Exergo-Economic Evaluation of the Cost for Solar Thermal Depuration of Water
Abstract
:1. Introduction
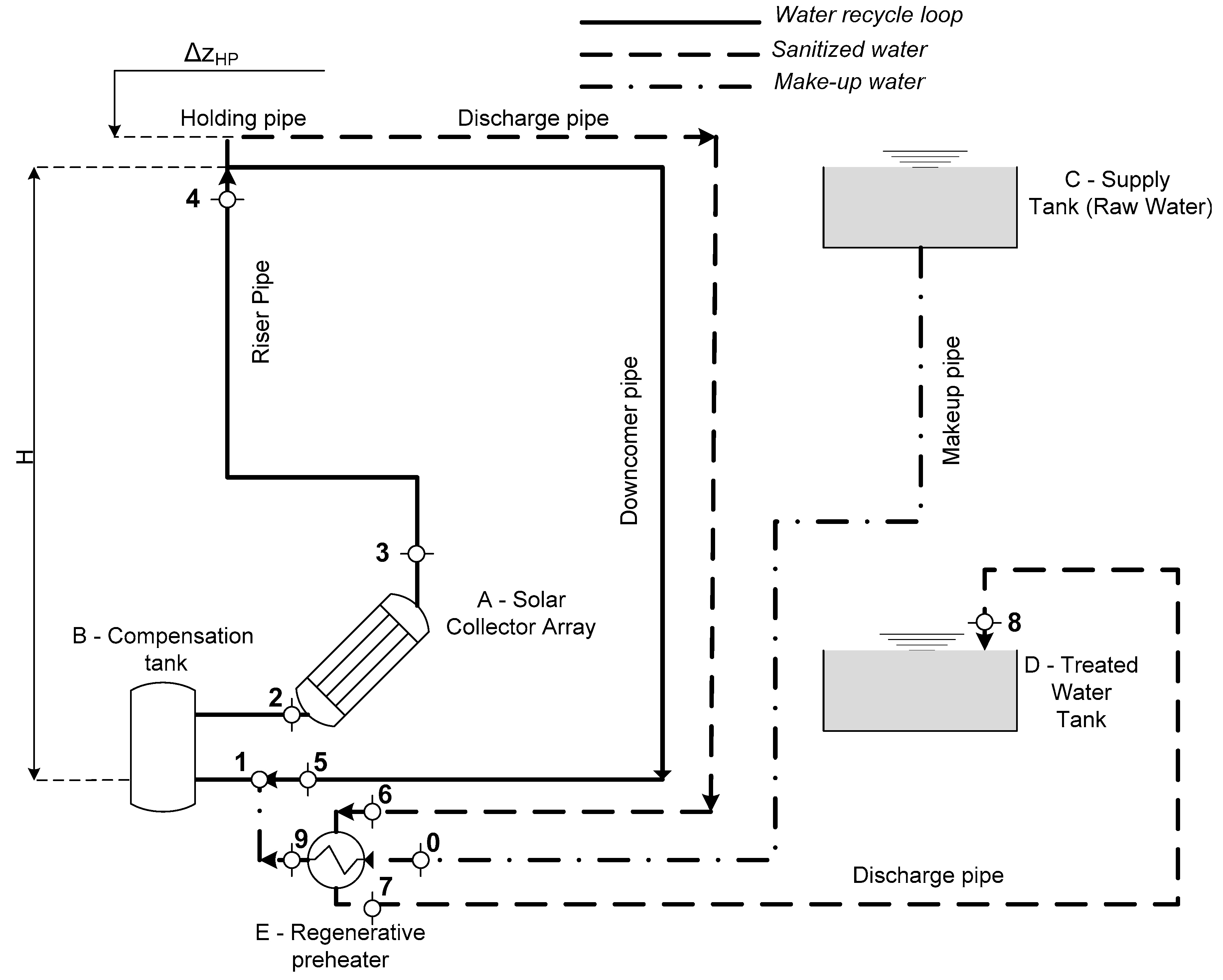
1.1. Pasteurization of Water with Solar Collectors in Recirculation System—Existing Research and Investigated Plant
2. Methodology
2.1. Design and Off-Design Simulation; Energy Analysis of the System
2.1.1. Sizing the Natural Circulation Pasteurisation System
2.1.2. Off-Design Simulation of the System
2.2. Exergy Analysis
- Solar collector exergy destruction—resulting from the exergy balance of solar collector component; as energy transferred to the fluid is conserved, this destruction is essentially a heat transfer irreversibility;
- Piping insulation exergy loss—resulting from standard heat transfer models for thermally insulated pipe lines
- Piping friction exergy destruction—predicted using turbulent flow pipe correlations; entropy calculated from the overall pressure loss
- Regenerative heat exchanger exergy destruction (spill-over)
- Make-up mixing exergy destruction (spill-over)
- Piping insulation exergy loss (spill-over; make-up and discharge pipes)
- Piping friction exergy destruction (spill-over; make-up and discharge pipes)
- Compensation tank unsteady exergy destruction—this results from the model of the compensation tank as a dynamic-effect carrier; the tank is modelled as a fixed volume with variable temperature, internal energy, and closed-system exergy accumulation [18]. The transient exergy destruction rate associated with the compensation tank is a difference between exergy rate of water flowing into the tank and water leaving the tank at given time step and change of internal exergy of the tank during the whole time step.
