A Novel Method to Directly Analyze Dissolved Acetic Acid in Transformer Oil without Extraction Using Raman Spectroscopy
Abstract
:1. Introduction
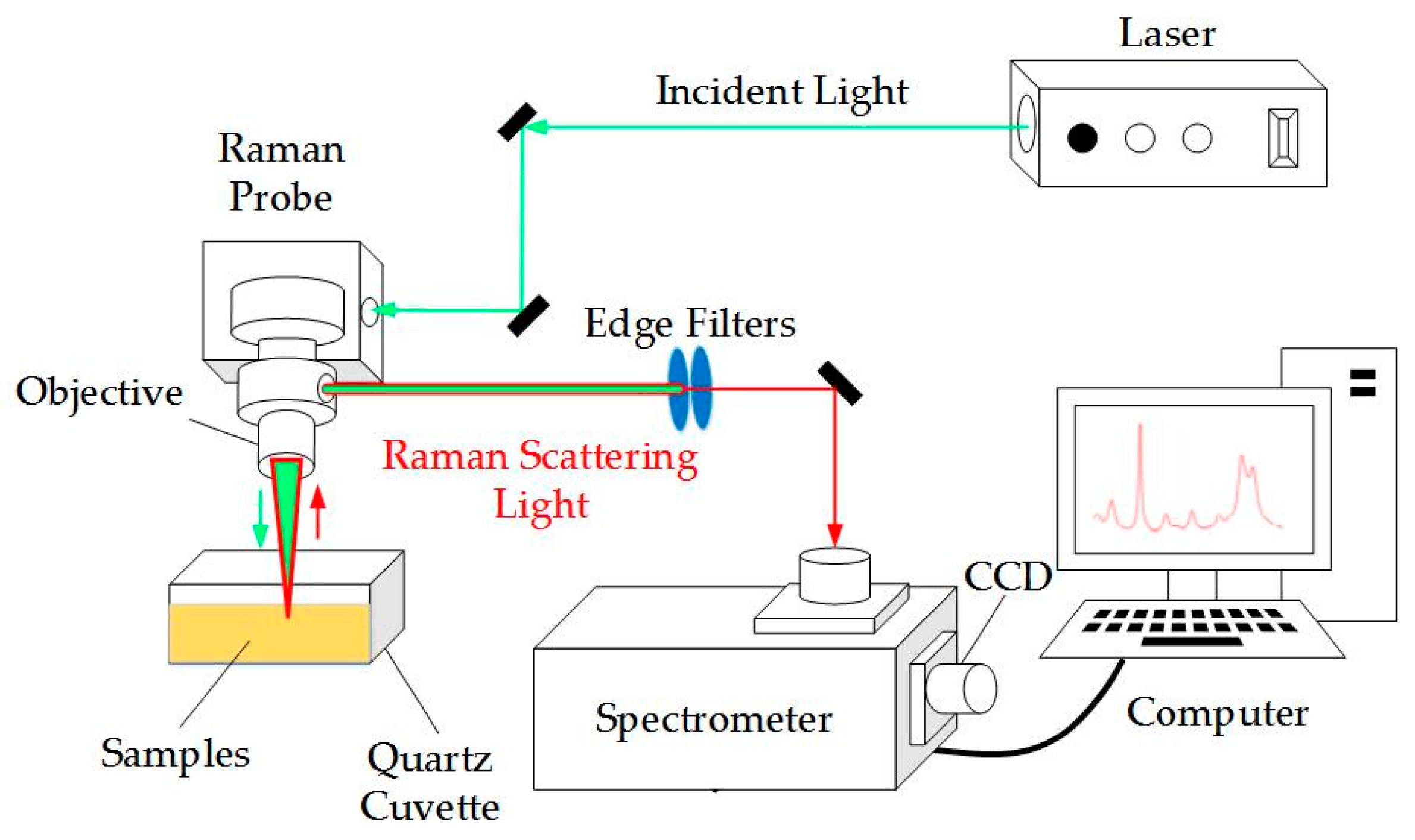
2. Experimental Setup
3. Analysis of Dissolved Acetic Acid in Transformer Oil
3.1. Sample Preparation
3.2. Raman Shift Spectral Lines of Acetic Acid
3.3. Characteristic Raman Shift Spectral Line of Oil-Dissolved Acetic Acid
- (1)
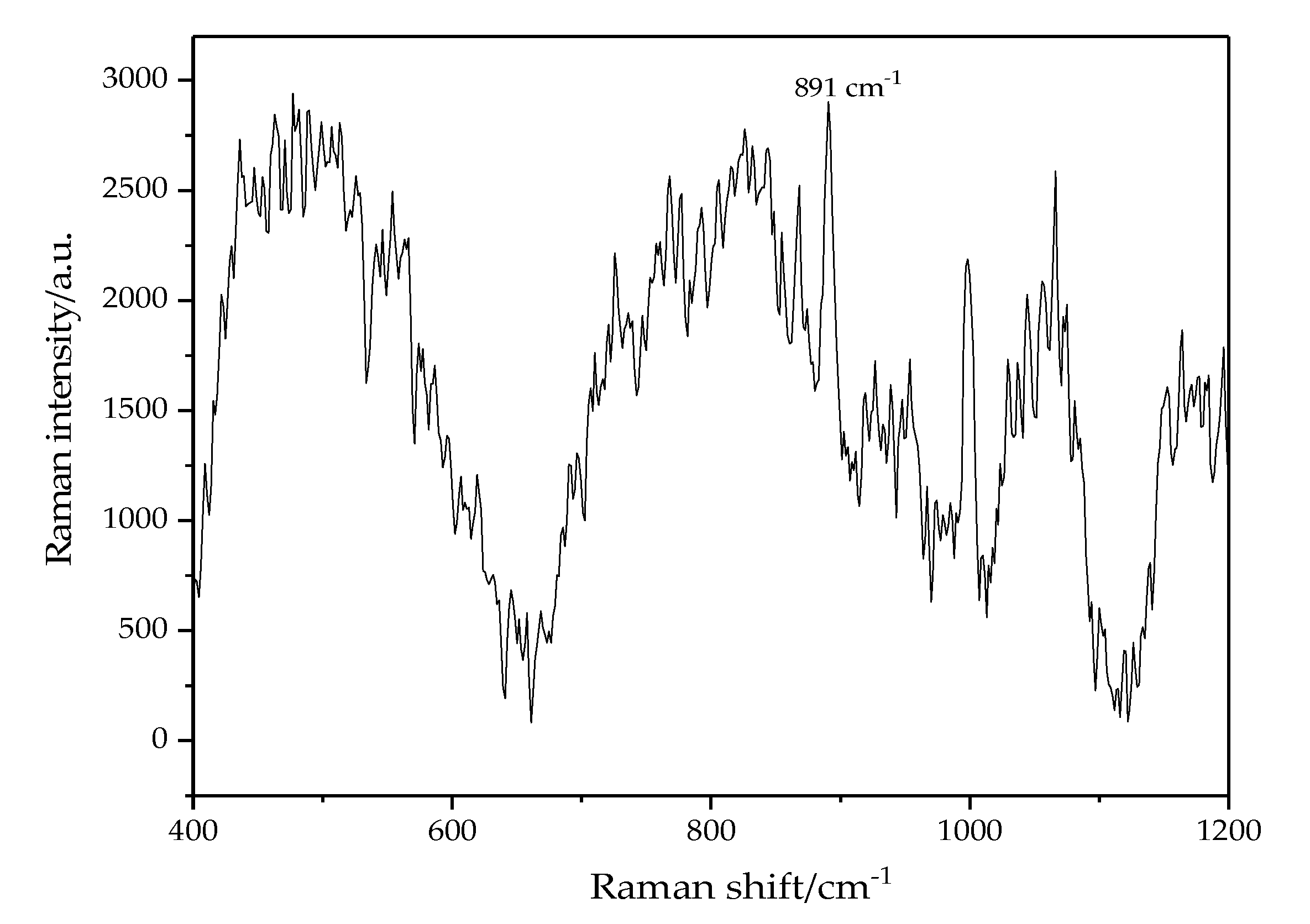
- The Raman lines of pure oil have strong interference and will cover many Raman lines of acetic acid. Only in a range between 400 cm−1 and 1200 cm−1, the pure oil has no Raman lines or with low intense lines, which has little effect on the measurement of oil-dissolved acetic acid. Therefore, this range is identified as the detection interval.
- (2)
- In this detection interval, obvious Raman shift lines of oil-dissolved acetic acid are at 443 cm−1, 599 cm−1, 618 cm−1 and 891 cm−1, which correspond to lines of pure acetic acid at 446 cm−1, 603 cm−1, 621 cm−1, 893 cm−1. The difference of 2~4 cm−1 is called blue-shift, mainly attributing to influence of transformer oil on Raman vibration mode of acetic acid.
- (3)
- The most intense Raman shift line of acetic acid solution is 891 cm−1, corresponding to H–C–H symmetrical swing and O–H swing modes. Since the concentration of pure oil is constant, its effect on intensity of Raman line at 891 cm−1 and 932 cm−1 can be also regarded as a constant value. The low interference of oil on oil-dissolved acetic acid detection at 891 cm−1 