Separation of Acetate Produced from C1 Gas Fermentation Using an Electrodialysis-Based Bioelectrochemical System
Abstract
1. Introduction
2. Materials and Methods
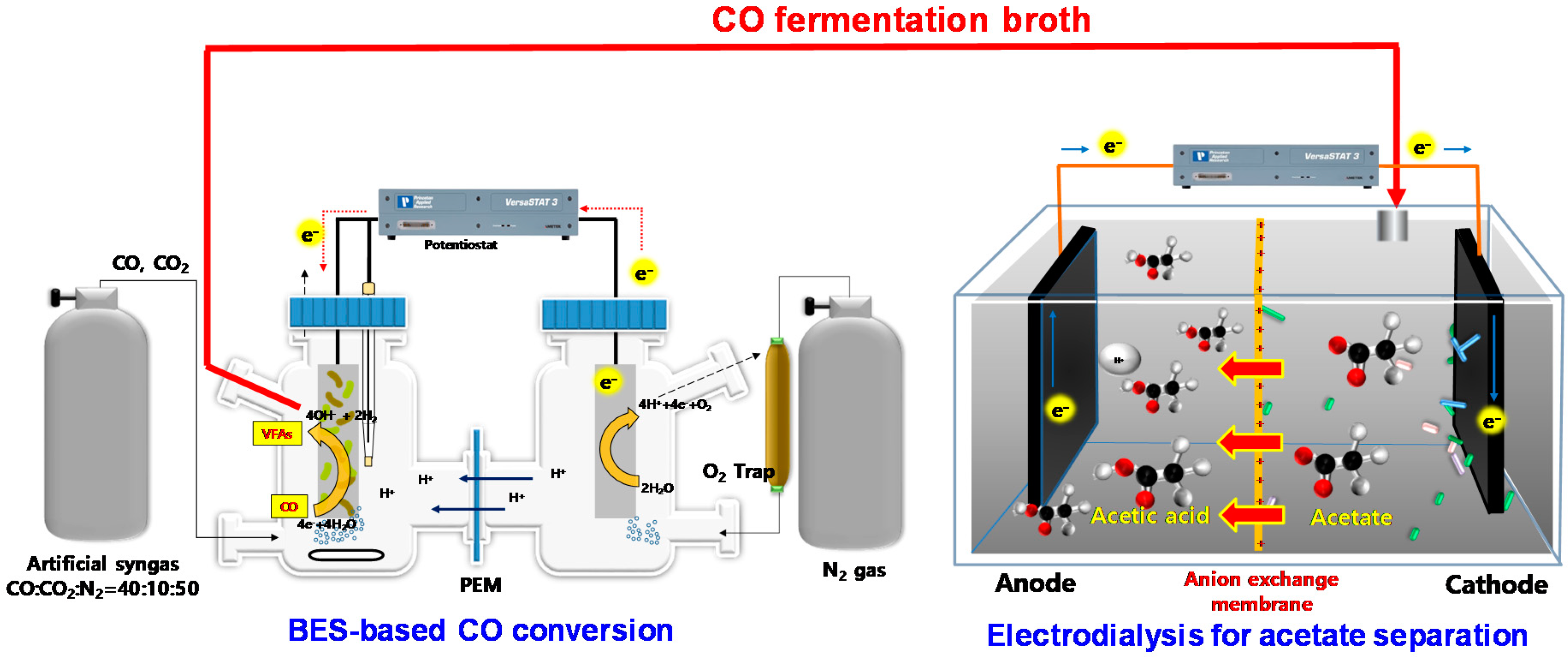
2.1. Configuration of Bioelectrochemical System and Electrodialysis Reactor
2.2. Composition of Electrolyte
2.3. Operation of Electrodialysis Reactor
2.4. Analyses
3. Results and Discussion
3.1. Different Applied Current on Acetate Transportation in BES
3.2. Effect of Different Acetate Concentration
3.3. Effect of Different Initial Anodic pH
3.4. Acetate Separation from the Actual Fermentation Broth
4. Implication and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ali, J.; Sohail, A.; Wang, L.; Haider, M.R.; Mulk, S.; Pan, G. Electro-microbiology as a promising approach towards renewable energy and environmental sustainability. Energies 2018, 11, 1822. [Google Scholar] [CrossRef]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microb. 2010, 8, 706. [Google Scholar] [CrossRef] [PubMed]
- Im, C.H.; Kim, C.; Song, Y.E.; Oh, S.-E.; Jeon, B.-H.; Kim, J.R. Electrochemically enhanced microbial CO conversion to volatile fatty acids using neutral red as an electron mediator. Chemosphere 2018, 191, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Im, C.H.; Song, Y.E.; Jeon, B.-H.; Kim, J.R. Biologically activated graphite fiber electrode for autotrophic acetate production from CO2 in a bioelectrochemical system. Carbon Lett. 2016, 20, 76–80. [Google Scholar] [CrossRef]
- Gildemyn, S.; Verbeeck, K.; Slabbinck, R.; Andersen, S.J.; Prévoteau, A.; Rabaey, K. Integrated production, extraction, and concentration of acetic acid from CO2 through microbial electrosynthesis. Environ. Sci. Technol. Lett. 2015, 2, 325–328. [Google Scholar] [CrossRef]