2.3. Exergo-Economic Analysis
2.4. Tools
3. Results
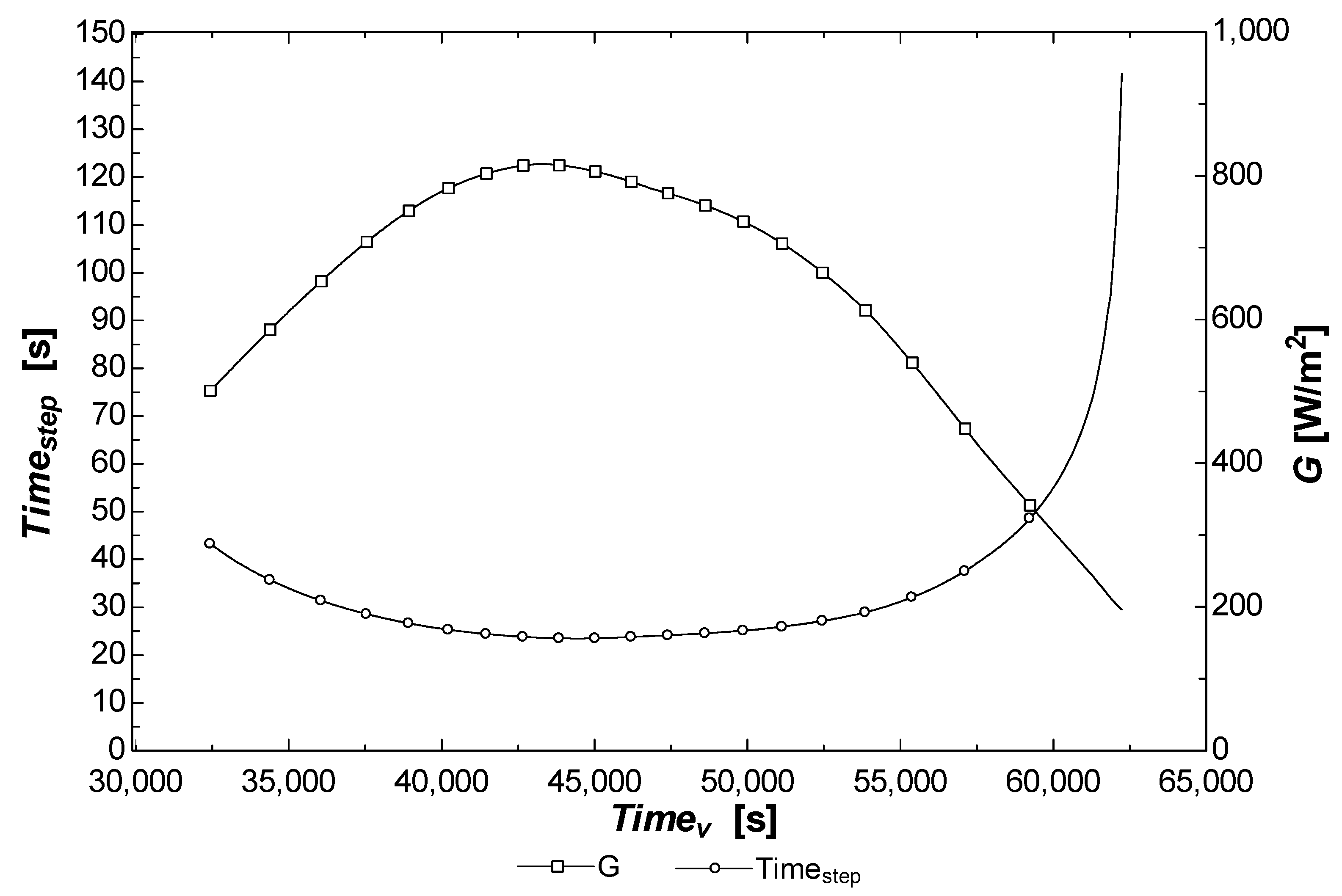
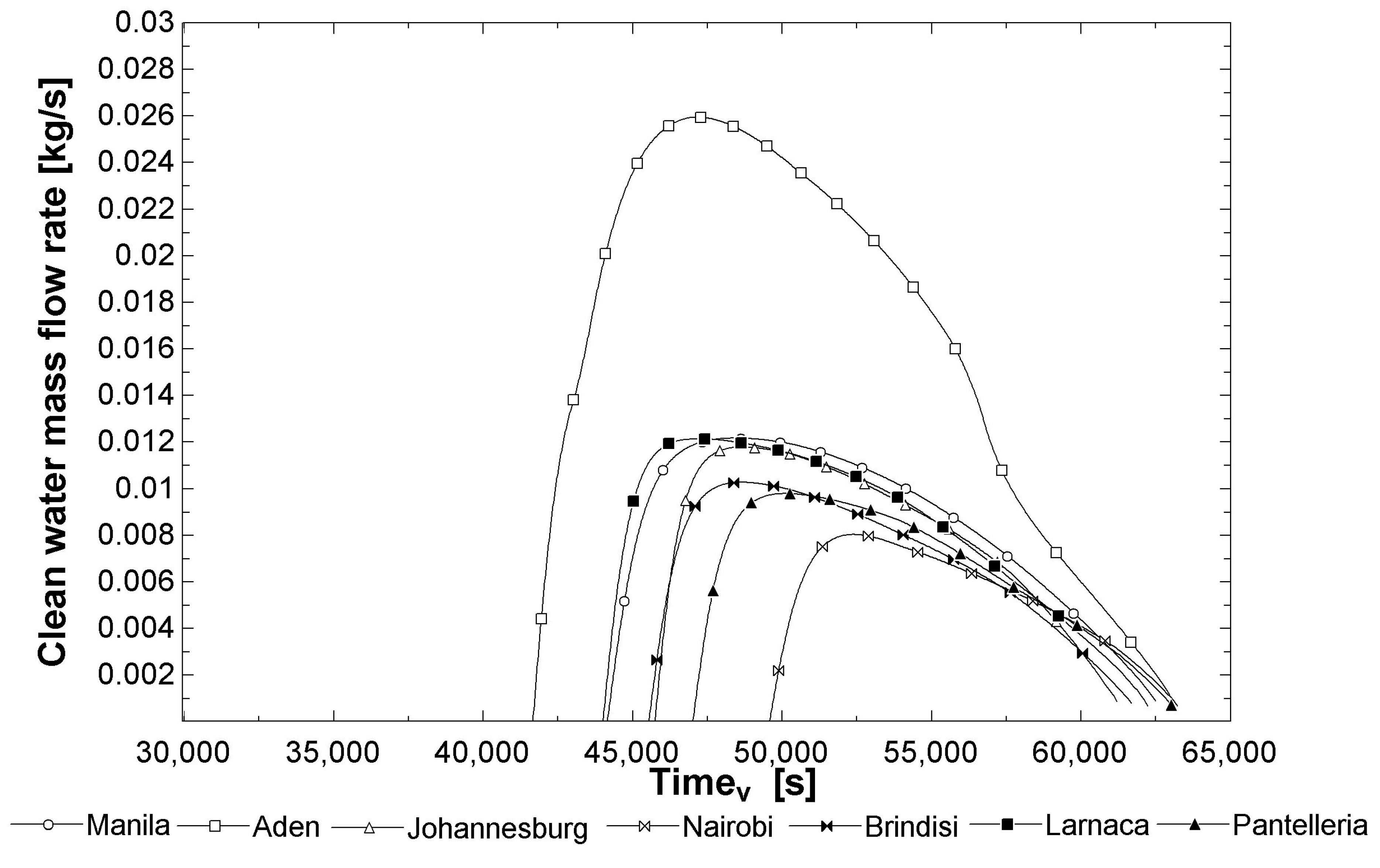
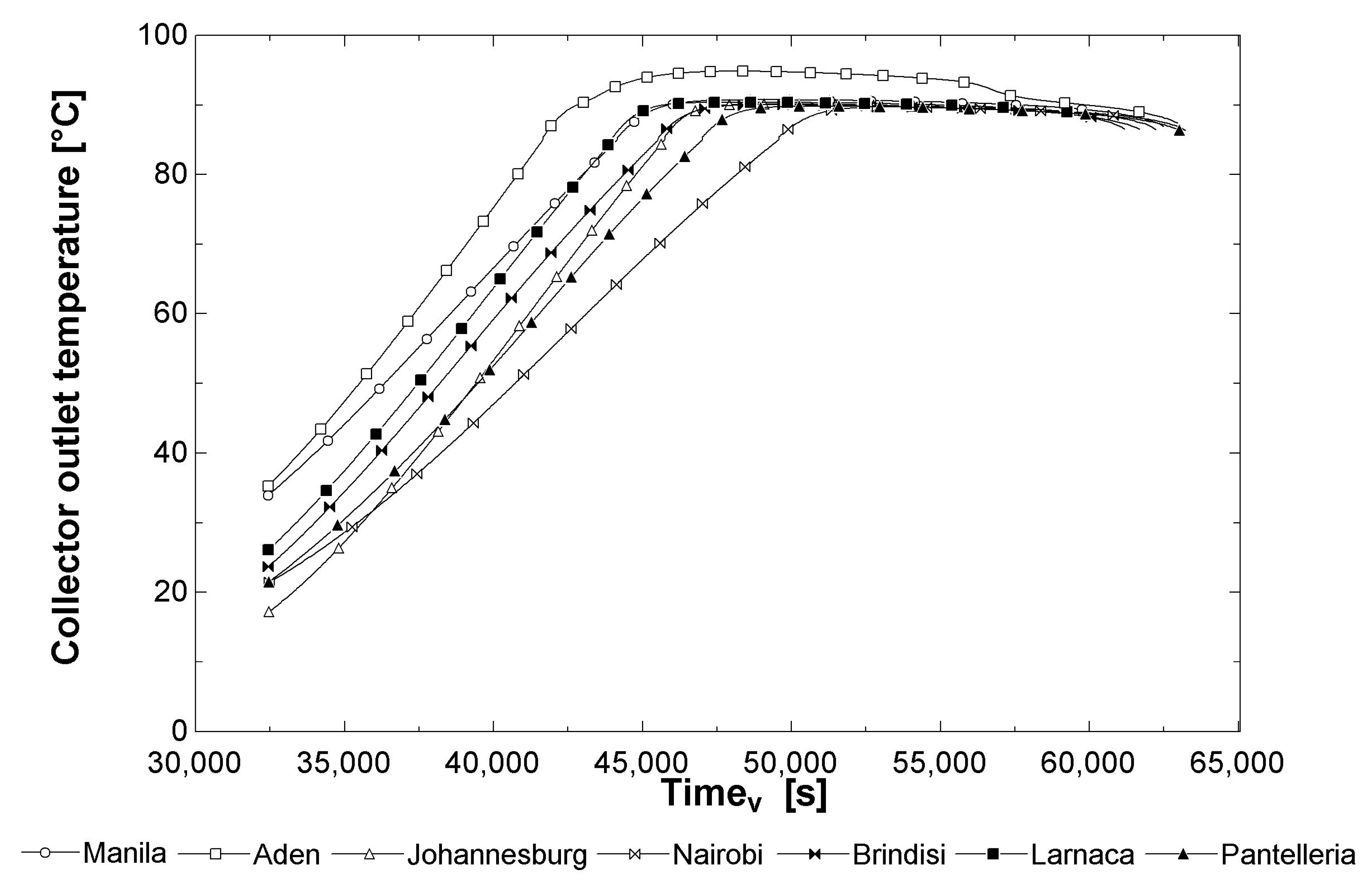
3.1. Results: Productivity (from Thermo-Fluid Off-Design Analysis)
3.2. Results—Exergy Analysis
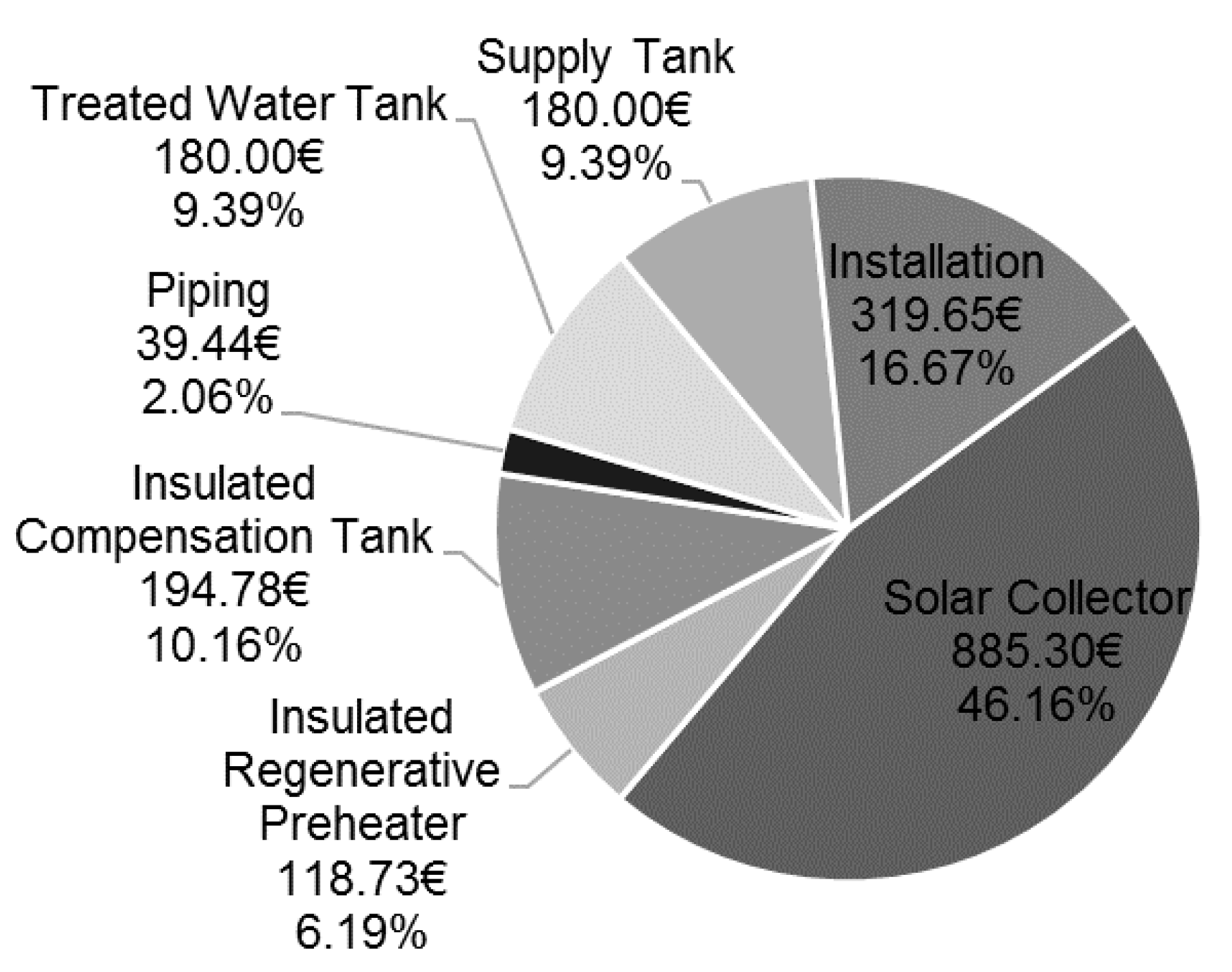
3.3. Results–Exergo-Economic Analysis
4. Comparison with PV Driven Reverse Osmosis System
5. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PET | Polyethylene terephthalate, common thermoplastic polymer |
| PVC | Polyvinyl chloride |
| RHE | Regenerative heat exchanger |
| SODIS | Solar Water Disinfection |
| UV | Ultraviolet |
Symbols
| Surface (solar collector or PV module) or cross section (pipe), m2 | |
| Cost rate associated with exergy transfer, €/s | |
| Cost rate associated with exergy transfer, €/day | |
| Collector constant (non-dimensional) | |
| Collector constant, W/(m2·K) | |
| Collector constant, W/(m2·K2) | |
| Cost per unit of exergy, €/J | |
| cp | Constant-pressure specific heat, J/(kg·K) |
| Specific cost, €/kg | |
| Exergy rate, W | |
| ExD | Exergy destruction, J |
| ExL | Exergy loss, J |
| g | Gravitational constant, m/s2 |
| G | Overall radiation, W/m2 |
| h | Enthalpy, kJ/kg |
| ir | Interest rate |
| m | Mass, kg |
| Mass flow rate, kg/s | |
| Mass flow rate, kg/year | |
| Mass flow rate, kg/day | |
| H | Driving head, m |
| k | Pressure constant |
| Operation period, year | |
