Application of Subcritical Water to Dechlorinate Polyvinyl Chloride Electric Wires
Abstract
1. Introduction
2. Materials and Methods
3. Results
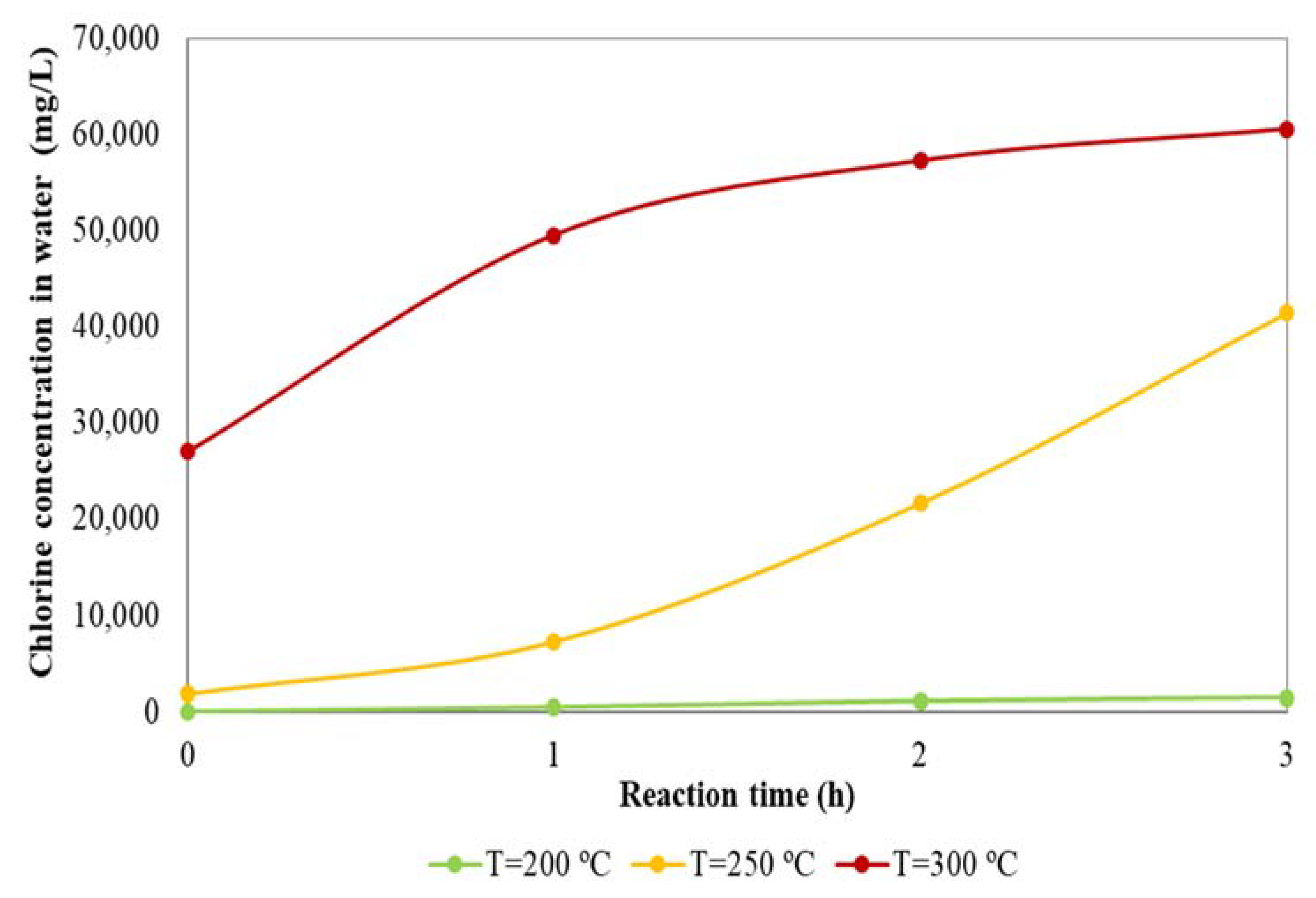
3.1. Dechlorination Treatment
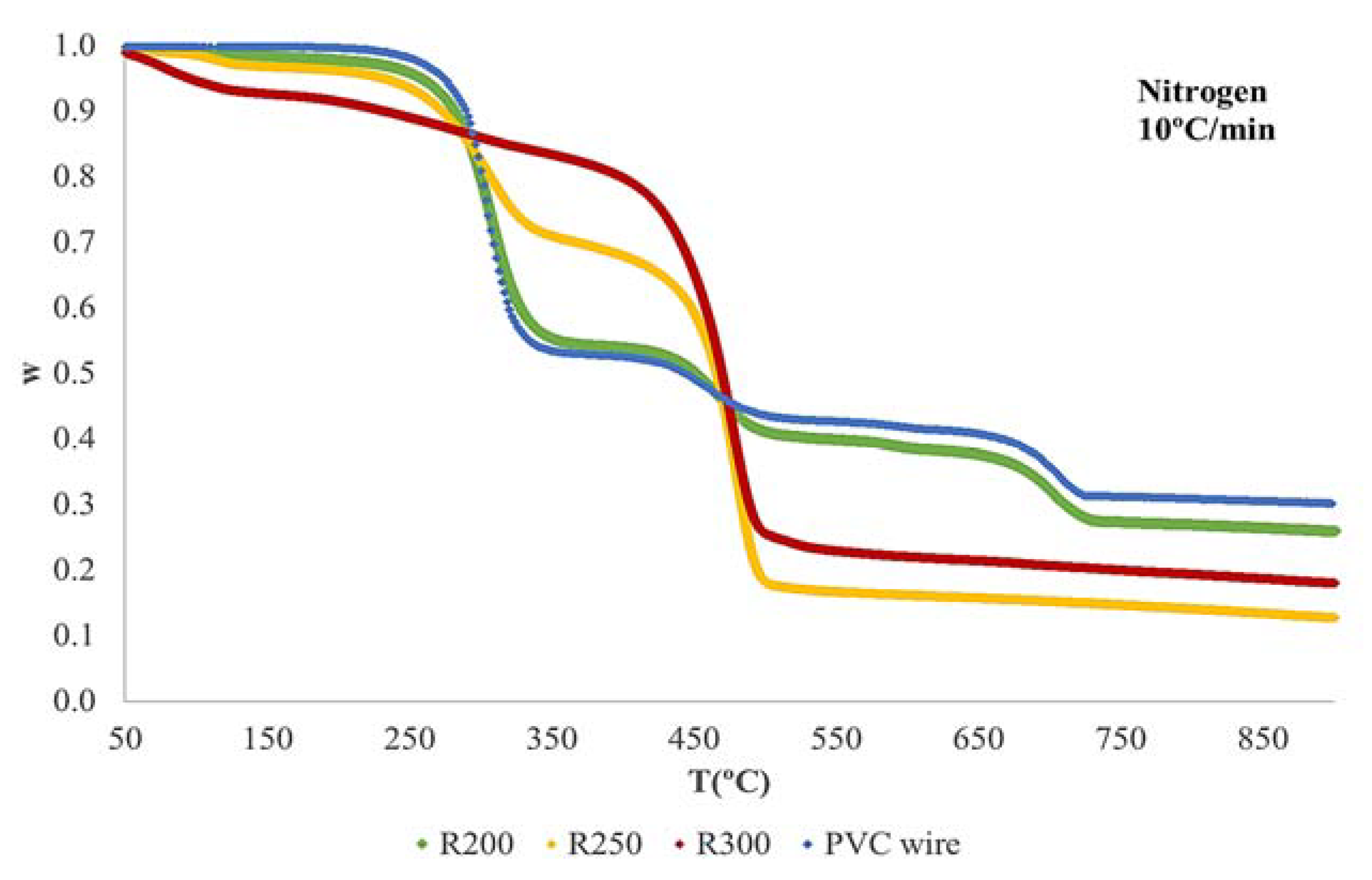
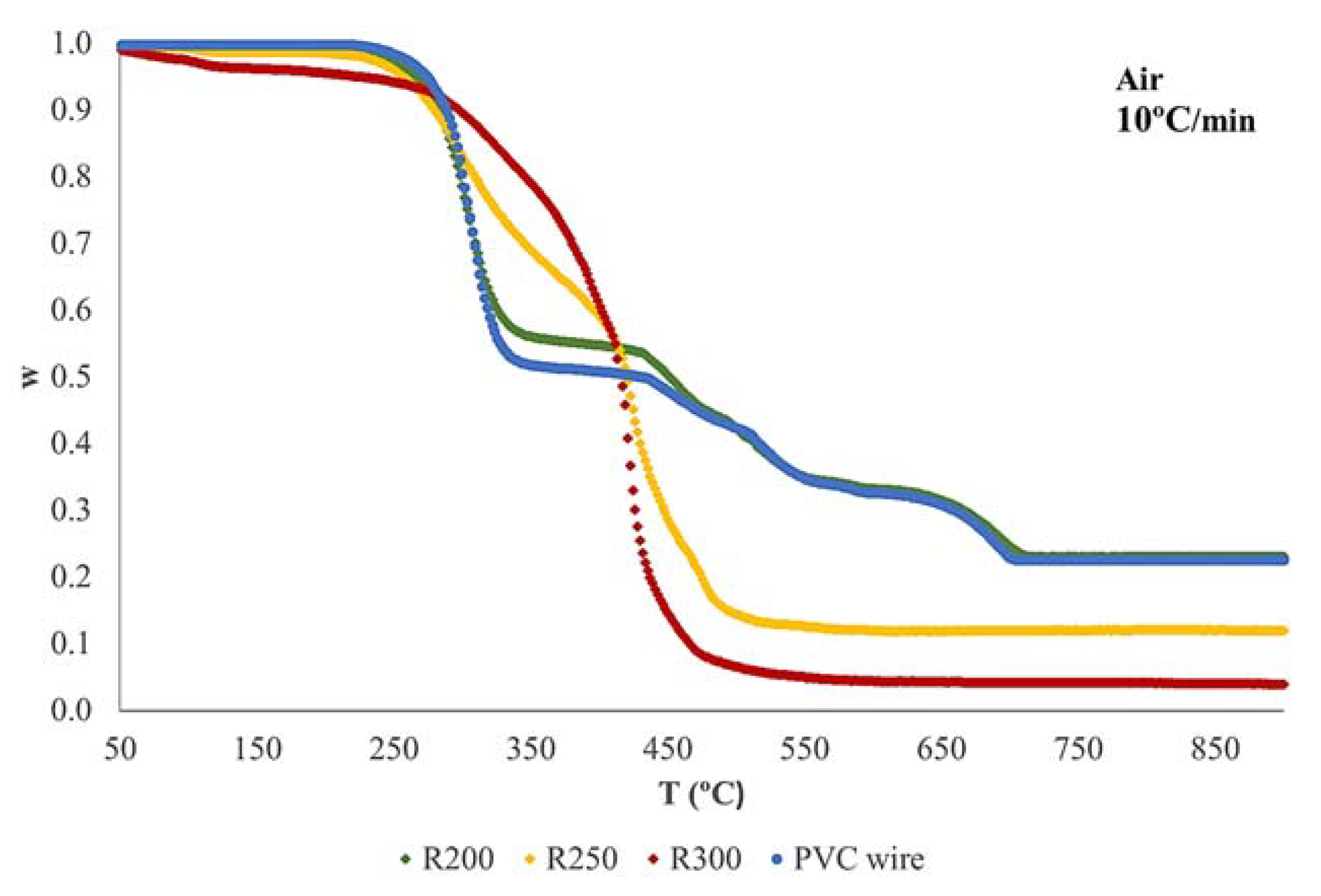
3.2. Thermogravimetric Analysis
3.3. Pollutant Emissions in Pyrolysis Runs
3.3.1. Hydrogen Halide and Halogen Emissions
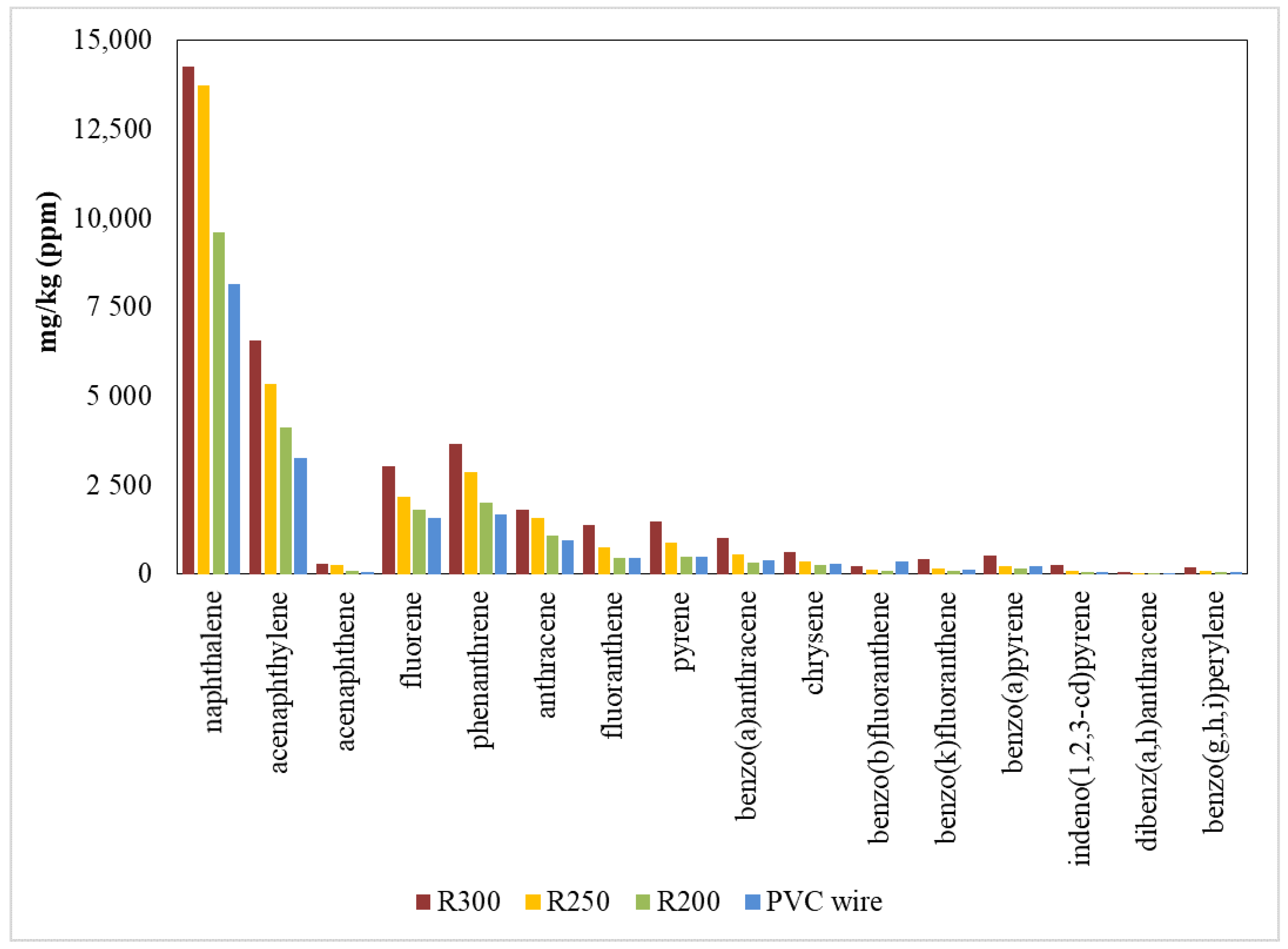
3.3.2. PAHs
3.3.3. ClBzs
3.3.4. ClPhs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sheih, S.W.; Tsai, M.S. Hot water separation process for copper and insulating material recovery from electric cable waste. Waste Manag. Res. 2000, 18, 478–484. [Google Scholar] [CrossRef]
- Hagstrom, B.; Hampton, R.N.; Helmesjo, B.; Hjertberg, T. Disposal of cables at the “end of life”; some of the environmental considerations. IEEE Electr. Insul. Mag. 2006, 22, 21–30. [Google Scholar] [CrossRef]
- Directive, E.C. Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on Waste Electrical and Electronic Equipment (WEEE). Off. J. Eur. Union L 2012, 197, 38–71. [Google Scholar]