can be eliminated when cutting down the constant value of Raman intensity of pure oil. In this experiment, for better detection limit of acetic acid, 891 cm−1 is taken as the characteristic Raman shift spectral line of oil-dissolved acetic acid and used for its qualitative and quantitative analysis. Besides, the Raman line of pure oil at 932 cm−1 is taken as an internal standard for improved detection accuracy.
3.4. Platform Parameters Optimization
3.4.1. Laser Power and Grating
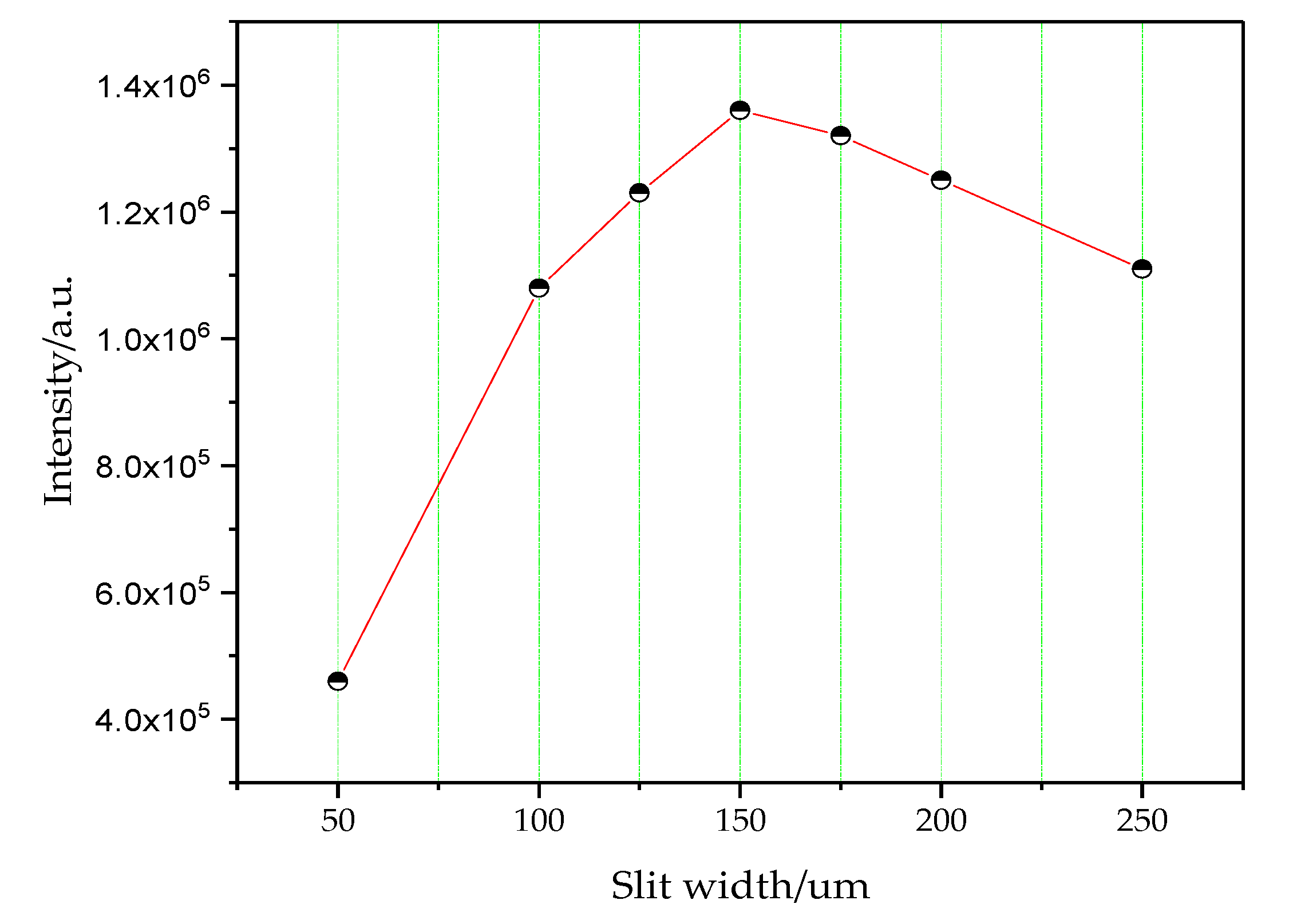
3.4.2. Selection of Slit Width
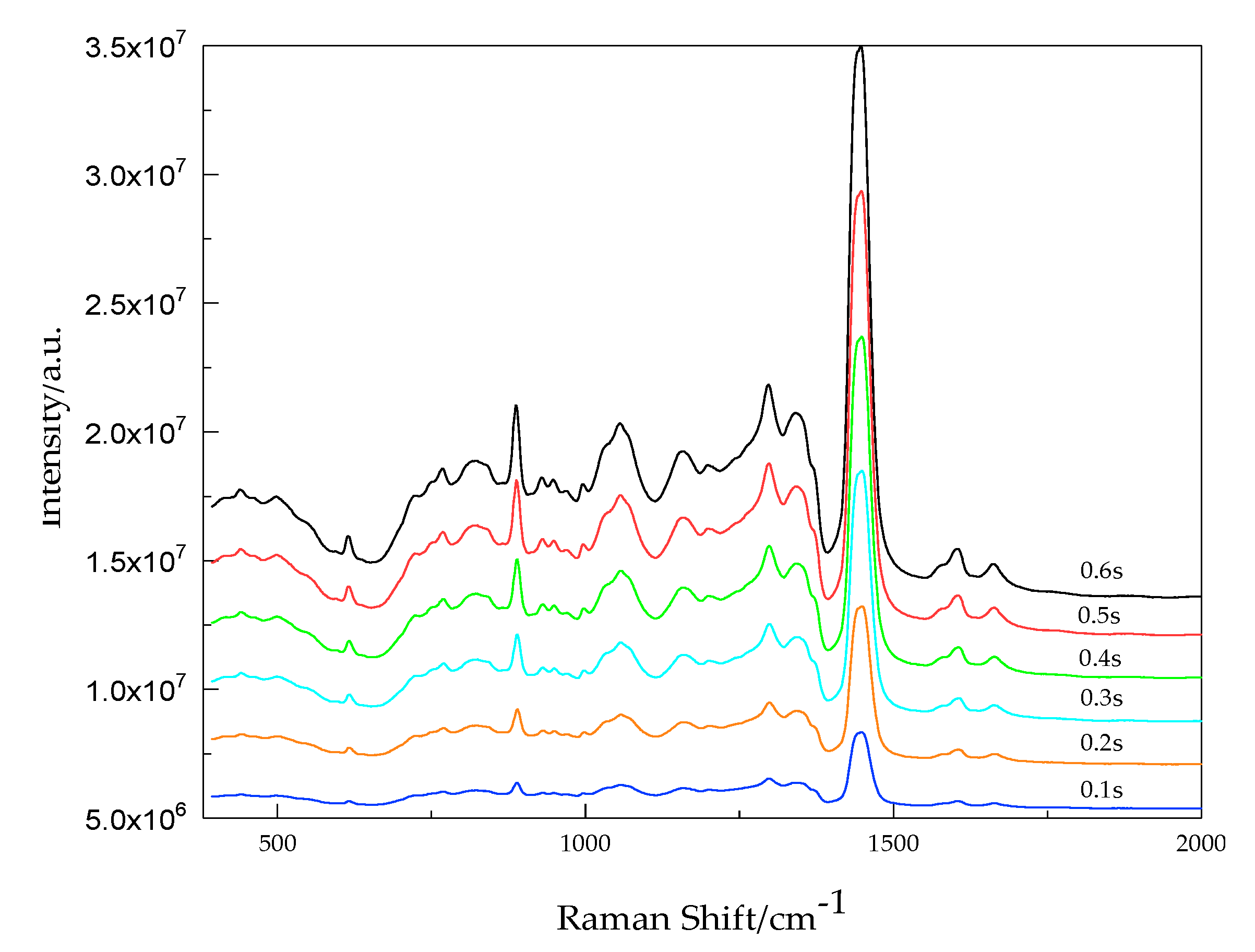
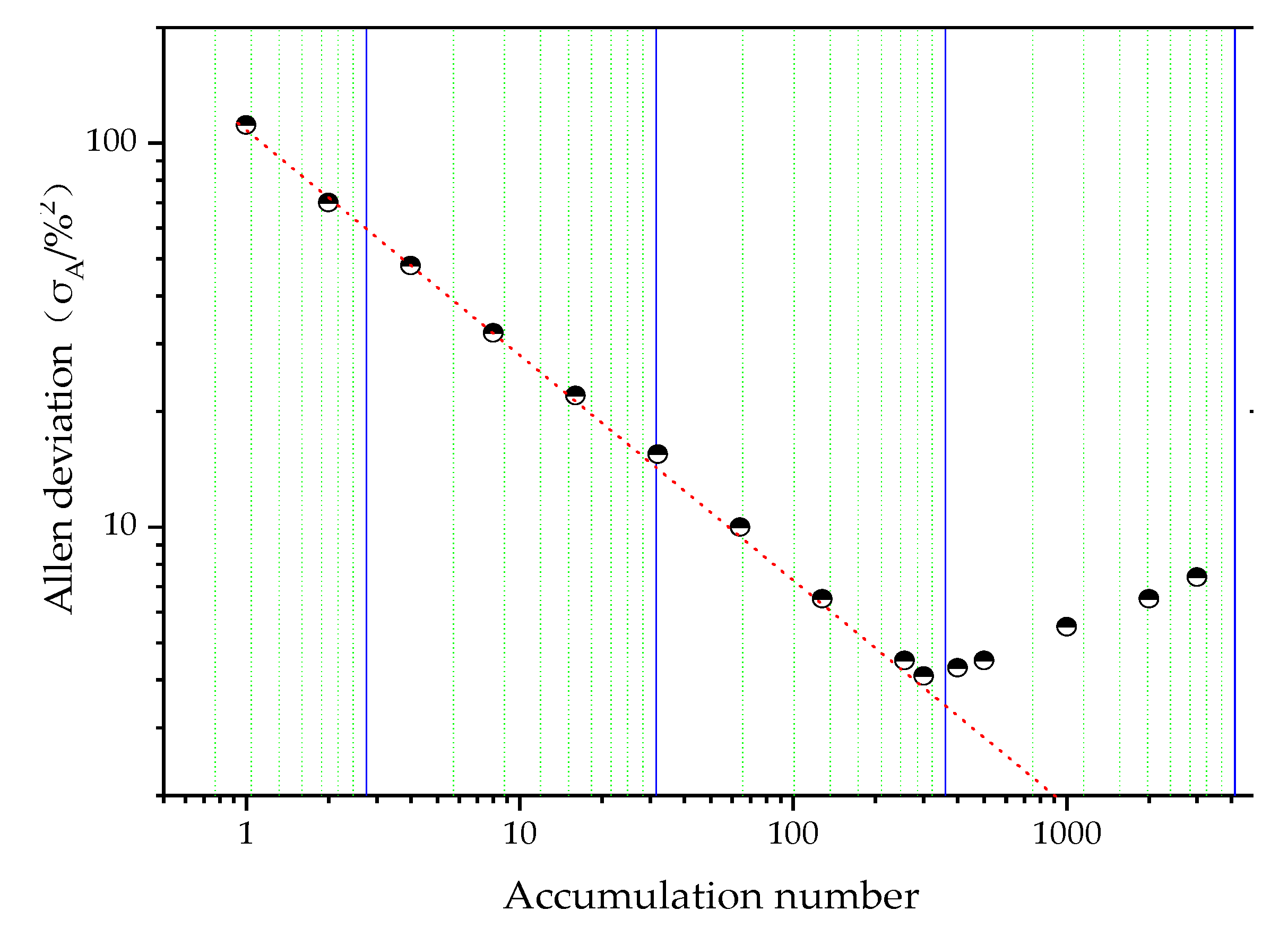
3.4.3. Selection of Exposure time and Accumulation Number
4. Results
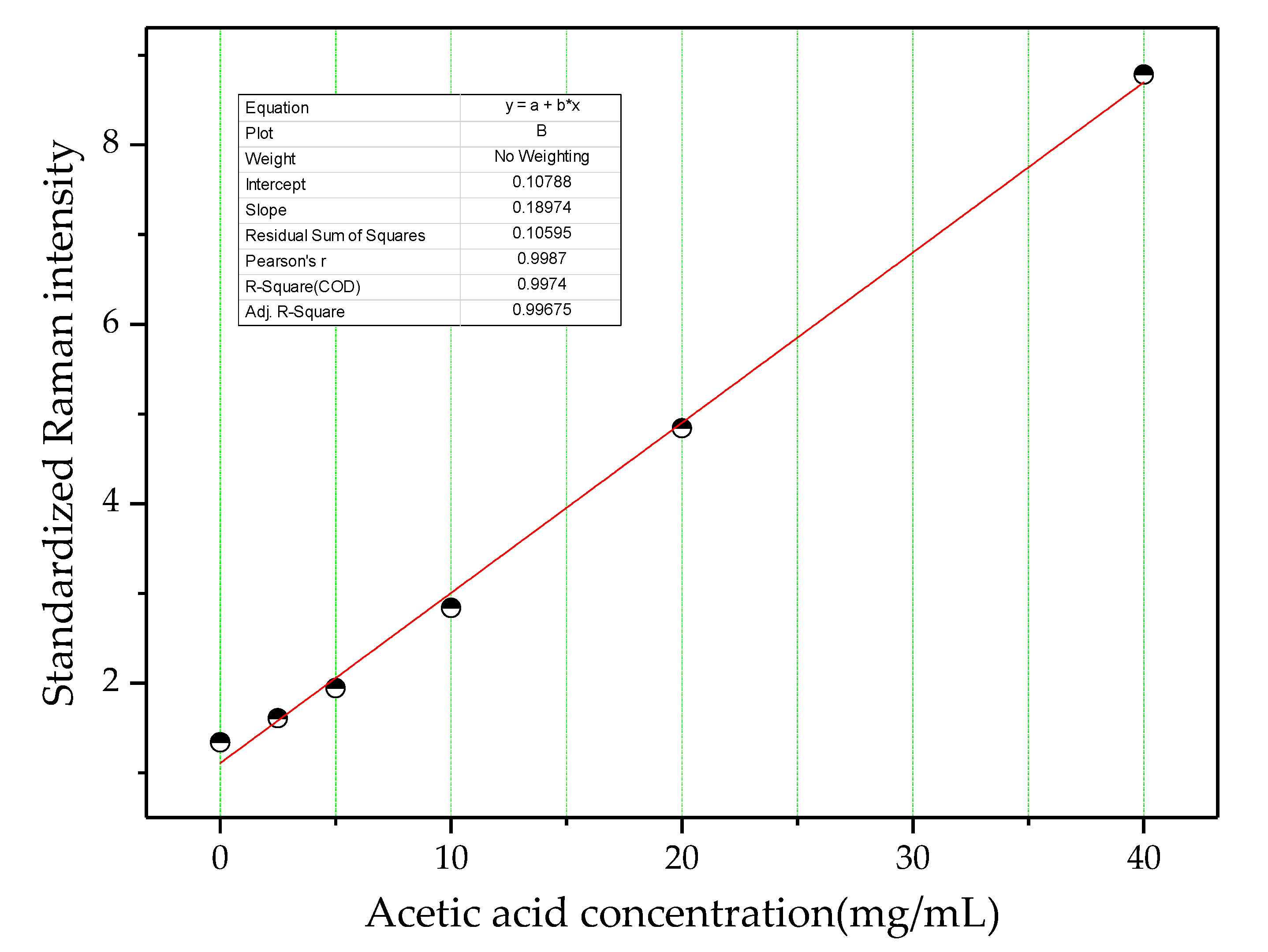
4.1. Quantitative Analysis
4.2. Detection Limit
4.3. Analysis of Oil-Dissolved Acetic Acid from an Operating Transformer
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Conseil International des Grands Réseaux Électriques (CIGRE). Aging of Cellulose in Mineral-Oil Insulated Transformers; Task Force D1.01.10; CIGRE: Paris, France, 2007. [Google Scholar]
- International Electrotechnical Commission (IEC). Guide to the Interpretation of Dissolved and Free Gases Analysis; IEC: Geneva, Switzerland, 2007. [Google Scholar]
- Wan, F.; Chen, W.G. Using a Sensitive Optical System to Analyze Gases Dissolved in Samples Extracted from Transformer Oil. IEEE Electr. Insul. Mag. 2014, 30, 15–22. [Google Scholar] [CrossRef]