| N | Number |
| p | Pressure, Pa |
| Overall Radiation heat rate, W | |
| Useful solar collector heat rate, W | |
| s | Entropy, J/(kgK) |
| T | Temperature ( average temperature), K |
| U | Internal energy, J/kg |
| v | Velocity, m/s |
| V | Volume, m3 |
| Power, W | |
| Cost rate associated with capital investment and operating and maintenance costs, €/s | |
| Cost rate associated with capital investment and operating and maintenance costs, €/s | |
| Cost rate associated with capital investment and operating and maintenance costs, €/day | |
| Z | Purchase cost, € |
| Vertical distance from ground level, m |
Greek Symbols
| Δ | Variation |
| η | Efficiency |
| ρ | Density, kg/m3 |
| τ | System time step (variable), s |
Subscripts
| amb | Ambient |
| av | Average |
| D | Destruction |
| Discharge pipe | |
| HP | Holding pipe |
| i | Time step i |
| in | Inlet |
| Installation | |
| Plant component | |
| L | Loss |
| MU | Make up (treated water) |
| Makeup pipe | |
| out | Outlet |
| Heat transfer associated | |
| rel | Relative (exergy loss or destruction) |
| ric | Recirculating |
| Solar Collector | |
| Supply tank | |
| tank | Tank |
| Treated water | |
| Treated water tank | |
| Work transfer associated | |
| x | Exergy |
References
- Hanouz, M.D.; Collins, A.; Marti, G.; Browne, C.; Di Battista, A.; Shaw, K.; Verin, S. The Global Risks Report 2017; The World Economic Forum: Geneva, Switzerland, 2017; pp. 91–93. ISBN 978-1-944835-07-1. [Google Scholar]