- Batlle-Vilanova, P.; Puig, S.; Gonzalez-Olmos, R.; Balaguer, M.D.; Colprim, J. Continuous acetate production through microbial electrosynthesis from CO2 with microbial mixed culture. J. Chem. Technol. Biotechnol. 2016, 91, 921. [Google Scholar] [CrossRef]
- Patil, S.A.; Arends, J.; Vanwonterghem, I.; Van Meerbergen, J.; Guo, K.; Tyson, G.; Rabaey, K. Selective enrichment establishes a stable performing community for microbial electrosynthesis of acetate from CO2. Environ. Sci. Technol. 2015, 49, 8833–8843. [Google Scholar] [CrossRef] [PubMed]
- LaBelle, E.V.; Marshall, C.W.; Gilbert, J.A.; May, H.D. Influence of acidic pH on hydrogen and acetate production by an electrosynthetic microbiome. PLoS ONE 2014, 9, e109935. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Singh, D.; Vanbroekhoven, K.; Pant, D.; Kumar, M.; Puri, S.K.; Ramakumar, S.S.V. Electro-biocatalytic conversion of carbon dioxide to alcohols using gas diffusion electrode. Bioresour. Technol. 2018, 265, 45–51. [Google Scholar] [CrossRef] [PubMed]
- del Pilar Anzola Rojas, M.; Zaiat, M.; Gonzalez, E.R.; De Wever, H.; Pant, D. Effect of the electric supply interruption on a microbial electrosynthesis system converting inorganic carbon into acetate. Bioresour. Technol. 2018, 266, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xu, T.; Zhang, Y.; Xue, Y.; Chen, G. Application of electrodialysis to the production of organic acids: State-of-the-art and recent developments. J. Membr. Sci. 2007, 288, 1–12. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, H.; Zhang, Y.; Feng, H.; Shehzad, M.A.; Wang, Y.; Xu, T. Complexation Electrodialysis as a general method to simultaneously treat wastewaters with metal and organic matter. Chem. Eng. J. 2018, 348, 952–959. [Google Scholar] [CrossRef]
