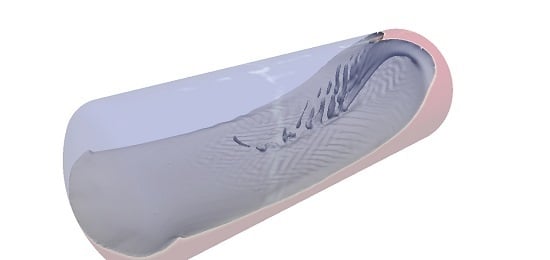
Numerical Simulations of Sloshing and the Thermodynamic Response Due to Mixing
Abstract
:1. Introduction
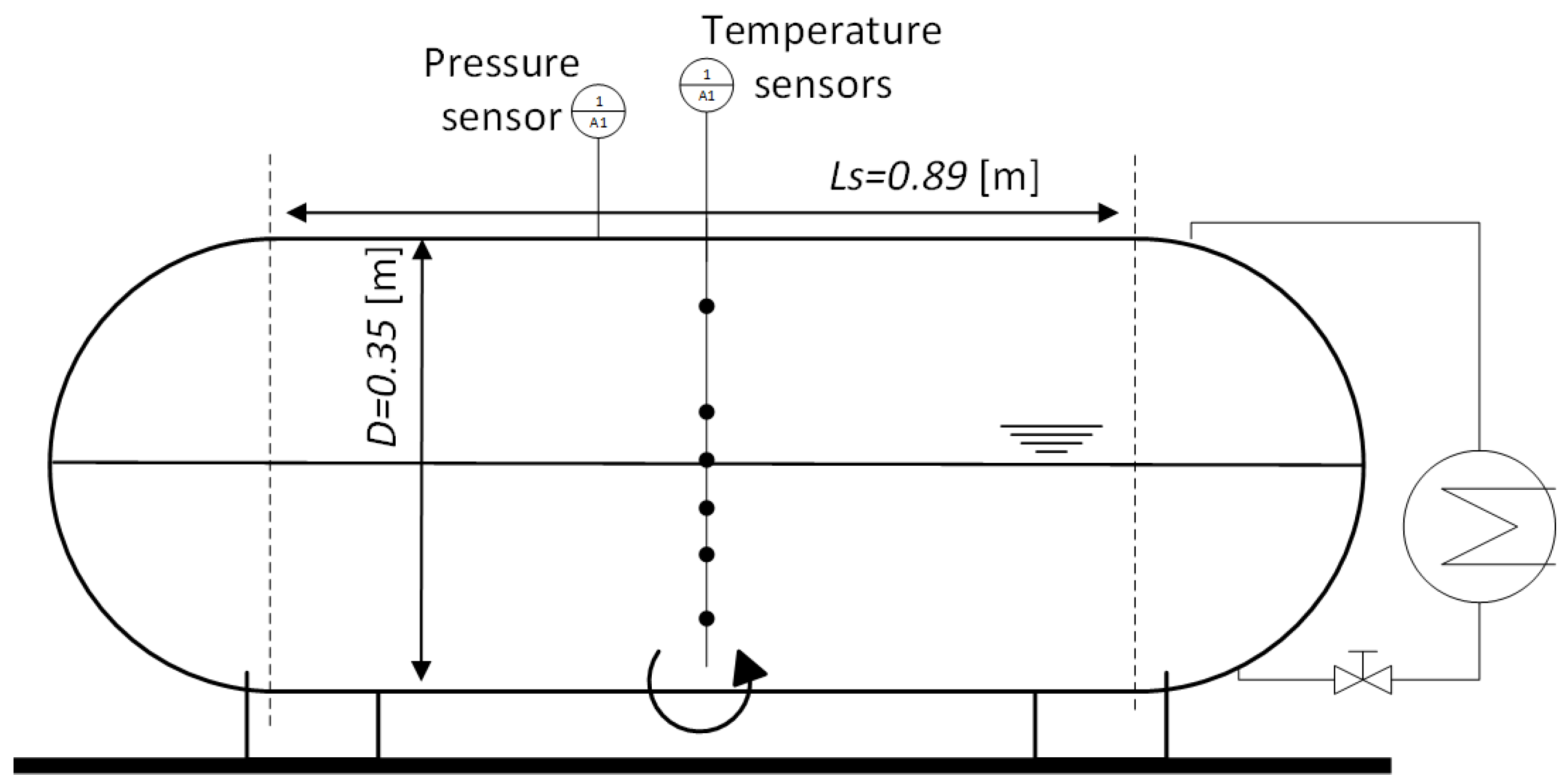
2. Experimental Work
3. Mathematical Models and Numerical Methods
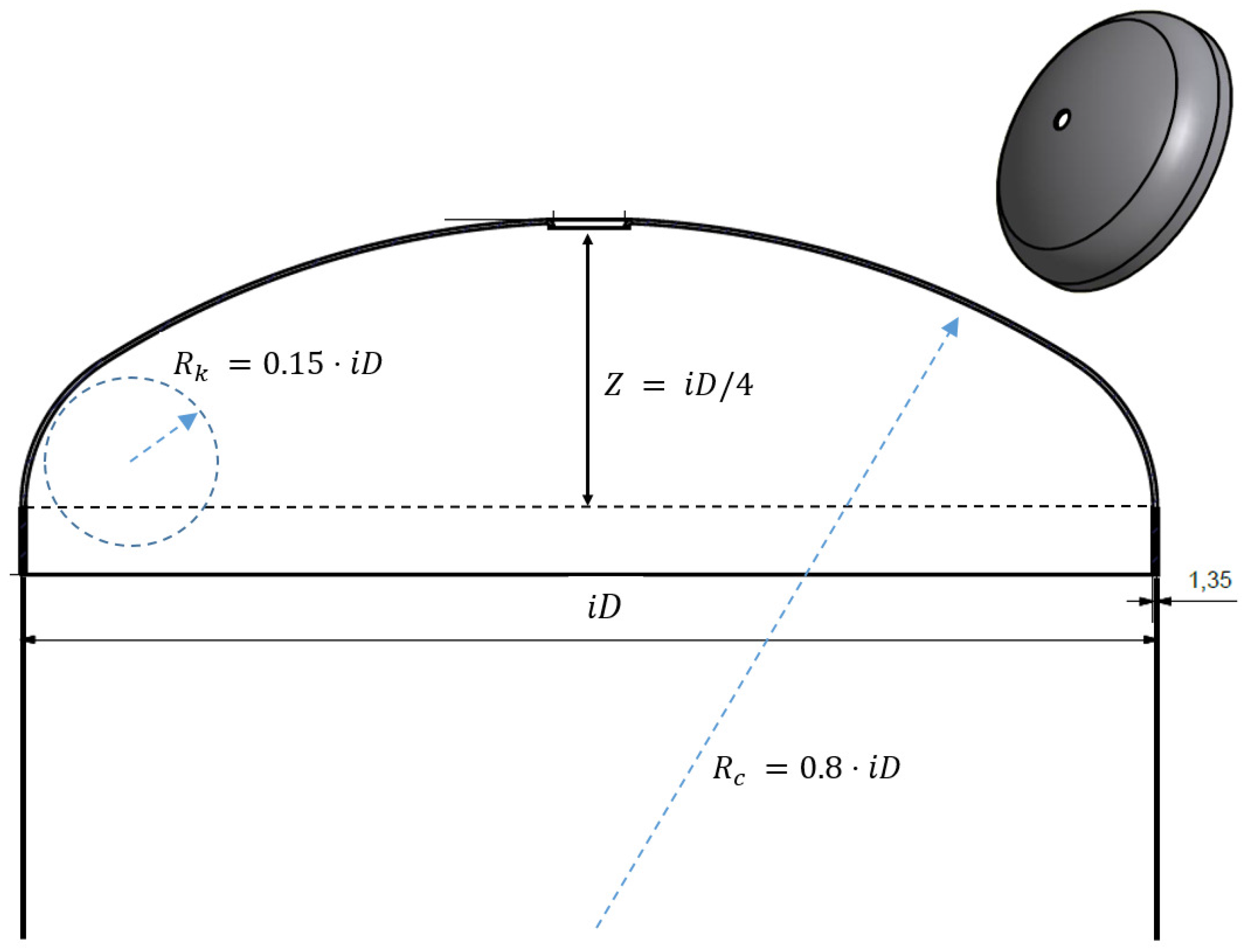
3.1. Problem Description
3.2. CFD-Model
3.3. OpenFOAM Solver InterDyMFoam
4. Results
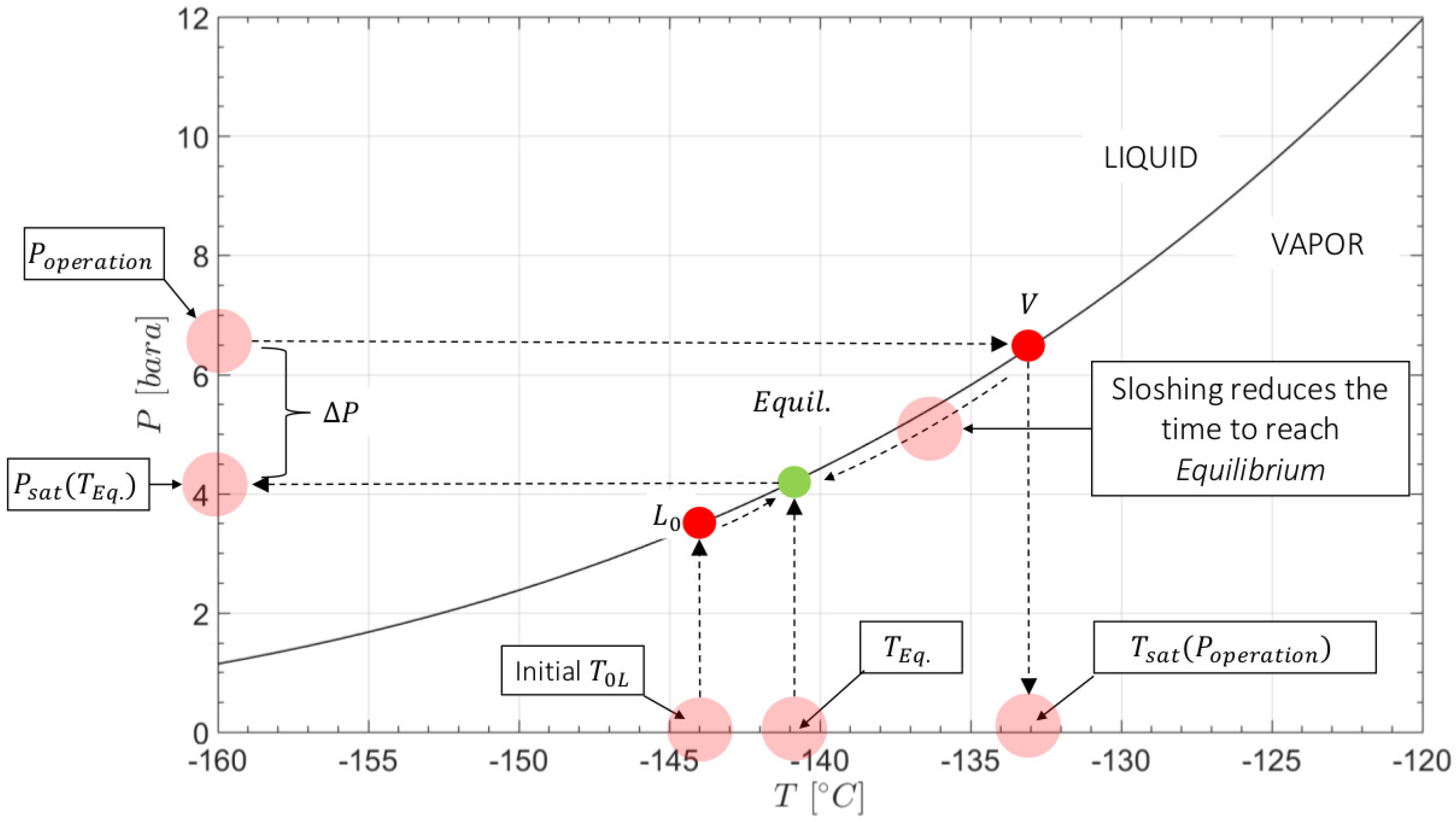
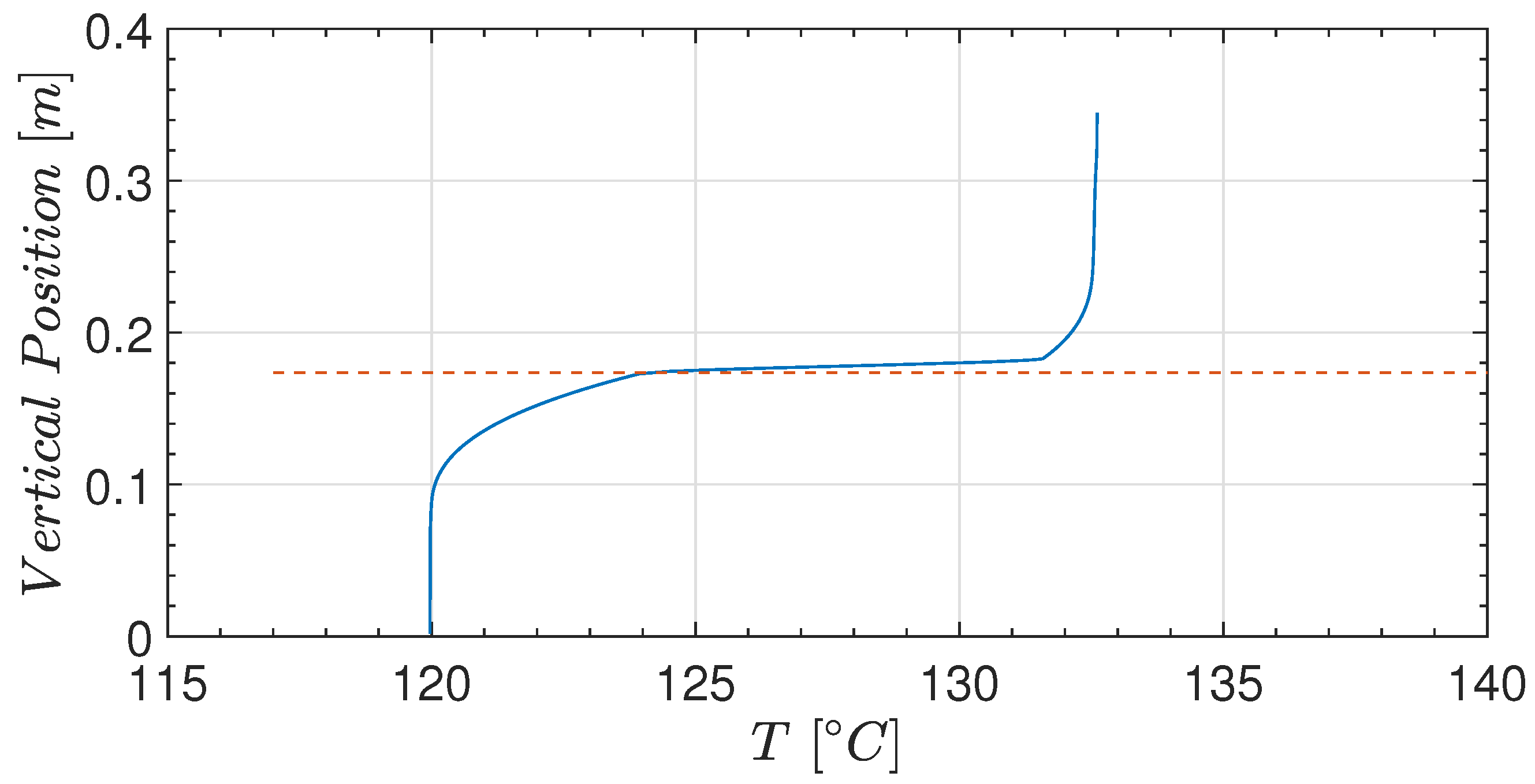
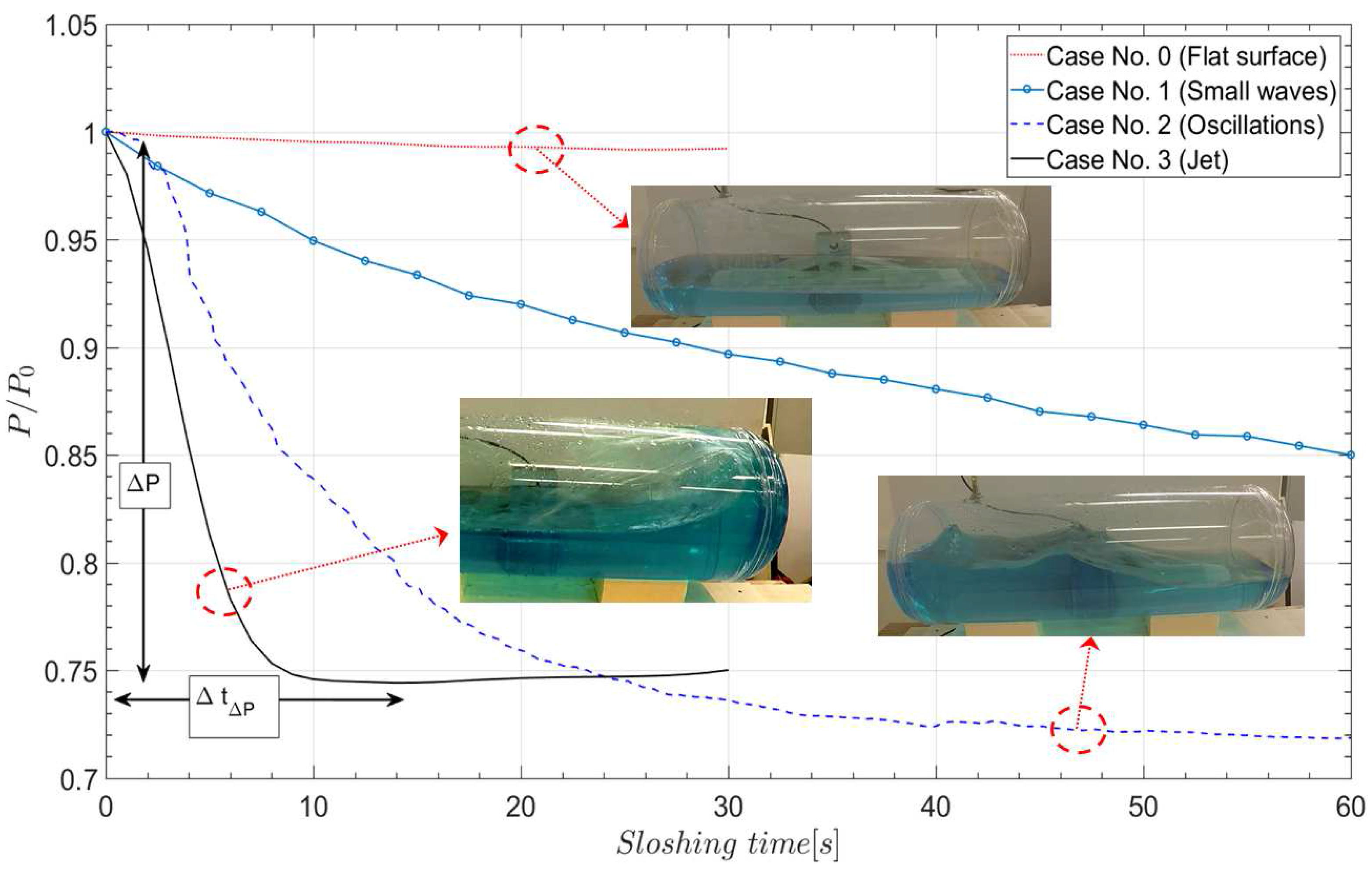
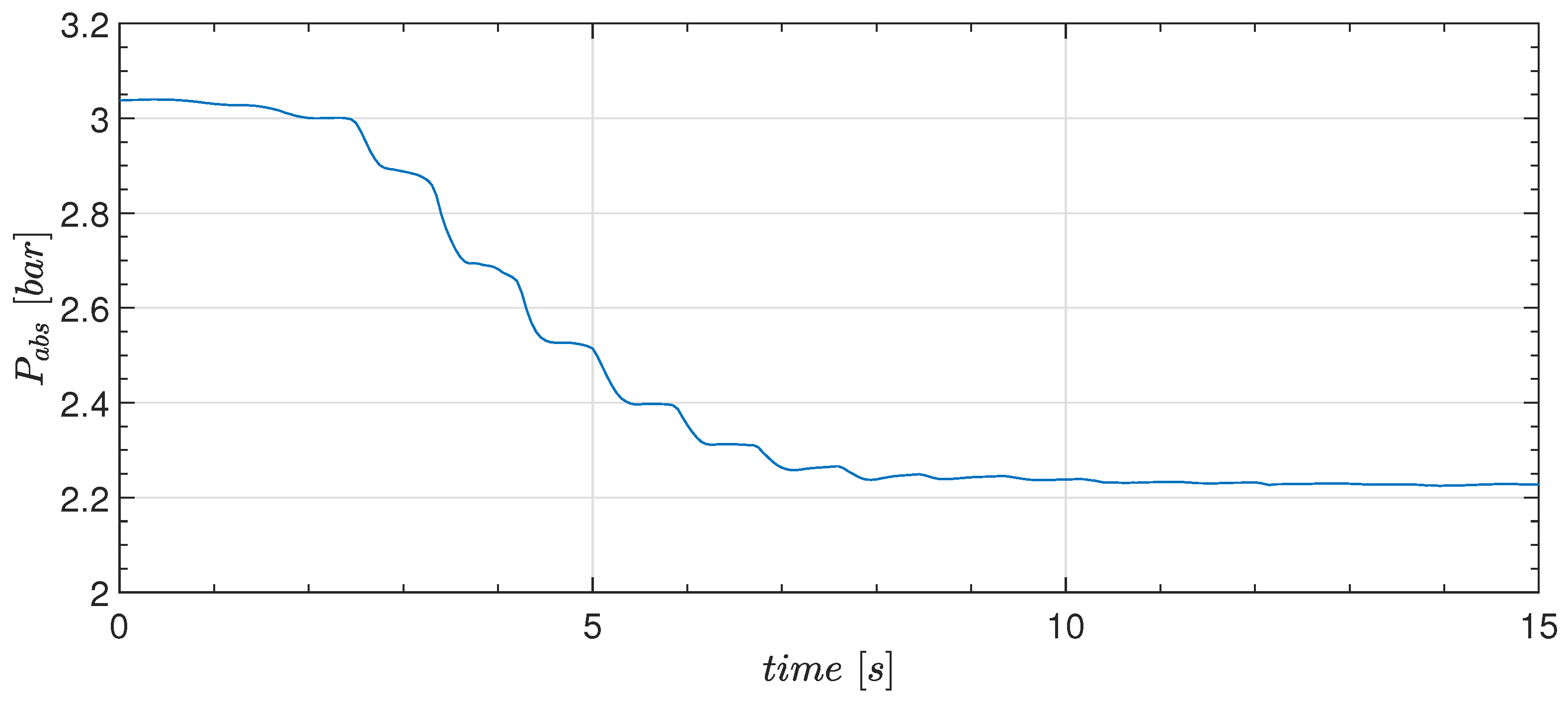
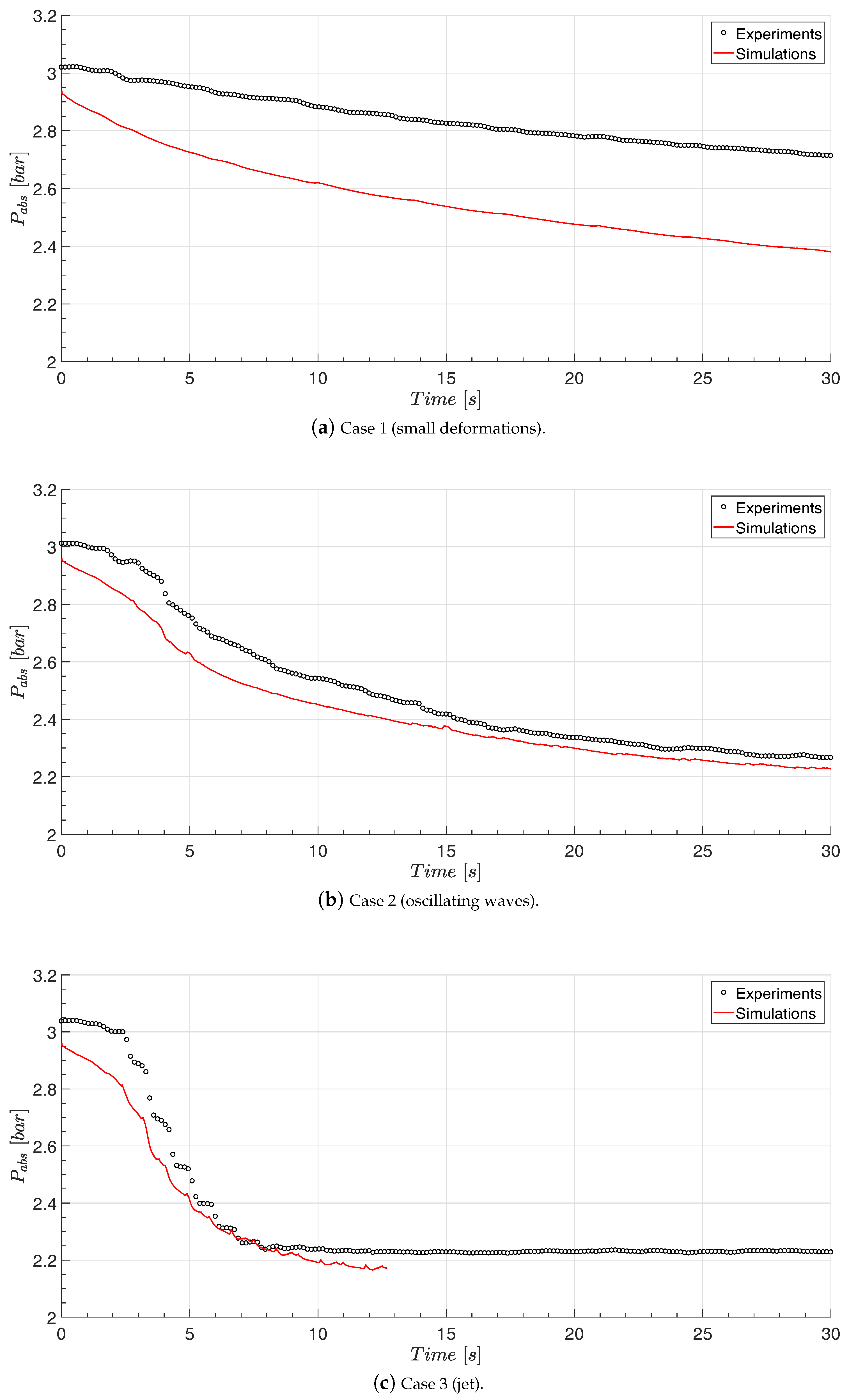