- Alsheghri, A.; Sharief, S.A.; Rabbani, S.; Aitzhan, N.Z. Design and Cost Analysis of a Solar Photovoltaic Powered Reverse Osmosis Plant for Masdar Institute. Energy Procedia 2015, 75, 319–324. [Google Scholar] [CrossRef]
- McGuigan, K.G.; Conroy, R.M.; Mosler, H.J.; du Preez, M.; Ubomba-Jaswa, E.; Fernandez-Ibañez, P. Solar water disinfection (SODIS): A review from bench-top to roof-top. J. Hazard. Mater. 2012, 235–236, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, K.R.; Kaushik, S.C. Energy, exergy and thermo-economic analysis of solar distillation systems : A review. Renew. Sustain. Energy Rev. 2013, 27, 709–723. [Google Scholar] [CrossRef]
- Anderson, R. Solar Water Disinfection. In Proceedings of the 1996 Annual Conference of the American Solar Energy Society, San Antonio, TX, USA, 31 March–3 April 1996. [Google Scholar]
- Duff, W.S.; Hodgson, J.A. A simple high efficiency solar water purification system. Sol. Energy 2005, 79, 25–32. [Google Scholar]
- Carielo da Silva, G.; Tiba, C.; Calazans, G.M.T. Solar pasteurizer for the microbiological decontamination of water. Renew. Energy 2016, 87, 711–719. [Google Scholar] [CrossRef]
- El Ghetany, H.; Abdel Dayem, A. Numerical simulation and experimental validation of a controlled flow solar water disinfection system. Desalin. Water Treat. 2010, 20, 11–21. [Google Scholar] [CrossRef]