- Buekens, A.; Yang, J. Recycling of WEEE plastics: A review. J. Mater. Cycles Waste Manag. 2014, 16, 415–434. [Google Scholar] [CrossRef]
- Conesa, J.A.; Egea, S.; Moltó, J.; Ortuño, N.; Font, R. Decomposition of two types of electric wires considering the effect of the metal in the production of pollutants. Chemosphere 2013, 91, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Aracil, I.; Font, R.; Conesa, J.A. Semivolatile and volatile compounds from the pyrolysis and combustion of polyvinyl chloride. J. Anal. Appl. Pyrolysis 2005, 74, 465–478. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, F.-S. Degradation of brominated flame retardant in computer housing plastic by supercritical fluids. J. Hazard. Mater. 2012, 205-206, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Soler, A.; Conesa, J.A.; Ortuño, N. Emissions of brominated compounds and polycyclic aromatic hydrocarbons during pyrolysis of E-waste debrominated in subcritical water. Chemosphere 2017, 186, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, H.; Zhang, J.; Jing, D. Degradation of carbon fiber-reinforced polymer using supercritical fluids. Fibers Polym. 2017, 18, 795–805. [Google Scholar] [CrossRef]
- Zhu, N.-M.; Wang, C.-F.; Zhang, F.-S. An integrated two-stage process for effective dechlorination of polychlorinated biphenyls in subcritical water in the presence of hydrogen donors. Chem. Eng. J. 2012, 197, 135–142. [Google Scholar] [CrossRef]
- Oh, S.C.; Kwak, H.; Bae, S.-Y. A Kinetic Analysis of Polymer Degradation in Supercritical Fluid. J. Chem. Eng. Jpn. 2006, 39, 1004–1009. [Google Scholar] [CrossRef]
- Zhao, P.; Li, Z.; Li, T.; Yan, W.; Ge, S. The study of nickel effect on the hydrothermal dechlorination of PVC. J. Clean. Prod. 2017, 152, 38–46. [Google Scholar] [CrossRef]
- Kubátová, A.; Lagadec, A.J.M.; Hawthorne, S.B. Dechlorination of lindane, dieldrin, tetrachloroethane, trichloroethene, and PVC in subcritical water. Environ. Sci. Technol. 2002, 36, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Marrone, P.A.; Arias, T.A.; Peters, W.A.; Tester, J.W. Solvation effects on kinetics of methylene chloride reactions in sub- and supercritical water: Theory, experiment, and ab initio calculations. J. Phys. Chem. A 1998, 102, 7013–7028. [Google Scholar] [CrossRef]