- Hongo, M.; Nomura, Y.; Iwahara, M. Novel method of lactic acid production by electrodialysis fermentation. Appl. Environ. Microb. 1986, 52, 314–319. [Google Scholar]
- Xue, S.; Wu, C.; Wu, Y.; Chen, J.; Li, Z. Bipolar membrane electrodialysis for treatment of sodium acetate waste residue. Sep. Purif. Technol. 2015, 154, 193–203. [Google Scholar] [CrossRef]
- Patil, R.; Truong, C.; Genco, J.; Pendse, H.; Van Heiningen, A. Applicability of electrodialysis to the separation of sodium acetate from synthetic alkaline hardwood extract. Tappi J. 2015, 14, 695–708. [Google Scholar]
- Kariduraganavar, M.; Nagarale, R.; Kittur, A.; Kulkarni, S. Ion-exchange membranes: Preparative methods for electrodialysis and fuel cell applications. Desalination 2006, 197, 225–246. [Google Scholar] [CrossRef]
- Fidaleo, M.; Moresi, M. Electrodialysis applications in the food industry. Adv. Food Nutr. Res. 2006, 51, 265–360. [Google Scholar] [PubMed]
- Wan, D.; Liu, H.; Qu, J.; Lei, P. Bio-electrochemical denitrification by a novel proton-exchange membrane electrodialysis system—A batch mode study. J. Chem. Technol. Biotechnol. 2010, 85, 1540–1546. [Google Scholar] [CrossRef]
- Mohan, S.V.; Srikanth, S. Enhanced wastewater treatment efficiency through microbially catalyzed oxidation and reduction: Synergistic effect of biocathode microenvironment. Bioresour. Technol. 2011, 102, 10210–10220. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liang, P.; Zhang, X.; Huang, X. Bioelectrochemical systems-driven directional ion transport enables low-energy water desalination, pollutant removal, and resource recovery. Bioresour. Technol. 