- Emsley, A.M.; Xiao, X.; Heywood, R.J.; Ali, M. Degradation of cellulosic insulation in power transformers. Part 2: Formation of furan products in insulating oil. IEE Proc. Sci. Meas. Technol. 2000, 147, 110–114. [Google Scholar] [CrossRef]
- Okabe, S.; Ueta, G.; Tsuboi, T. Investigation of Aging Degradation Status of Insulating Elements in Oil-immersed Transformer and its Diagnostic Method Based on Field Measurement Data. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 346–355. [Google Scholar] [CrossRef]
- Ekonomou, L.; Skafidas, P.D.; Oikonomou, D.S. Transformer oil’s service life identification using neural networks. In Proceedings of the 8th WSEAS International Conference on Electric Power Systems, High Voltages, Electric Machines (POWER’ 08), Venice, Italy, 21–23 November 2008; pp. 222–226. [Google Scholar]
- Dervos, C.T.; Vassiliou, P.; Skafidas, P.; Paraskevas, C. Service life estimation of transformer oil. In Proceedings of the International Conference on Protection and Restoration of the Environment VI, Skiathos, Greece, 1–5 July 2002; pp. 1239–1246. [Google Scholar]
- N’cho, J.S.; Fofana, I.; Hadjadj, Y.; Beroual, A. Review of physicochemical based diagnostic techniques for assessing insulation condition in aged transformers. Energies 2016, 9, 367. [Google Scholar] [CrossRef]
- Lipshteǐn, R.A.; Shakhnovich, M.I. Transformer Oil, 2nd ed.; Israel Program for Scientific Translations (IPST): Jerusalem, Israel, 1970. [Google Scholar]
- Hill, G.C.; Holman, J.S. Chemistry in Context, 5th ed.; Nelson: Walton-on Thames, UK, 2000. [Google Scholar]
