | 1.0 | 22.7 | 62.9 |
| T4 | 10.6 | 1.1 | 40.9 | 72.3 |
| T5 | 9.8 | 1.0 | 12.9 | 66.2 |
| T6 | 9.6 | 1.0 | 21.0 | 60.9 |
| T7 | 7.7 | 0.8 | 7.5 | 59.6 |
| Sample | SiO2 | P2O5 | CaO | MgO | K2O | Al2O3 | Fe2O3 | MnO | Na2O | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | 11.00 | 1.18 | 17.60 | 12.93 | 1.35 | 5.23 | 6.40 | 0.00 | 4.23 | 0.00 |
| T1 | 18.03 | 0.43 | 15.68 | 8.05 | 1.35 | 8.15 | 11.53 | 0.00 | 1.65 | 0.18 |
| T4 | 23.63 | 1.00 | 11.10 | 5.13 | 2.15 | 9.80 | 9.63 | 0.00 | 1.98 | 0.75 |
| T5 | 23.08 | 0.63 | 12.53 | 5.93 | 1.83 | 8.35 | 8.68 | 0.13 | 1.83 | 0.00 |
| T6 | 36.50 | 1.97 | 7.03 | 3.07 | 2.30 | 9.07 | 5.03 | 0.00 | 1.83 | 0.00 |
| T7 | 34.78 | 11.45 | 15.00 | 2.40 | 1.73 | 1.00 | 0.85 | 0.80 | 0.00 | 0.00 |
| * MS | 54.71–63.12 | 6.03–10.60 | 7.61–10.35 | 3.46–6.07 | 16.39–21.79 | 0.45–0.69 | 0.18–0.37 | 0.20–0.36 | 0.28–0.85 | 0.01–0.04 |
| Sample | Alkali Index(AI) | Base to Acid ratio, (Rb/a) | Bed Agglomeration Index (BAI) |
|---|---|---|---|
| T0 | 0.00017 | 2.61941 | 1.14798 |
| T1 | 0.00008 | 1.45161 | 3.84167 |
| T4 | 0.00009 | 0.87710 | 2.33333 |
| T5 | 0.00008 | 0.97932 | 2.37671 |
| T6 | 0.00003 | 0.42282 | 1.21774 |
| T7 | 0.00001 | 0.55835 | 0.49275 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, P.; Dutta, A.; Gallant, J. Hydrothermal Carbonization of Peat Moss and Herbaceous Biomass (Miscanthus): A Potential Route for Bioenergy. Energies 2018, 11, 2794. https://doi.org/10.3390/en11102794
Roy P, Dutta A, Gallant J. Hydrothermal Carbonization of Peat Moss and Herbaceous Biomass (Miscanthus): A Potential Route for Bioenergy. Energies. 2018; 11(10):2794. https://doi.org/10.3390/en11102794
Chicago/Turabian StyleRoy, Poritosh, Animesh Dutta, and Jim Gallant. 2018. "Hydrothermal Carbonization of Peat Moss and Herbaceous Biomass (Miscanthus): A Potential Route for Bioenergy" Energies 11, no. 10: 2794. https://doi.org/10.3390/en11102794
APA StyleRoy, P., Dutta, A., & Gallant, J. (2018). Hydrothermal Carbonization of Peat Moss and Herbaceous Biomass (Miscanthus): A Potential Route for Bioenergy. Energies, 11(10), 2794. https://doi.org/10.3390/en11102794