2016, 215, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.J.; Hennebel, T.; Gildemyn, S.; Coma, M.; Desloover, J.; Berton, J.; Tsukamoto, J.; Stevens, C.; Rabaey, K. Electrolytic membrane extraction enables production of fine chemicals from biorefinery sidestreams. Environ. Sci. Technol. 2014, 48, 7135–7142. [Google Scholar] [CrossRef] [PubMed]
- Długołęcki, P.; Anet, B.; Metz, S.J.; Nijmeijer, K.; Wessling, M. Transport limitations in ion exchange membranes at low salt concentrations. J. Membr. Sci. 2010, 346, 163–171. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Ismail, M.; Rahimnejad, M.; Ismail, A.F.; Leong, J.X.; Miskan, M.; Liew, K.B. Effect of pre-treatment and biofouling of proton exchange membrane on microbial fuel cell performance. Int. J. Hydrogen Energy 2013, 38, 5480–5484. [Google Scholar] [CrossRef]
- Choi, M.-J.; Chae, K.-J.; Ajayi, F.F.; Kim, K.-Y.; Yu, H.-W.; Kim, C.-W.; Kim, I.S. Effects of biofouling on ion transport through cation exchange membranes and microbial fuel cell performance. Bioresour. Technol. 2011, 102, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Dass, L.A.; Alhoshan, M.S.; Ghasemi, M.; Mohammad, A.W. Development of polyaniline-modified polysulfone nanocomposite membrane. Appl. Water Sci. 2012, 2, 37–46. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.; Gadhamshetty, V. Appreciating the role of carbon nanotube composites in preventing biofouling and promoting biofilms on material surfaces in environmental engineering: A review. Biotechnol. Adv. 2010, 28, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Ainala, S.K.; Oh, Y.-K.; Jeon, B.-H.; Park, S.; Kim, J.R. Metabolic flux change in Klebsiella pneumoniae L17 by anaerobic respiration in microbial fuel cell. Biotechnol. Bioprocess Eng. 2016, 21, 250–260. [Google Scholar] [CrossRef]