- Abraham, J.P.; Plourde, B.D.; Minkowycz, W.J. Continuous flow solar thermal pasteurization of drinking water: Methods, devices, microbiology and analysis. Renew. Energy 2015, 81, 795–803. [Google Scholar] [CrossRef]
- Gill, L.W.; Price, C. Preliminary observations of a continuous flow solar disinfection system or a rural community in Kenya. Energy 2010, 35, 4607–4611. [Google Scholar] [CrossRef]
- Manfrida, G.; Petela, K.; Rossi, F. Natural circulation solar thermal system for water disinfection. In Proceedings of the 4th International Conference on Contemporary Problems of Thermal Engineering (CPOTE 2016), Katowice, Poland, 14–16 September 2016. [Google Scholar]
- Burch, J.D.; Thomas, K.E. Water disinfection for developing countries and potential for solar thermal pasteurization. Sol. Energy 1998, 64, 87–97. [Google Scholar] [CrossRef]
- Technical Data Sheet–LT-Power: For Thermal Applications 45 °C to 100 °C. Available online: www.tvpsolar.com (accessed on 13 July 2017).
- Bejan, A.; Tsatsaronis, G.; Moran, M. Thermal Design and Optimization; John Wiley & Sons, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Szargut, J.; Petela, R. Exergy; WNT: Warsaw, Poland, 1965. [Google Scholar]
- Ge, Z.; Wang, H.; Wang, H.; Zhang, S.; Guan, X. Exergy analysis of flat plate solar collectors. Entropy 2014, 16, 2549–2567. [Google Scholar] [CrossRef]
- Manfrida, G.; Kawambwa, S. Exergy control for a flat-plate Collector/Rankine Cycle Solar Power System. ASME J. Sol. Energy Eng. 1991, 113, 89–93. [Google Scholar] [CrossRef]