4.1. Experiments—Thermodynamic Response with Water
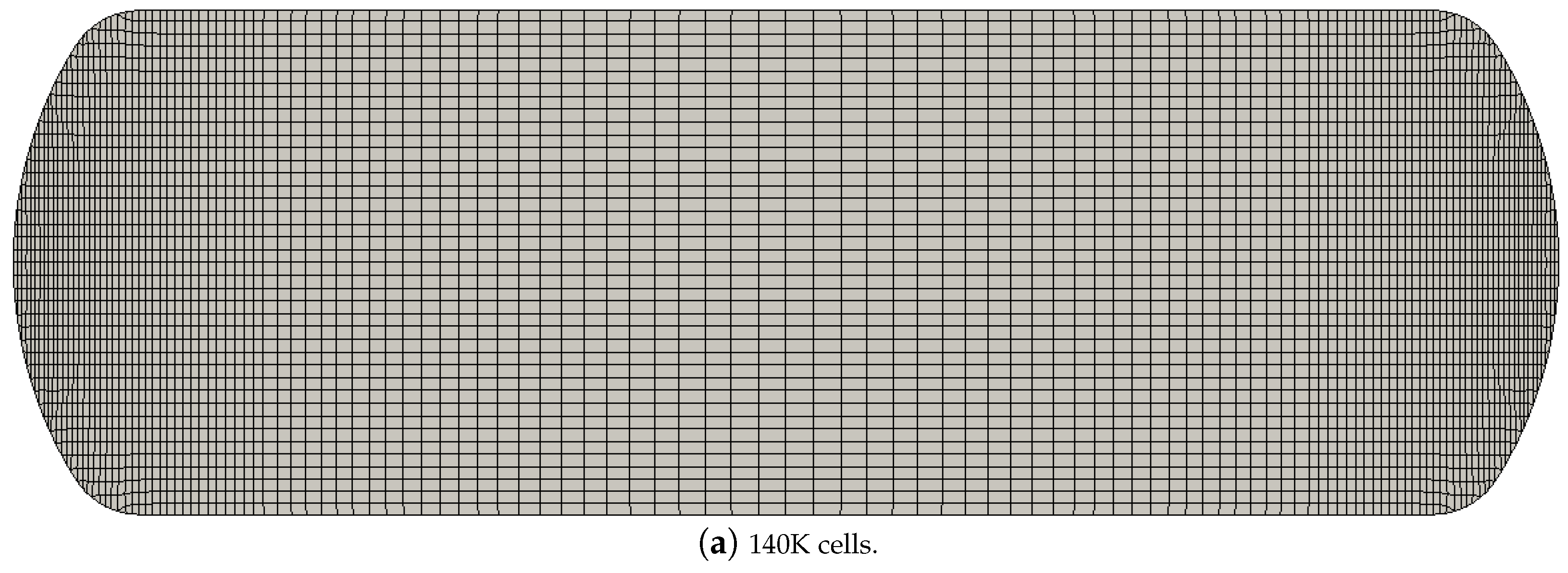
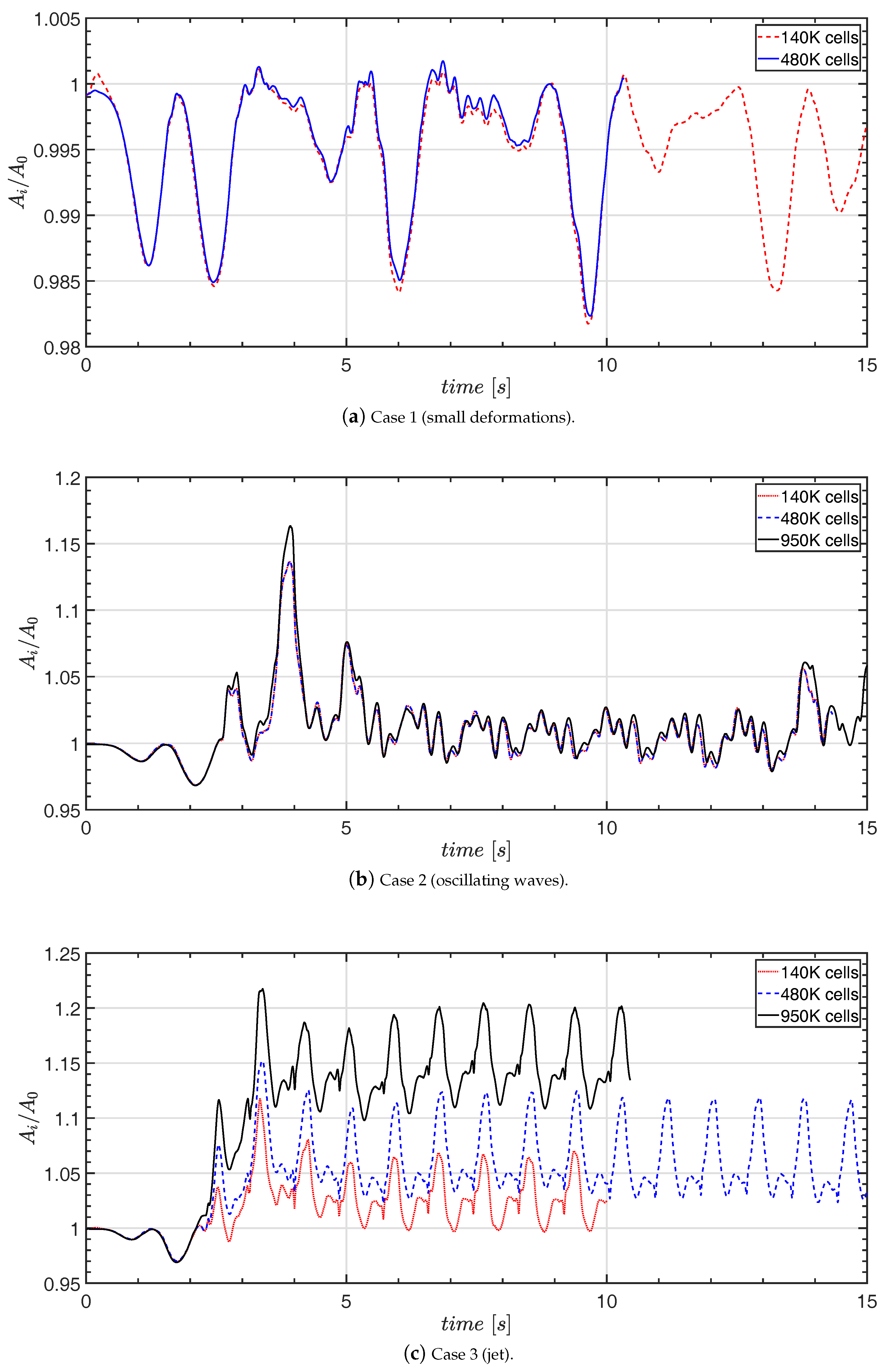
4.2. Grid Independence Study
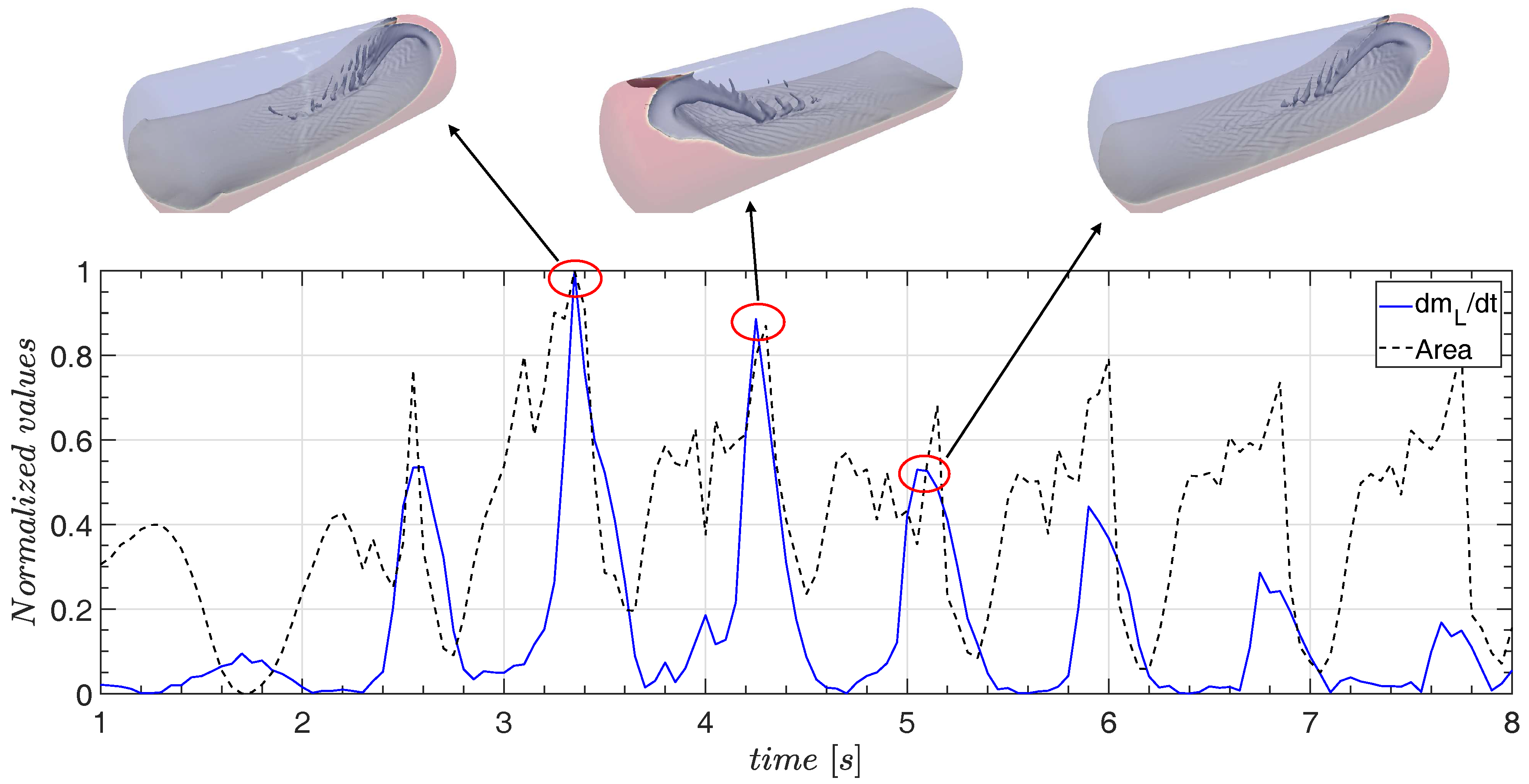
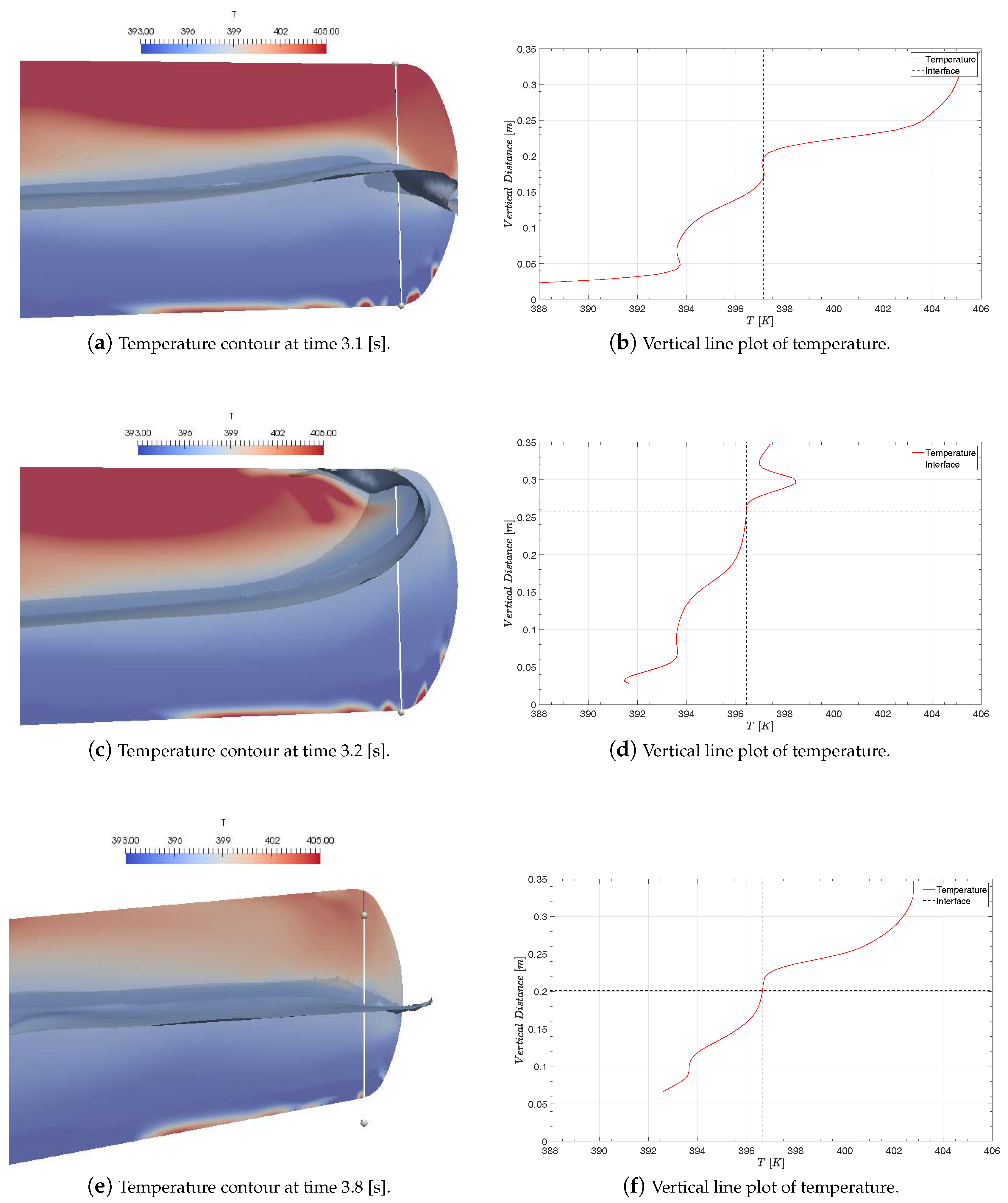
4.3. The Influence of the Liquid Jet
4.4. Prediction of Pressure and Temperature
5. Discussion
6. Conclusions
- The grid independence study indicates that the area can be estimated when the free surface does not break. Grid convergence was not found in case 3.