- Jaime-Ferrer, J.S.; Couallier, E.; Viers, P.; Durand, G.; Rakib, M. Three-compartment bipolar membrane electrodialysis for splitting of sodium formate into formic acid and sodium hydroxide: Role of diffusion of molecular acid. J. Membr. Sci. 2008, 325, 528–536. [Google Scholar] [CrossRef]
- Chukwu, U.; Cheryan, M. Electrodialysis of Acetate Fermentation Broths. In Twentieth Symposium on Biotechnology for Fuels and Chemicals; Humana Press: Totowa, NJ, USA, 1999; pp. 485–499. [Google Scholar]
- Wei, P.; Xia, A.; Liao, Q.; Sun, C.; Huang, Y.; Fu, Q.; Zhu, X.; Lin, R. Enhancing fermentative hydrogen production with the removal of volatile fatty acids by electrodialysis. Bioresour. Technol. 2018, 263, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pinoy, L.; Meesschaert, B.; Van der Bruggen, B. Separation of small organic ions from salts by ion-exchange membrane in electrodialysis. AIChE J. 2011, 57, 2070–2078. [Google Scholar] [CrossRef]
- Kim, J.R.; Cheng, S.; Oh, S.-E.; Logan, B.E. Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ. Sci. Technol. 2007, 41, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Drews, A. Membrane fouling in membrane bioreactors—Characterisation, contradictions, cause and cures. J. Membr. Sci. 2010, 363, 1–28. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, S.; Cao, H.; Li, Y.; Van der Bruggen, B. Layer-by-layer assembly of anion exchange membrane by electrodeposition of polyelectrolytes for improved antifouling performance. J. Membr. Sci. 2018, 558, 1–8. [Google Scholar] [CrossRef]
- Lee, H.-M.; Jeon, B.-Y.; Oh, M.-K. Microbial production of ethanol from acetate by engineered Ralstonia eutropha. Biotechnol. Bioprocess Eng. 2016, 21, 402–407. [Google Scholar] [CrossRef]