- Lundgaard, L.E.; Hansen, W.; Linhjell, D. Aging of oil-impregnated paper in power transformers. IEEE Trans. Power Syst. Deliv. 2004, 19, 230–238. [Google Scholar] [CrossRef]
- Mander, L.; Liu, H.W. Comprehensive Natural Products II: Chemistry and Biology; Elsevier Science: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Ingebrigtsen, S.; Dahlund, M.; Hansen, W.; Linhjell, D.; Lundgaard, L.E. Solubility of carboxylic acids in paper (Kraft)-oil insulation systems. In Proceedings of the 2004 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Boulder, CO, USA, 20–20 October 2004; pp. 253–257. [Google Scholar]
- Lelekakis, N.; Wijaya, J.; Martin, D. The effect of acid accumulation in power-transformer oil on the aging rate of paper insulation. IEEE Electr. Insul. Mag. 2014, 30, 19–26. [Google Scholar] [CrossRef]
- Liao, R.J.; Sang, F.M.; Liu, G.; Yang, L.J. Study on neutral acid and water dissolved in oil for different types of oil-paper insulation compositions of transformers in accelerated ageing tests. Proc. CSEE 2010, 30, 125–131. [Google Scholar]
- Du, L.L.; Chen, W.G.; Gu, Z.L. Analysis of acetic acid dissolved in transformer oil based on laser Raman spectroscopy. In Proceedings of the 2016 IEEE International Conference on High Voltage Engineering and Application (ICHVE), Chengdu, China, 19–22 September 2016; pp. 1–4. [Google Scholar]
- Lundgaard, L.E.; Hansen, W.; Ingebrigtsen, S. Ageing of mineral oil impregnated cellulose by acid catalysis. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 540–546. [Google Scholar] [CrossRef]