- The increase of the interface area is approximately 2.5% in case 2 when the sloshing is closer to a quasi-steady condition.
- The interface area is not accurately determined in case 3, but the results indicate that the area is larger than in case 2, but may be less than an increase of 50% relative to the undisturbed area.
- The pressure measurement in case 3 shows correspondence with the sloshing hydrodynamics, and the occurrence of the jet clearly influences the pressure drop.
- Results from simulations with the extended OpenFOAM solver shows acceptable agreement with the measured pressure, and may be used for further investigation. However, it is quite uncertain to what extent the model is applicable for other cases with other components, initial conditions, etc.
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. OpenFOAM Code Snippets
Appendix A.1. Weighted Average of the Gas Temperature
Tsat = sum((scalar(1)-alpha1)*T)/sum(scalar(1)-alpha1);
Appendix A.2. Energy Transport Equation
fvScalarMatrix TEqn ( fvm::ddt(rho,T) +fvm::div(rhoPhi,T) −fvm::laplacian(kappaF/cp,T) == fvm::Su(alpha1*(scalar(1)-alpha1)*rho*evap*Tsat, T) +fvm::Sp(-alpha1*(scalar(1)-alpha1)*rho*evap, T) ); TEqn.solve()
Appendix A.3. Code to Calculate the Interface Area
volScalarField gradGamma = mag(fvc::grad(alpha1)); dimensionedScalar totalSurfaceArea = fvc::domainIntegrate(gradGamma);
References
- Æsøy, V.; Stenersen, D. Low emission LNG fuelled ships for environmental friendly operations in arctic areas. In Proceedings of the ASME 2013 32nd International Conference on Ocean, Offshore and Arctic Engineering, Nantes, France, 9–14 June 2013; p. V006T07A028. [Google Scholar]
- Faltinsen, O.M.; Timokha, A.N. Sloshing; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Æsøy, V.; Einang, P.M.; Stenersen, D.; Hennie, E.; Valberg, I. LNG-fuelled engines and fuel systems for medium-speed engines in maritime applications. SAE Tech. Paper 2011. [Google Scholar] [CrossRef]
- Grotle, E.; Æsøy, V.; Pedersen, E. Modelling of LNG fuel systems for simulations of transient operations. In Maritime-Port Technology and Development; CRC Press: Cleveland, OH, USA, 2014; p. 205. [Google Scholar]
- Lemmon, E.W.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP; version 9.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2013.