- López-Garzón, C.S.; Straathof, A.J. Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.E.; Boghani, H.C.; Kim, H.S.; Kim, B.G.; Lee, T.; Jeon, B.-H.; Premier, G.C.; Kim, J.R. Electricity Production by the Application of a Low Voltage DC-DC Boost Converter to a Continuously Operating Flat-Plate Microbial Fuel Cell. Energies 2017, 10, 596. [Google Scholar] [CrossRef]
- Song, Y.E.; Boghani, H.C.; Kim, H.S.; Kim, B.G.; Lee, T.; Jeon, B.H.; Premier, G.C.; Kim, J.R. Maximum Power Point Tracking to Increase the Power Production and Treatment Efficiency of a Continuously Operated Flat-Plate Microbial Fuel Cell. Energy Technol. 2016, 4, 1427–1434. [Google Scholar] [CrossRef]
- Matemadombo, F.; Puig, S.; Ganigué, R.; Ramírez-García, R.; Batlle-Vilanova, P.; Dolors Balaguer, M.; Colprim, J. Modelling the simultaneous production and separation of acetic acid from CO2 using an anion exchange membrane microbial electrosynthesis system. J. Chem. Technol. Biotechnol. 2017, 92, 1211–1217. [Google Scholar] [CrossRef]
- Bajracharya, S.; Van den Burg, B.; Vanbroekhoven, K.; De Wever, H.; Buisman, C.J.; Pant, D.; Strik, D.P. In situ acetate separation in microbial electrosynthesis from CO2 using ion-exchange resin. Electrochim. Acta 2017, 237, 267–275. [Google Scholar] [CrossRef]
- Prochaska, K.; Antczak, J.; Regel-Rosocka, M.; Szczygiełda, M. Removal of succinic acid from fermentation broth by multistage process (membrane separation and reactive extraction). Sep. Purif. Technol. 2018, 192, 360–368. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhang, X.; Feng, H.; Xu, T. In-situ combination of fermentation and electrodialysis with bipolar membranes for the production of lactic acid: Continuous operation. Bioresour. Technol. 2013, 147, 442–448. [Google Scholar] [CrossRef] [PubMed]