- International Electrotechnical Commission (IEC). Insulating Liquids-Determination of Acidity; IEC: Geneva, Switzerland, 2014. [Google Scholar]
- Tur’yan, Y.I.; Strochkova, E.; Berezin, O.Y.; Kuselman, I.; Shenhar, A. pH-metric determination of acid numbers in petroleum oils without titration. Talanta 2000, 47, 53–58. [Google Scholar] [CrossRef]
- Lundgaard, L.E.; Hansen, W.; Ingebrigtsen, S.; Linhjell, D.; Dahlund, M. Aging of Kraft paper by acid catalyzed hydrolysis. In Proceedings of the IEEE International Conference on Dielectric Liquids, Coimbra, Portugal, 26 June–1 July 2005; pp. 381–384. [Google Scholar]
- Kiefer, J. Recent advances in the characterization of gaseous and liquid fuels by vibrational spectroscopy. Energies 2015, 8, 3165–3197. [Google Scholar] [CrossRef]
- Irawan, R.; Scelsi, G.; Woolsey, G. Continuous monitoring of SF6 degradation in high voltage switchgear using Raman scattering. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 815–820. [Google Scholar] [CrossRef]
- Li, X.Y.; Xia, Y.X.; Zhan, L.; Huang, J.M. Near-confocal cavity-enhanced Raman spectroscopy for multitrace-gas detection. Opt. Lett. 2008, 33, 2143–2145. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Xia, Y.X.; Zhan, L.; Leng, J.H. A Raman system for gas-species analysis in power transformer. Appl. Phys. B 2008, 93, 665–669. [Google Scholar] [CrossRef]
- Somekawa, T.; Kasaoka, M.; Kawachi, F.; Nagano, Y.; Fujita, M.; Izawa, Y. Analysis of dissolved C2H2 in transformer oils using laser Raman spectroscopy. Opt. Lett. 2013, 38, 1086–1088. [Google Scholar] [CrossRef] [PubMed]