- Grotle, E.L.; Halse, K.H.; Pedersen, E.; Li, Y. Non-Isothermal Sloshing in Marine Liquefied Natural Gas Fuel Tanks. In Proceedings of the 26th International Ocean and Polar Engineering Conference, Rhodes, Greece, 26 June–1 July 2016; International Society of Offshore and Polar Engineers: Mountain View, CA, USA, 2016. [Google Scholar]
- Moran, M.E.; Mcnelis, N.B.; Kudlac, M.T.; Haberbusch, M.S.; Satornino, G.A. Experimental results of hydrogen slosh in a 62 cubic foot (1750 liter) tank. In Proceedings of the 30th Joint Propulsion Conference, Indianapolis, IN, USA, 27–29 June 1994. [Google Scholar]
- Lacapere, J.; Vieille, B.; Legrand, B. Experimental and numerical results of sloshing with cryogenic fluids. Progress in Propulsion Physics. EDP Sci. 2009, 1, 267–278. [Google Scholar]
- Arndt, T. Sloshing of Cryogenic Liquids in a Cylindrical Tank under Normal Gravity Conditions; Universtität Bremen: Bremen, Germany, 2011. [Google Scholar]
- Ludwig, C.; Dreyer, M.; Hopfinger, E. Pressure variations in a cryogenic liquid storage tank subjected to periodic excitations. Int. J. Heat Mass Transf. 2013, 66, 223–234. [Google Scholar] [CrossRef]
- Lin, C.S.; Hasan, M.; Nyland, T. Mixing and transient interface condensation of a liquid hydrogen tank. In Proceedings of the 29th Joint Propulsion Conference and Exhibit, Monterey, CA, USA, 28–30 June 1993. [Google Scholar]
- Juric, D.; Tryggvason, G. Computations of boiling flows. Int. J. Multiph. Flow 1998, 24, 387–410. [Google Scholar] [CrossRef]
- Welch, S.W.; Wilson, J. A volume of fluid based method for fluid flows with phase change. J. Comput. Phys. 2000, 160, 662–682. [Google Scholar] [CrossRef]
- Youngs, D.L. Time-dependent multi-material flow with large fluid distortion. In Numerical Methods for Fluid Dynamics; Academic Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Hardt, S.; Wondra, F. Evaporation model for interfacial flows based on a continuum-field representation of the source terms. J. Comput. Phys. 2008, 227, 5871–5895. [Google Scholar] [CrossRef]
- Gibou, F.; Chen, L.; Nguyen, D.; Banerjee, S. A level set based sharp interface method for the multiphase incompressible Navier–Stokes equations with phase change. J. Comput. Phys. 2007, 222, 536–555. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Jin, Y. Pressurization performance and temperature stratification in cryogenic final stage propellant tank. Appl. Therm. Eng. 2016, 106, 211–220. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Jin, Y.; Li, C. Thermodynamic performance of pre-pressurization in a cryogenic tank. Appl. Therm. Eng. 2017, 112, 801–810. [Google Scholar] [CrossRef]
- Haider, J. Numerical Modelling of Evaporation and Condensation Phenomena-Numerische Modellierung von Verdampfungs-und Kondensationsphänomenen. Ph.D. Thesis, Universität Stuttgart, Stuttgart, Germany, 2013. [Google Scholar]
- Kunkelmann, C. Numerical Modeling and Investigation of Boiling Phenomena. Ph.D. Thesis, Technische Universität, Munich, Germany, 2011. [Google Scholar]
- Georgoulas, A.; Andredaki, M.; Marengo, M. An Enhanced VOF Method Coupled with Heat Transfer and Phase Change to Characterise Bubble Detachment in Saturated Pool Boiling. Energies 2017, 10, 272. [Google Scholar] [CrossRef]
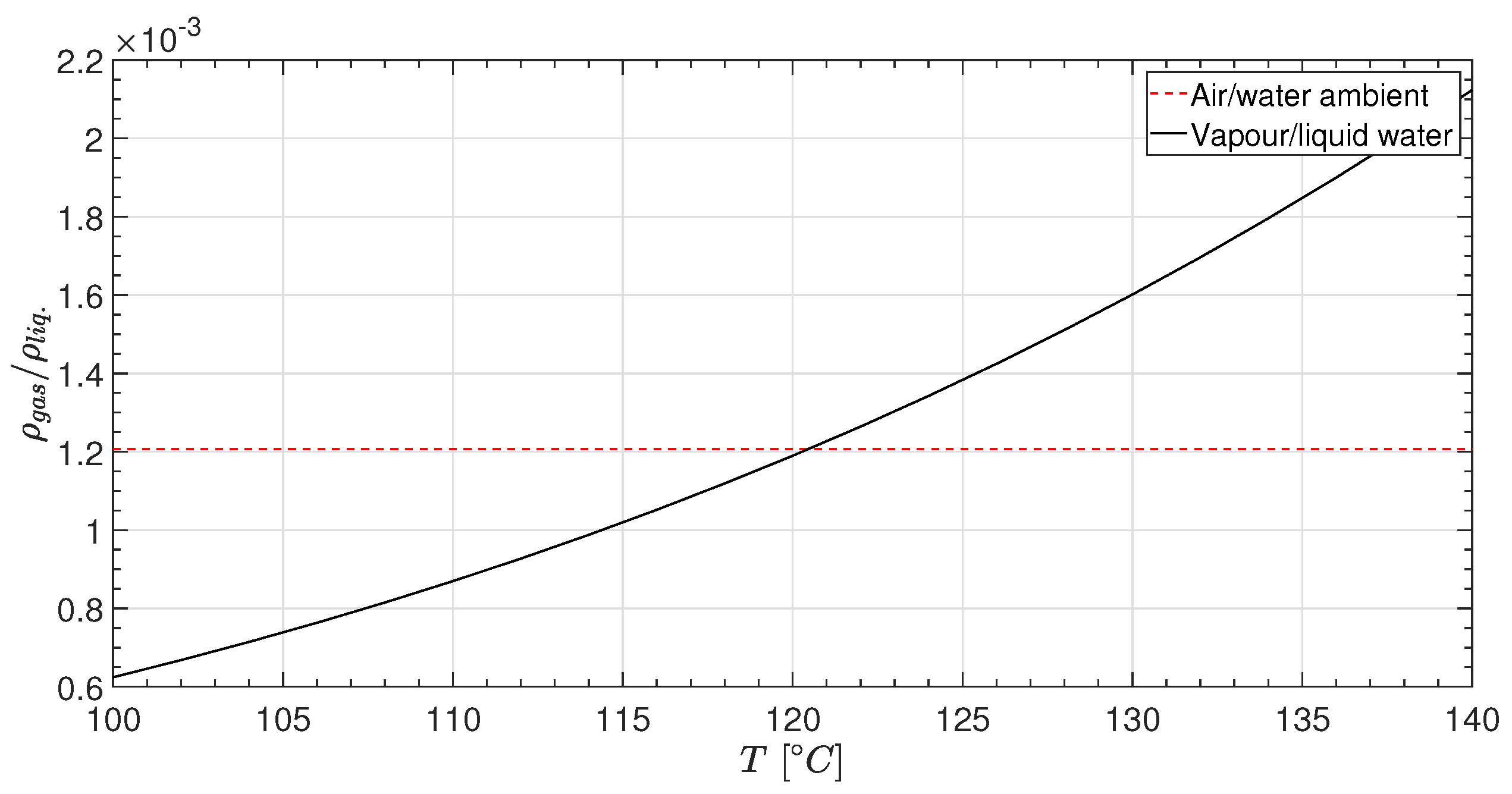
- Maillard, S.; Brosset, L. Influence of DR Between Liquid and Gas on Sloshing Model Test Results. Int. J. Offshore Polar Eng. 2009, 19, 167–175. [Google Scholar]
- Moukalled, F.; Mangani, L.; Darwish, M. The Finite Volume Method in Computational Fluid Dynamics: An Advanced Introduction with OpenFOAM® and Matlab; Springer: Berlin, Germany, 2015. [Google Scholar]
- Menter, F.R. Two-equation eddy-viscosity turbulence models for engineering applications. AIAA J. 1994, 32, 1598–1605. [Google Scholar] [CrossRef]
- Menter, F.R.; Esch, T. Elements of Industrial Heat Transfer Prediction. In Proceedings of the 16th Brazilian Congress of Mechanical Engineering (COBEM), Minas Gerais, Brazil, 26–30 November 2001. [Google Scholar]
- Menter, F.; Kuntz, M.; Langtry, R. Ten years of industrial experience with the SST turbulence model. Turbul. Heat Mass Trans. 2003, 4, 625–632. [Google Scholar]
- The OpenFOAM Foundation Ltd. kOmegaSSTBase.H File Reference. 2017. Available online: https://cpp.openfoam.org/v5/classFoam_1_1kOmegaSST.html#details (accessed on 27 August 2017).