- Baldini, A.; Manfrida, G.; Tempesti, D.; Sergio, E.; Lombroso, C. Model of a Solar Collector / Storage System for Industrial Thermal Applications. Int. J. Thermodyn. 2009, 12, 83–88. [Google Scholar]
- Tsatsaronis, G. Thermoeconomic analysis and optimization of energy systems. Prog. Energy Combust. Sci. 1993, 19, 227–257. [Google Scholar] [CrossRef]
- POLYFLEX Insulation Systems. Available online: http://www.fiorettisrl.it/online/wp-content/uploads/2013/10/CATALOGO-POLYFLEX-2013-A.pdf (accessed on 13 July 2017).
- Catalogo Generale. Available online: http://www.rototec.it/aspimg/download/31032016172032_CatalogoGeneraleRototec2016rev.01.pdf (accessed on 13 July 2017).
- Multistrato, Tubazioni e Raccorderia, Multilayer Pipes and Fitting. Available online: http://tubi.net/wp-content/uploads/2015/09/MULTISTRATO-TUBAZIONI-E-RACCORDERIE.pdf (accessed on 13 July 2017).
- Scambiatore di Calore Acciao inox Hrale 50 Dischi max 90 kw Scambiatore Termico. Available online: https://www.manomano.it/scambiatori-per-caldaie/scambiatore-di-calore-acciao-inox-hrale-50-dischi-max-90-kw-scambiatore-termico-3214353 (accessed on 13 July 2017).
- Alloggiamento Isolante Hrale per Scambiatore di Calore 50 Piastre B3-12A-50. Available online: https://www.manomano.it/scambiatori-per-caldaie/alloggiamento-isolante-hrale-per-scambiatore-di-calore-50-piastre-b3-12a-50-3214417 (accessed on 13 July 2017).