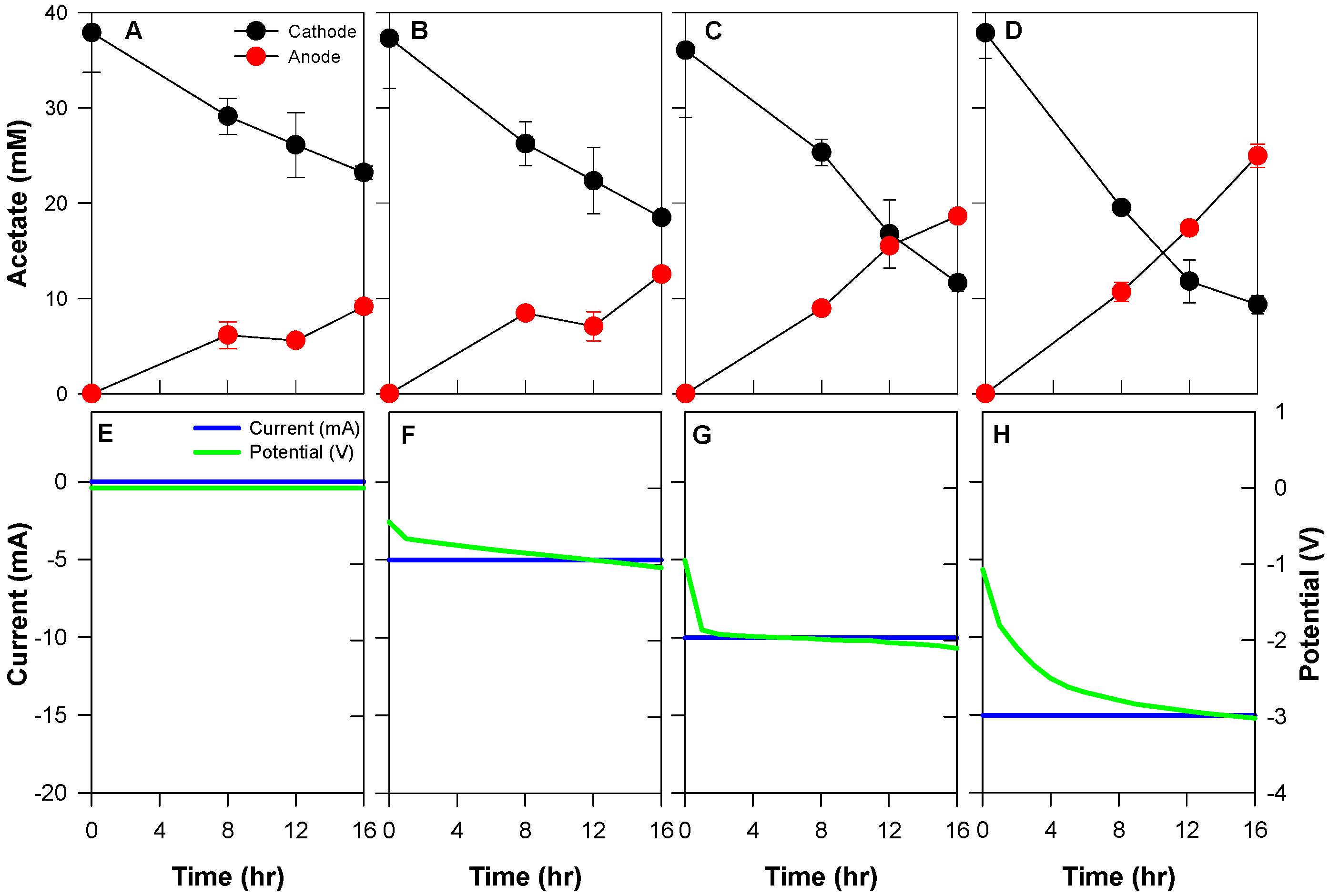

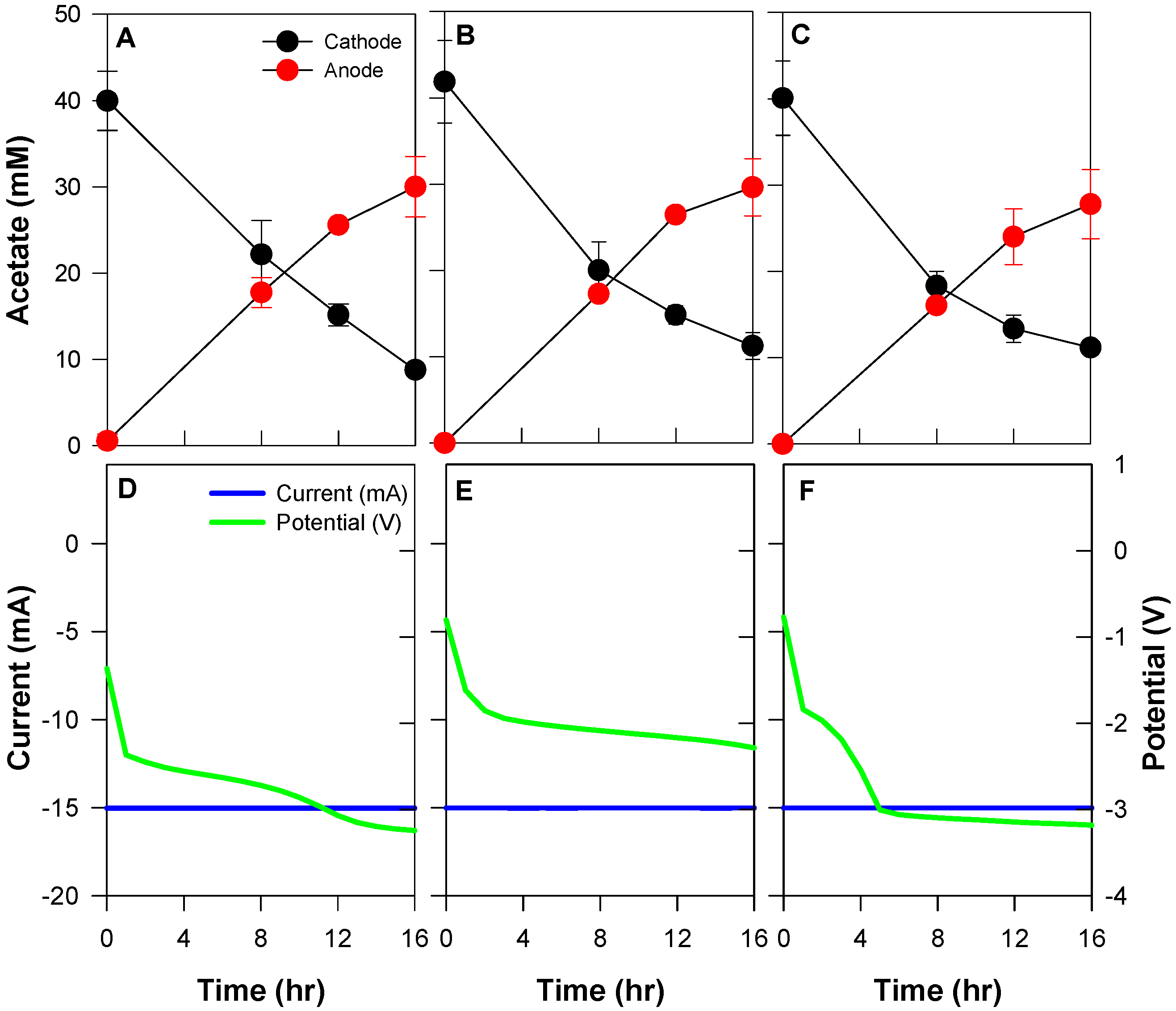
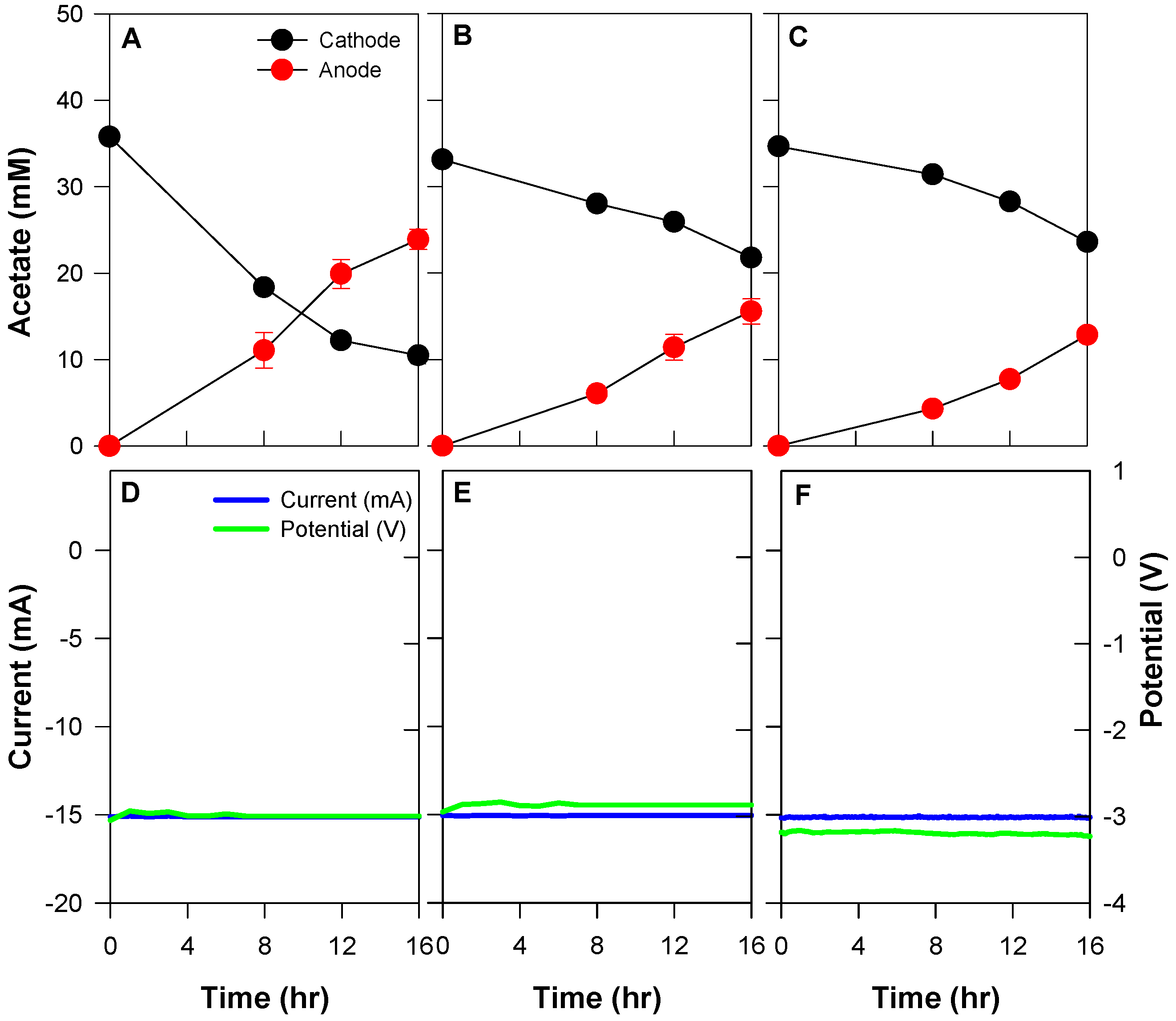
| Conditions | Acetate Migration from the Cathode (mM) | Total Applied Current (C) | Current Efficiency (%) | Acetate Flux (mmol/m2·h) | |
|---|---|---|---|---|---|
| Applied current | 0 mA | 14.7 ± 4.9 | - | - | 8.8 ± 0.4 |
| −5 mA | 18.8 ± 5.2 | 288.0 | 54.4 ± 0.2 | 12.0 ± 0.0 | |
| −10 mA | 24.4 ± 7.9 | 576.0 | 40.4 ± 0.6 | 17.8 ± 0.3 | |
| −15 mA | 28.5 ± 3.6 | 864.0 | 36.1 ± 1.2 | 23.9 ± 0.8 | |
| Acetate concentration | 20 mM | 16.3 ± 2.1 | 864.0 | 23.8 ± 0.6 | 15.8 ± 0.4 |
| 40 mM | 30.1 ± 4.5 | 40.2 ± 1.2 | 26.6 ± 0.8 | ||
| 80 mM | 44.2 ± 9.3 | 63.1 ± 2.6 | 41.8 ± 1.7 | ||
| 100 mM | 55.9 ± 11.3 | 73.4 ± 4.6 | 48.6 ± 3.0 | ||
| pH test | 2.0 | 31.2 ± 3.1 | 864.0 | 43.4 ± 2.8 | 28.2 ± 1.8 |
| 4.0 | 30.6 ± 6.3 | 42.8 ± 3.4 | 28.4 ± 2.2 | ||
| 6.0 | 28.9 ± 3.9 | 40.1 ± 4.1 | 26.6 ± 2.7 | ||
| Catholyte composition | Synthetic | 25.3 ± 0.9 | 864.0 | 34.5 ± 1.2 | 22.9 ± 0.8 |
| Fermented with centrifuge | 11.4 ± 1.2 | 22.5 ± 1.5 | 14.9 ± 1.0 | ||
| Fermented without centrifuge | 11.1 ± 0.3 | 18.6 ± 0.7 | 12.3 ± 0.4 |
| Volatile Fatty Acids | Separated Acetate Titer | Separation Material & Membrane | Applied Potential or Current | Current Efficiency (%) | Reference |
|---|---|---|---|---|---|
| Acetate | 1.21 mol/m3 during 32 days | Anion exchange membrane (1.77 cm2, AMI-7001) | −800 mV (vs. SHE) | - | [39] |
| Acetate | 0.379 kg/m2·d | Anion exchange membrane (64 cm2, AM-7001) | 20 Am−2 (vs. Ag/AgCl) | 36% (coulombic efficiency | [21] |