- Poerschmann, J.; Weiner, B.; Woszidlo, S.; Koehler, R.; Kopinke, F.-D. Hydrothermal carbonization of poly(vinyl chloride). Chemosphere 2015, 119, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Xiu, F.-R.; Qi, Y.; Zhang, F.-S. Co-treatment of waste printed circuit boards and polyvinyl chloride by subcritical water oxidation: Removal of brominated flame retardants and recovery of Cu and Pb. Chem. Eng. J. 2014, 237, 242–249. [Google Scholar] [CrossRef]
- Endo, K.; Emori, N. Dechlorination of poly(vinyl chloride) without anomalous units under high pressure and at high temperature in water. Polym. Degrad. Stab. 2001, 74, 113–117. [Google Scholar] [CrossRef]
- Conesa, J.A.; Moltó, J.; Font, R.; Egea, S. Polyvinyl chloride and halogen-free electric wires thermal decomposition. Ind. Eng. Chem. Res. 2010, 49, 11841–11847. [Google Scholar] [CrossRef]
- Solid Biofuels-Determination of Ash Content (UNE-EN-14775:2009); European Committee for Standardization: Brussels, Belgium, 2010.
- Semivolatile Organic Compounds by GC/MS; United States Environmental Protection Agency, Office of Solid Waste: Washington, DC, USA, 2007.
- Soler, A.; Conesa, J.A.; Iñiguez, M.E.; Ortuño, N. Pollutant formation in the pyrolysis and combustion of materials combining biomass and e-waste. Sci. Total Environ. 2018, 622, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Moltó, J.; Egea, S.; Conesa, J.A.; Font, R. Thermal decomposition of electronic wastes: Mobile phone case and other parts. Waste Manag. 2011, 31, 2546–2552. [Google Scholar] [CrossRef] [PubMed]
- Determination of Hydrogen Halide and Halogen Emissions from Stationary Sources. Non-Isokinetic Method; United States Environmental Protection Agency, Emissions Measurement Center: Washington, DC, USA, 1994.
- Pressurized Fluid Extraction (PFE); United States Environmental Protection Agency, Office of Solid Waste: Washington, DC, USA, 2000.
- Handbook for Air Toxic Emission Inventory Development; United States Environmental Protection Agency, Office of Air Quality Planning and Standards: Washington, DC, USA, 1998.