- Vasi di Espansione Autoclavi a Membrana. Available online: http://diazilla.com/doc/369155/vasi-di-espansione-autoclavi-a-membrana (accessed on 13 July 2017).
- Technology Data for Energy Plants. Available online: https://energiatalgud.ee/img_auth.php/4/42/Energinet.dk._Technology_Data_for_Energy_Plants._2012.pdf (accessed on 13 July 2017).
- EES (Engineering Equation Solver) Information. Available online: http://www.fchart.com/ees/ (accessed on 13 July 2017).
- Trnsys16 Information. Available online: https://sel.me.wisc.edu/trnsys/features/ (accessed on 13 July 2017).
- Nellis, G.; Klein, S. Heat Transfer; Cambridge University Press: New York, NY, USA, 2009. [Google Scholar]
- Meteonorm Information. Available online: http://www.meteonorm.com/ (accessed on 13 July 2017).
- Helal, A.M.; Al-Malek, S.A.; Al-Katheeri, E.S. Economic feasibility of alternative designs of a PV-RO desalination unit for remote areas in the United Arab Emirates. Desalination 2008, 221, 1–16. [Google Scholar] [CrossRef]
- Bilton, A.M.; Wiesman, R.; Arif, A.F.M.; Zubair, S.M.; Dubowsky, S. On the feasibility of community-scale photovoltaic-powered reverse osmosis desalination systems for remote locations. Renew. Energy 2011, 36, 3246–3256. [Google Scholar] [CrossRef]
- Renewable Energy Technologies: Cost Analysis Series, International Renewable Energy Agency (IRENA). Available online: https://www.irena.org/DocumentDownloads/Publications/RE_Technologies_Cost_Analysis-SOLAR_PV.pdf#page=1&zoom=auto,475,874 (accessed on 24 July 2017).
- Bilton, A.M.; Kelley, L.C.; Dubowsky, S. Photovoltaic reverse osmosis—Feasibility and a pathway to develop technology. Desalination Water Treat. 2011, 31, 24–34. [Google Scholar] [CrossRef]
- Carvalho, P.C.M.; Riffel, D.B.; Freire, C.; Damasceno Montenegro, F.F. The Brazilian Experience with a Photovoltaic Powered Reverse Osmosis Plant. Prog. Photovolt. Res. Appl. 2004, 385, 373–385. [Google Scholar] [CrossRef]

| Mixer | Solar collector | RHE | Tank | Pipes | |
|---|---|---|---|---|---|
| Relative Exergy Destruction | 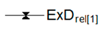 | 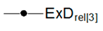 |  | 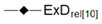 | 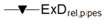 |
| Relative Exergy Loss | 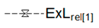 | 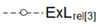 | 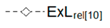 | 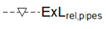 |