- Somekawa, T.; Fujita, M.; Izawa, Y.; Kasaoka, M.; Nagano, Y. Furfural analysis in transformer oils using laser Raman spectroscopy. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 229–231. [Google Scholar] [CrossRef]
- Wan, F.; Chen, W.G.; Gu, Z.L.; Zou, J.Z. The research for Raman analysis of dissolved gases in transformer oil and optimization of quantitative detection. Trans. China Electr. Soc. 2016, 31, 236–243. [Google Scholar]
- Chen, W.G.; Gu, Z.L.; Zou, J.Z. Analysis of furfural dissolved in transformer oil based on confocal laser Raman spectroscopy. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 915–921. [Google Scholar] [CrossRef]
- Atkins, P. Physical Chemistry; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Dirschmid, H.; Fischer, F.D. Generalized Gaussian error functions and their applications. Acta Mech. 2015, 226, 2887–2897. [Google Scholar] [CrossRef]
- Kiefer, J.; Seeger, T.; Steuer, S.; Schorsch, S.; Leipertz, A. Design and characterization of a Raman-scattering-based sensor system for temporally resolved gas analysis and its application in a gas turbine power plant. Meas. Sci. Technol. 2008, 19, 085408. [Google Scholar] [CrossRef]
- El-Sheimy, N.; Hou, H.; Niu, X. Analysis and modeling of inertial sensors using Allan variance. IEEE Trans. Instrum. Meas. 2008, 57, 140–149. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. Raman intensities of geochemically relevant gas mixtures for a laser Raman microprobe. Anal. Chem. 1987, 59, 2165–2170. [Google Scholar] [CrossRef]