- Yoshiyuki, S.; Kiyoshi, K.; Yukitoshi, T.; Kazue, T.; Shiro, N. Decomposition of Ployvinylchloride using Supercritical Water. Jpn. J. Appl. Phys. 1998, 37, 6270. [Google Scholar]
- Takeshita, Y.; Kato, K.; Takahashi, K.; Sato, Y.; Nishi, S. Basic study on treatment of waste polyvinyl chloride plastics by hydrothermal decomposition in subcritical and supercritical regions. J. Supercrit. Fluids 2004, 31, 185–193. [Google Scholar] [CrossRef]
- Nagai, Y.; Smith, R.L.; Inomata, H.; Arai, K. Direct observation of polyvinylchloride degradation in water at temperatures up to 500 °C and at pressures up to 700 mpa. J. Appl. Polym. Sci. 2007, 106, 1075–1086. [Google Scholar] [CrossRef]
- Lu, J.; Ma, S.; Gao, J. Study on the pressurized hydrolysis dechlorination of PVC. Energy Fuels 2002, 16, 1251–1255. [Google Scholar] [CrossRef]
- Caballero, J.A.; Conesa, J.A. Mathematical considerations for nonisothermal kinetics in thermal decomposition. J. Anal. Appl. Pyrolysis 2005, 73, 85–100. [Google Scholar] [CrossRef]
- Conesa, J.A.; Rey, L. Thermogravimetric and kinetic analysis of the decomposition of solid recovered fuel from municipal solid waste. J. Therm. Anal. Calorim. 2015, 120, 1233–1240. [Google Scholar] [CrossRef]
- Conesa, J.A.; Soler, A. Decomposition kinetics of materials combining biomass and electronic waste. J. Therm. Anal. Calorim. 2017, 128, 225–233. [Google Scholar] [CrossRef]
- Ortuño, N.; Conesa, J.A.; Moltó, J.; Font, R. Pollutant emissions during pyrolysis and combustion of waste printed circuit boards, before and after metal removal. Sci. Total. Environ. 2014, 499, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Conesa, J.A.; Font, R.; Fullana, A.; Martín-Gullón, I.; Aracil, I.; Gálvez, A.; Moltó, J.; Gómez-Rico, M.F. Comparison between emissions from the pyrolysis and combustion of different wastes. J. Anal. Appl. Pyrolysis 2009, 84, 95–102. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, A.; Long, Y.; Li, Q.; Zhang, Y. A review of dioxin-related substances during municipal solid waste incineration. Waste Manag. 2015, 36, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Wornat, M.J. The effects of oxygen on the yields of polycyclic aromatic hydrocarbons formed during the pyrolysis and fuel-rich oxidation of catechol. Fuel 2008, 87, 768–781. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Zhang, H.; Xu, M.Z. Transfer behavior of PAHs and PCBs from sewage sludge in the thermal treatment process. China Environ. Sci. 2011, 31, 933–937. [Google Scholar]
- Kaivosoja, T.; Virén, A.; Tissari, J.; Ruuskanen, J.; Tarhanen, J.; Sippula, O.; Jokiniemi, J. Effects of a catalytic converter on pcdd/f, chlorophenol and pah emissions in residential wood combustion. Chemosphere 2012, 88, 278–285. [Google Scholar] [CrossRef] [PubMed]



| wt % | Cover | Insulation | PVC Wire |
|---|---|---|---|
| Ashes | 51.22 | 0.50 | 42.52 |
| Elemental analysis | |||
| C | 34.58 | 84.57 | 43.16 |
| H | 4.45 | 14.68 | 6.20 |
| N | nd | nd | nd |
| S | nd | nd | nd |
| O (by difference) | 9.75 | 0.25 | 8.12 |
| Fluorescence analysis | |||
| Na | 0.05 | 0.02 | 0.04 |
| Mg | 0.12 | nd | 0.10 |
| Al | 0.14 | 0.04 | 0.12 |