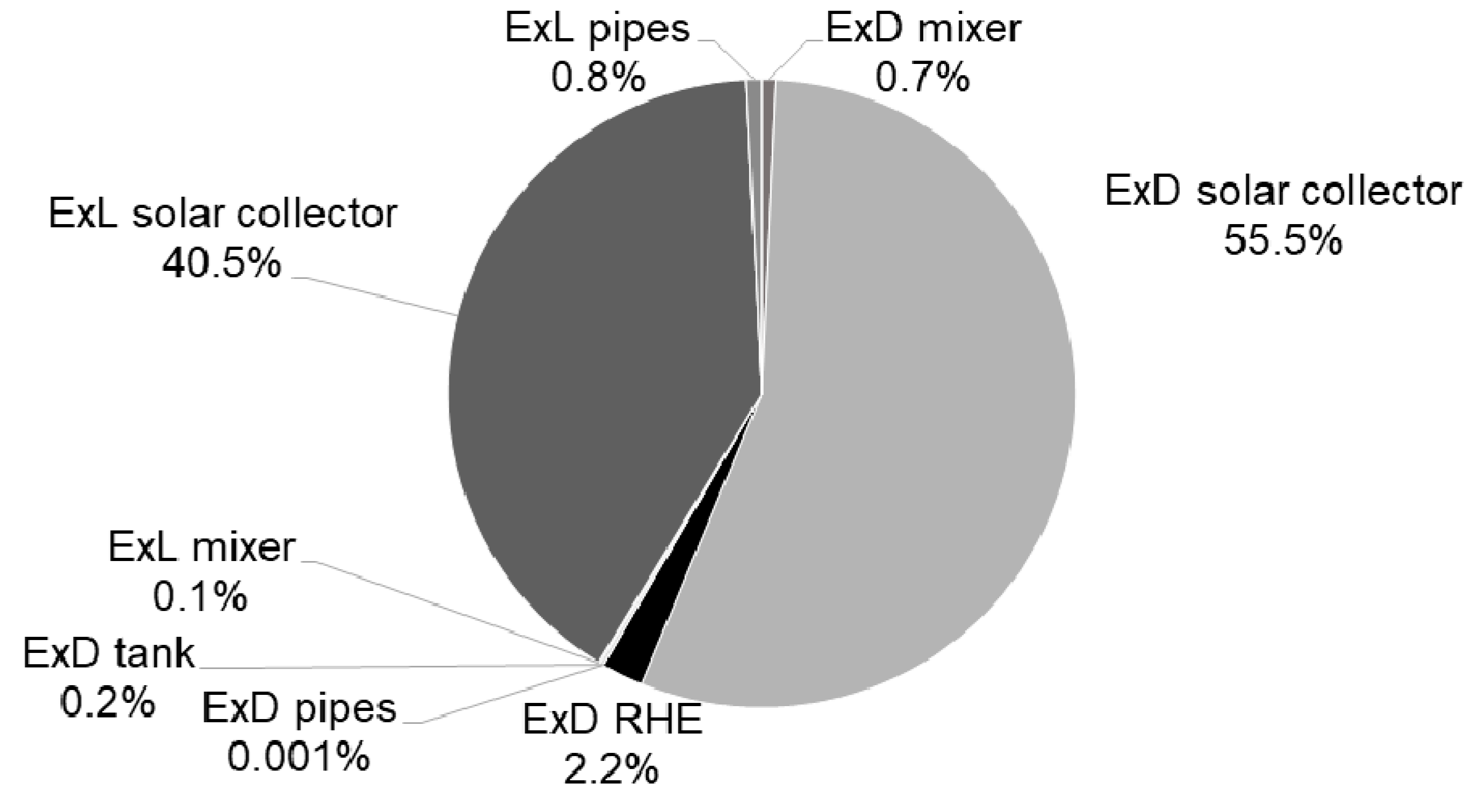
| System Parameters | |||||
|---|---|---|---|---|---|
| C0 | 0.78 | C1 | 1.75 W/(m2·K) | C2 | 0.00625 W/(m2·K2) |
| Tamb | 30°C | G | 1000 W/m2 | ASC | 2·1.95 m2 |
| L/H | 5.3 | H | 1.5 m | DHP | 0.15 m |
| Design Sizing Results | |||||
|---|---|---|---|---|---|
| D | 0.02 m | L | 8 m | 0.0255 kg/s | |
| Δp | 360 Pa | k | 552,000 | η | 0.692 |
| 2.7 kW | AHP | 0.785 cm2 | ΔzHP | 0.147 m | |
| No | Location | Manila (Philippines) | Aden (Yemen) | Johannesburg (South Africa) | Nairobi (Kenya) | Brindisi (Italy) | Pantelleria (Italy) | Larnaca (Cyprus) |
|---|---|---|---|---|---|---|---|---|
| 1 | Coordinates | 14.52° N 121°E | 12.83° N 45.03° E | 26.13° S 28.23° E | 1.3° S 36.75° E | 40.65° N 17.95° E | 36.82° N 11.97° E | 34.88° N 33.63° E |
| 2 | Annual solar radiation, MWh/(m2·year) | 1.818 | 2.244 | 2.254 | 1.933 | 1.701 | 2.111 | 1.702 |
| 3 | Annual productivity, m3 | 48.792 | 106.354 | 56.819 | 41.961 | 28.342 | 28.347 | 59.525 |
| 4 | Solar collector: annual exergy destruction, MWh/(m2·year) | 0.869 | 1.121 | 1.084 | 0.931 | 0.787 | 0.910 | 0.799 |
| 5 | Solar collector: annual exergy loss, MWh/(m2·year) | 0.626 | 0.739 | 0.777 | 0.677 | 0.584 | 0.642 | 0.617 |
| 6 | Water production cost—Natural circulation, €/m3 | 4.64 | 2.24 | 4.19 | 5.20 | 6.80 | 6.77 | 4.01 |
| 7 | Water production cost—ROPV, €/m3 | 5.34 | 4.02 | 3.81 | 5.13 | 5.96 | 6.87 | 4.83 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dainelli, N.; Manfrida, G.; Petela, K.; Rossi, F. Exergo-Economic Evaluation of the Cost for Solar Thermal Depuration of Water. Energies 2017, 10, 1395. https://doi.org/10.3390/en10091395
Dainelli N, Manfrida G, Petela K, Rossi F. Exergo-Economic Evaluation of the Cost for Solar Thermal Depuration of Water. Energies. 2017; 10(9):1395. https://doi.org/10.3390/en10091395
Chicago/Turabian StyleDainelli, Nicola, Giampaolo Manfrida, Karolina Petela, and Federico Rossi. 2017. "Exergo-Economic Evaluation of the Cost for Solar Thermal Depuration of Water" Energies 10, no. 9: 1395. https://doi.org/10.3390/en10091395
APA StyleDainelli, N., Manfrida, G., Petela, K., & Rossi, F. (2017). Exergo-Economic Evaluation of the Cost for Solar Thermal Depuration of Water. Energies, 10(9), 1395. https://doi.org/10.3390/en10091395