| Acetate | 225 mM during 43 days | Anion exchange membrane (Fumatech FAB) | −50 mA (vs. SHE) | - | [5] |
| Acetate | 100 mg/g (acetate sorption) | Anion exchange resin (35 g, Amberlite TM FPA53) | - | - | [40] |
| Succinic acid | 15.7 g/dm3 during 180 min | EDMB stack: 10 Bipolar (PC 200bip), 10 Anion exchange (PC 200D), Cation exchange(PC-SK), (207 cm2 each) | 120 A/m2 | 14.3% | [41] |
| Lactic acid | 1.46 mol/L during 15 h | EDMB stack: Bipolar(40 cm2, BMP-1), Anion exchange(40 cm2, FAS-PET-130), Cation exchange(40 cm2, JCM-II-05) | 40 mA/cm2 | - | [42] |
| Acetate | 12.3 ± 0.4 mmol/m2·h | Anion exchange membrane (49 cm2, FKB-PK-130) | −15 mA | 18.6 ± 0.7% | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, J.; Kim, C.; Song, Y.E.; Im, H.S.; Sakuntala, M.; Kim, J.R. Separation of Acetate Produced from C1 Gas Fermentation Using an Electrodialysis-Based Bioelectrochemical System. Energies 2018, 11, 2770. https://doi.org/10.3390/en11102770
Baek J, Kim C, Song YE, Im HS, Sakuntala M, Kim JR. Separation of Acetate Produced from C1 Gas Fermentation Using an Electrodialysis-Based Bioelectrochemical System. Energies. 2018; 11(10):2770. https://doi.org/10.3390/en11102770
Chicago/Turabian StyleBaek, Jiyun, Changman Kim, Young Eun Song, Hyeon Sung Im, Mutyala Sakuntala, and Jung Rae Kim. 2018. "Separation of Acetate Produced from C1 Gas Fermentation Using an Electrodialysis-Based Bioelectrochemical System" Energies 11, no. 10: 2770. https://doi.org/10.3390/en11102770
APA StyleBaek, J., Kim, C., Song, Y. E., Im, H. S., Sakuntala, M., & Kim, J. R. (2018). Separation of Acetate Produced from C1 Gas Fermentation Using an Electrodialysis-Based Bioelectrochemical System. Energies, 11(10), 2770. https://doi.org/10.3390/en11102770