| Si | 0.06 | 0.09 | 0.07 |
| P | 0.01 | nd | 0.01 |
| Cl | 27.25 | 0.07 | 22.59 |
| K | nd | nd | nd |
| Ca | 23.45 | 0.22 | 19.47 |
| Ti | 0.06 | nd | 0.05 |
| Fe | 0.03 | 0.01 | 0.03 |
| Cu | nd | 0.01 | nd |
| Zn | 0.05 | 0.03 | 0.04 |
| W | nd | 0.01 | nd |
| Sr | 0.01 | nd | nd |
| Experiment | Initial Wire (g) | Solid Residue (g) | Cl in Solid Residue (g) | Cl in Final Liquid (g) | DE (%) |
|---|---|---|---|---|---|
| Dechlorination at 300 °C | 50.06 | 11.98 | 0.46 | 10.78 | 95.96 |
| Dechlorination at 250 °C | 50.01 | 20.12 | 3.04 | 8.25 | 73.08 |
| Dechlorination at 200 °C | 50.04 | 39.65 | 11.06 | 0.28 | 2.09 |
| wt % | C | H | O | Ash | H/C | Net Calorific Value (kJ/kg) |
|---|---|---|---|---|---|---|
| R200 | 34.46 | 4.66 | 19.50 | 41.38 | 0.14 | 17,269 |
| R250 | 63.20 | 8.08 | 10.44 | 18.27 | 0.13 | 32,281 |
| R300 | 74.40 | 8.41 | 8.72 | 8.46 | 0.11 | 37,766 |
| PVC Wire | R200 | R250 | R300 | |
|---|---|---|---|---|
| Compound | mg/kg Sample | |||
| HCl | 155,300 | 149,200 | 18,750 | 2140 |
| Cl2 | 10,780 | 10,210 | 11,320 | 10,550 |
| PVC Wire | R200 | R250 | R300 | |
|---|---|---|---|---|
| Compound | mg/kg Sample | |||
| mono- | 919 | 1360 | 125 | 11.3 |
| 1,3- | nd | nd | nd | nd |
| 1,4- | 5.48 | 4.27 | 1.14 | 0.26 |
| 1,2- | 826 | 814 | 802 | 488 |
| 1,3,5- | 0.03 | 0.02 | 0.02 | nd |
| 1,2,4- | 0.03 | 0.02 | 0.02 | nd |
| 1,2,3- | 0.03 | 0.03 | 0.02 | 0.01 |
| 1,2,3,5-+1,2,4,5- | 0.02 | 0.02 | 0.02 | 0.01 |
| 1,2,3,4- | 0.04 | 0.03 | 0.02 | 0.02 |
| penta- | 0.04 | 0.01 | 0.02 | 0.01 |
| hexa- | nd | nd | nd | nd |
| TOTAL | 1751 | 2178 | 928 | 500 |
| Compound | PVC Wire | R200 | R250 | R300 |
|---|---|---|---|---|
| mg/kg Sample | ||||
| 2- | nd | nd | nd | nd |
| 3-+4- | 61.7 | 57.6 | 55.1 | 54.5 |
| 2,4- | nd | nd | nd | nd |
| 2,5- | 0.13 | 0.07 | 0.07 | 0.06 |
| 2,3- | nd | nd | nd | nd |
| 2,6- | 0.03 | nd | nd | nd |
| 3,5- | 51.0 | 42.7 | 2.15 | 2.13 |
| 3,4- | 0.26 | 0.16 | 0.10 | 0.10 |
| 2,3,5- | nd | nd | nd | nd |
| 2,4,6- | nd | nd | nd | nd |
| 2,4,5- | nd | nd | nd | nd |
| 2,3,4- | nd | nd | nd | nd |
| 2,3,6- | nd | nd | nd | nd |
| 3,4,5- | nd | 0.11 | nd | nd |
| 2,3,5,6- | 0.04 | 0.03 | 0.02 | 0.02 |
| 2,3,4,5- | 0.04 | 0.04 | 0.02 | 0.02 |
| 2,3,4,6- | 0.03 | 0.03 | 0.02 | 0.02 |
| penta- | nd | nd | nd | nd |
| TOTAL | 113.2 | 100.7 | 57.5 | 56.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soler, A.; Conesa, J.A.; Ortuño, N. Application of Subcritical Water to Dechlorinate Polyvinyl Chloride Electric Wires. Energies 2018, 11, 2612. https://doi.org/10.3390/en11102612
Soler A, Conesa JA, Ortuño N. Application of Subcritical Water to Dechlorinate Polyvinyl Chloride Electric Wires. Energies. 2018; 11(10):2612. https://doi.org/10.3390/en11102612
Chicago/Turabian StyleSoler, Aurora, Juan A. Conesa, and Nuria Ortuño. 2018. "Application of Subcritical Water to Dechlorinate Polyvinyl Chloride Electric Wires" Energies 11, no. 10: 2612. https://doi.org/10.3390/en11102612
APA StyleSoler, A., Conesa, J. A., & Ortuño, N. (2018). Application of Subcritical Water to Dechlorinate Polyvinyl Chloride Electric Wires. Energies, 11(10), 2612. https://doi.org/10.3390/en11102612