- Li, D.; Zhu, H.; Chang, Z. Quantitative method study on the pertechnetate in aqueous solution by Raman spectroscopy. Anal. Methods 2016, 8, 246–258. [Google Scholar]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef] [PubMed]
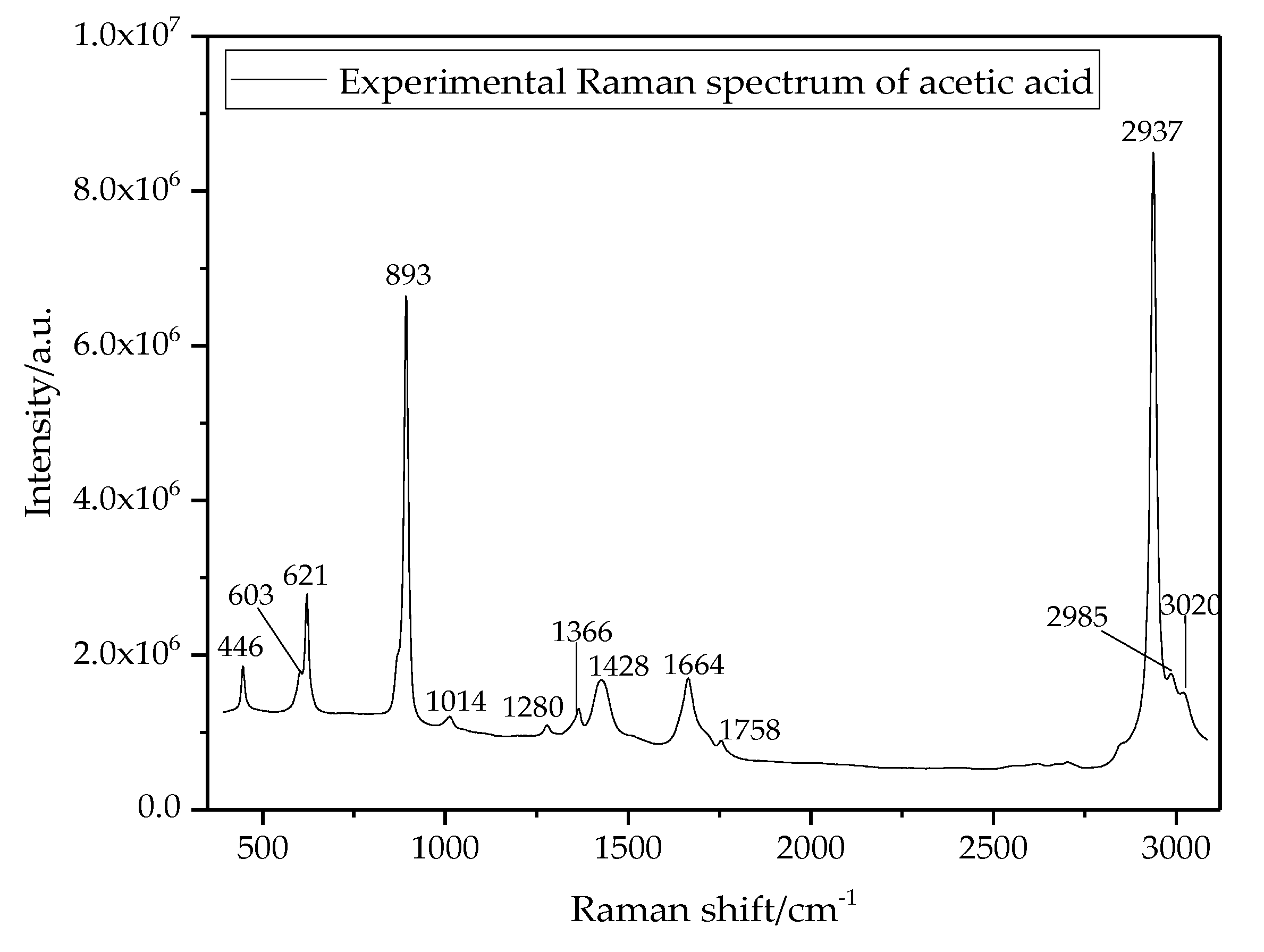
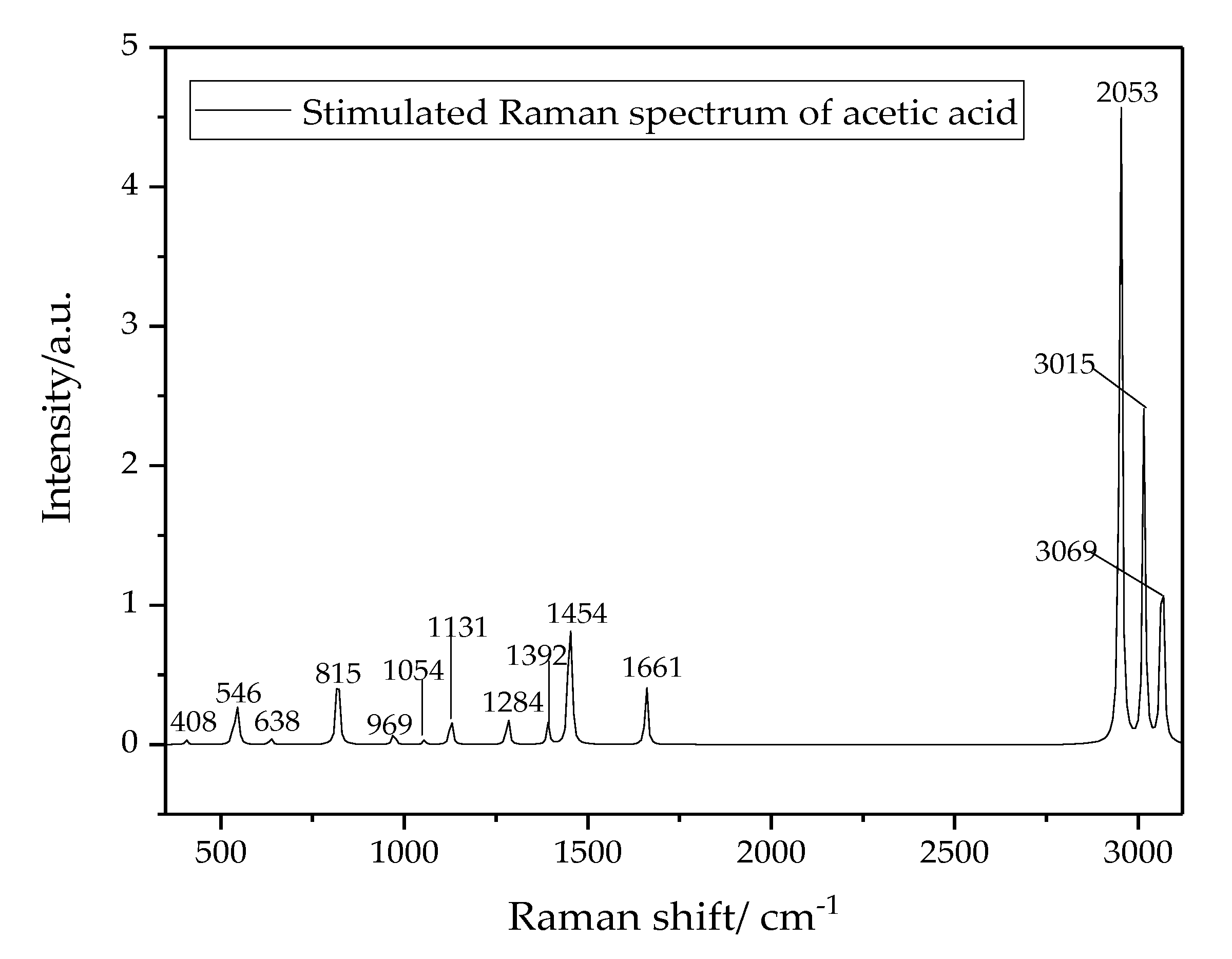
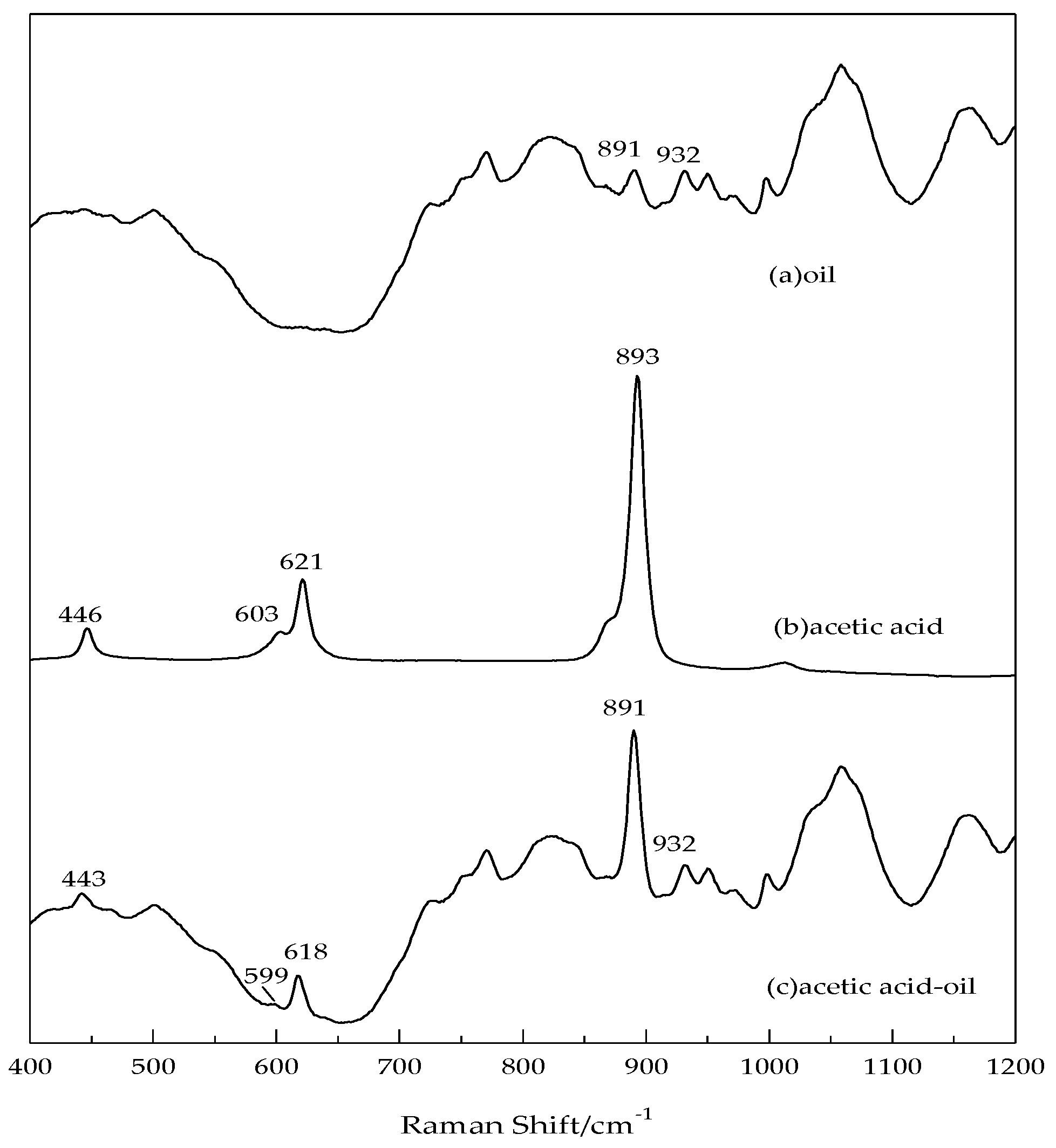

| No. | Stimulated Value (cm−1) | Pure Acetic Acid (cm−1) | Oil-Dissolved Acetic Acid (cm−1) | Vibration Modes |
|---|---|---|---|---|
| 1 | 408 | 446 | 443 | H–C–H symmetrical swing and O–H swing |
| 2 | 546 | - | - | O–H swing and C=O swing |
| 3 | 638 | 603/621 | 599/618 | O=C–O bending and C–C stretching |
| 4 | 815 | 893 | 891 | H–C–H symmetrical swing and O–H swing |
| 5 | 969 | 1014 | 932 | O–C–C asymmetric stretching and H–C–H symmetrical swing |
| 6 | 1054 | - | - | C–H asymmetric swing |
| 7 | 1131 | - | - | O–H swing and C–H swing |
| 8 | 1284 | 1280 | 1289 | O–H swing and C–C swing |
| 9 | 1392 | 1366 | 1375 | H–C–H bending |
| 10 | 1454 | 1428 | 1422 | H–C–H asymmetric swing |
| 11 | 1661 | 1664/1758 | 1654/1741 | C=O stretching vibration |
| 12 | 2953 | 2937 | 2935 | CH3 stretching vibration |
| 13 | 3015 | 2985 | 2990 | C–H asymmetric stretching |
| 14 | 3069 | 3020 | 3031 | H–C–H asymmetric stretching |
| Main Characteristics of Power Transformers from Which the Oil Samples Are Obtained | Raman Spectroscopy (mg/mL) | IPT (mg/mL) | Detection Error (%) | |||
|---|---|---|---|---|---|---|
| Substation | Model | Operation Time | Fault or Not | |||