- Menter, F.R.; Carregal Ferreira, J.; Esch, T.; Konno, B. The SST Turbulence Model with Improved Wall Treatment for Heat Transfer Predictions in Gas Turbines. In Proceedings of the International Gas Turbine Congress, Tokyo, Japan, 2–7 November 2003. [Google Scholar]
- Rusche, H. Computational Fluid Dynamics of Dispersed Two-Phase Flows at High Phase Fractions. Ph.D. Thesis, Imperial College London (University of London), London, UK, 2003. [Google Scholar]
- Murray, F.W. On the Computation of Saturation Vapor Pressure; American Meteorological Society: Boston, MA, USA, 1966. [Google Scholar]
- Greenshields, C. OpenFOAM User Guide, version 4.0; OpenFOAM Foundation Ltd.: London, UK, 2016. [Google Scholar]
- Issa, R.I. Solution of the implicitly discretised fluid flow equations by operator-splitting. J. Comput. Phys. 1986, 62, 40–65. [Google Scholar] [CrossRef]
- Rhie, C.; Chow, W. Numerical study of the turbulent flow past an airfoil with trailing edge separation. AIAA J. 1983, 21, 1525–1532. [Google Scholar] [CrossRef]
- Kärrholm, F.P. Rhie-Chow Interpolation in Openfoam; Department of Applied Mechanics, Chalmers University of Technology: Goteborg, Sweden, 2006. [Google Scholar]
- Stallo. 2017. Available online: https://www.sigma2.no/content/stallo (accessed on 14 August 2017).
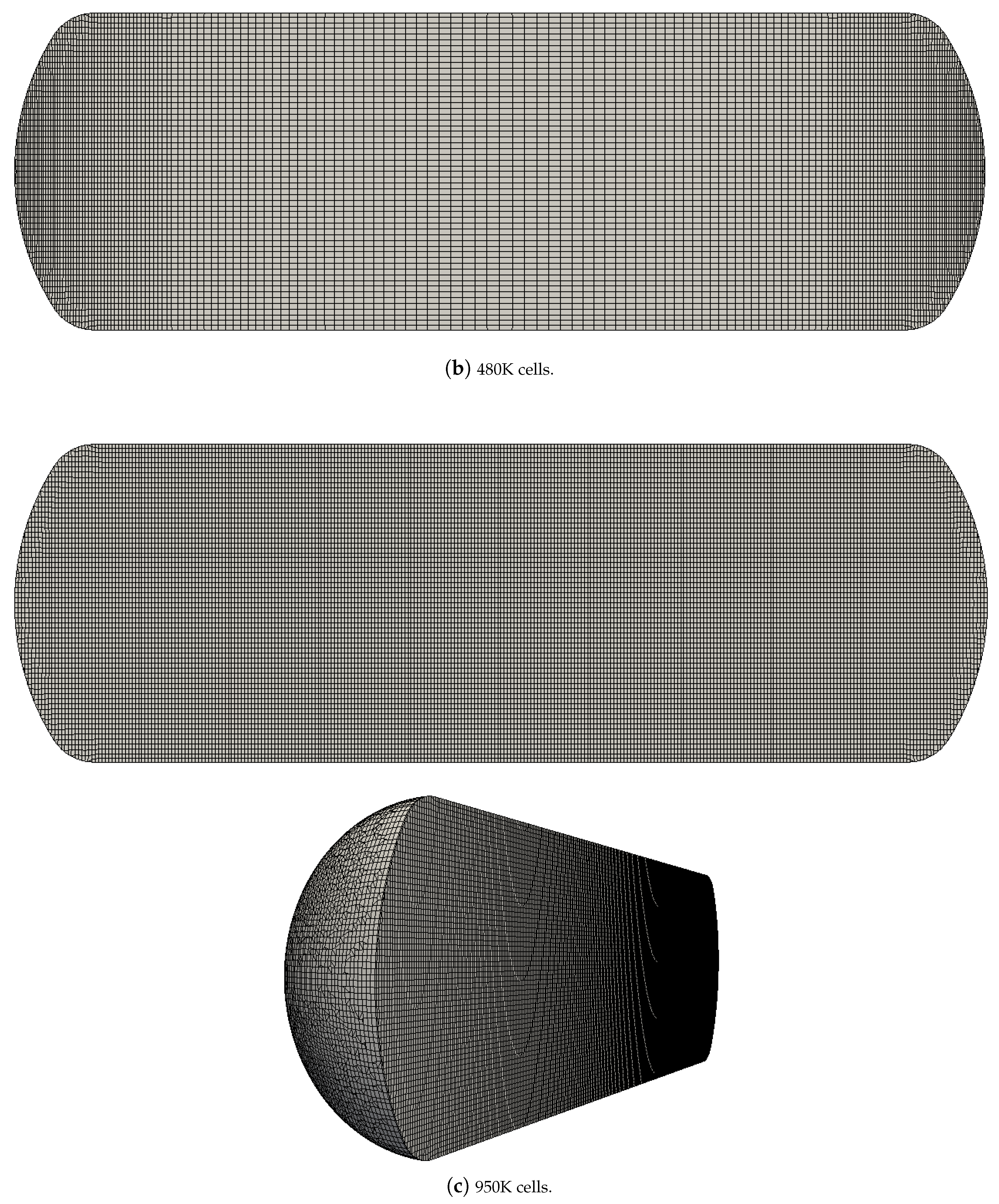
| Case No. | Description | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.50 | 0.190 | N/A | N/A | N/A | N/A | No motion |
| 1 | 0.50 | 0.190 | 0.29 | 857 | 0.427 | 40 | Small deformation |
| 2 | 0.50 | 0.190 | 0.40 | 1200 | 0.495 | 31 | Oscillating waves |
| 3 | 0.50 | 0.190 | 0.57 | 1714 | 0.840 | 30 | Jet formed |
| Phase | ||||
|---|---|---|---|---|
| Liquid | 997.56 | 4180 | 0.62 | |
| Gas | 1.18 | 1500 | 0.03 |
| % Liquid Volume | Volume [L] | N Drops | Surface Area () Drops | |
|---|---|---|---|---|
| 1.0 | 0.48 | 907 | 0.29 | 0.78 |
| 2.5 | 1.19 | 2268 | 0.71 | 1.95 |
| 5.0 | 2.38 | 4536 | 1.43 | 3.90 |
| 10.0 | 4.75 | 9072 | 2.85 | 7.81 |
| 15.0 | 7.13 | 13,608 | 4.28 | 11.71 |
| 20.0 | 9.50 | 18,144 | 5.70 | 15.62 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grotle, E.L.; Æsøy, V. Numerical Simulations of Sloshing and the Thermodynamic Response Due to Mixing. Energies 2017, 10, 1338. https://doi.org/10.3390/en10091338
Grotle EL, Æsøy V. Numerical Simulations of Sloshing and the Thermodynamic Response Due to Mixing. Energies. 2017; 10(9):1338. https://doi.org/10.3390/en10091338
Chicago/Turabian StyleGrotle, Erlend Liavåg, and Vilmar Æsøy. 2017. "Numerical Simulations of Sloshing and the Thermodynamic Response Due to Mixing" Energies 10, no. 9: 1338. https://doi.org/10.3390/en10091338
APA StyleGrotle, E. L., & Æsøy, V. (2017). Numerical Simulations of Sloshing and the Thermodynamic Response Due to Mixing. Energies, 10(9), 1338. https://doi.org/10.3390/en10091338