| Sigongli | SFSZ-240000/220 | 20 October 2004 | N | 18.26 | 20.11 | 9.20 |
| Dazhulin | SSZ11-180000/220 | 27 July 2007 | N | 11.15 | 10.04 | 11.06 |
| Yuping | SFSZ9-120000/220 | 30 June 2008 | N | 4.31 | 5.02 | 14.15 |
| Laisu | SFPSZ10-M-120000/220 | 5 January 2009 | N | 9.73 | 10.28 | 5.35 |
| Nanchuan | SFPSZ10-180000/220 | 10 May 2011 | N | 3.22 | 3.43 | 6.12 |
| Longjing | SFPSZ10-180000/220 | 2 April 2014 | N | 1.24 | 1.19 | 4.20 |
| Youting | ODFS-250000/500 | 15 April 2015 | N | <0.68 | 0.15 | / |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, F.; Du, L.; Chen, W.; Wang, P.; Wang, J.; Shi, H. A Novel Method to Directly Analyze Dissolved Acetic Acid in Transformer Oil without Extraction Using Raman Spectroscopy. Energies 2017, 10, 967. https://doi.org/10.3390/en10070967
Wan F, Du L, Chen W, Wang P, Wang J, Shi H. A Novel Method to Directly Analyze Dissolved Acetic Acid in Transformer Oil without Extraction Using Raman Spectroscopy. Energies. 2017; 10(7):967. https://doi.org/10.3390/en10070967
Chicago/Turabian StyleWan, Fu, Lingling Du, Weigen Chen, Pinyi Wang, Jianxin Wang, and Haiyang Shi. 2017. "A Novel Method to Directly Analyze Dissolved Acetic Acid in Transformer Oil without Extraction Using Raman Spectroscopy" Energies 10, no. 7: 967. https://doi.org/10.3390/en10070967
APA StyleWan, F., Du, L., Chen, W., Wang, P., Wang, J., & Shi, H. (2017). A Novel Method to Directly Analyze Dissolved Acetic Acid in Transformer Oil without Extraction Using Raman Spectroscopy. Energies, 10(7), 967. https://doi.org/10.3390/en10070967





