Numerical Modeling of Oxygen Carrier Performances (NiO/NiAl2O4) for Chemical-Looping Combustion
Abstract
:1. Introduction
2. Experimental Part
2.1. Material
2.2. Experimental Set-UP
2.3. Experimental Design
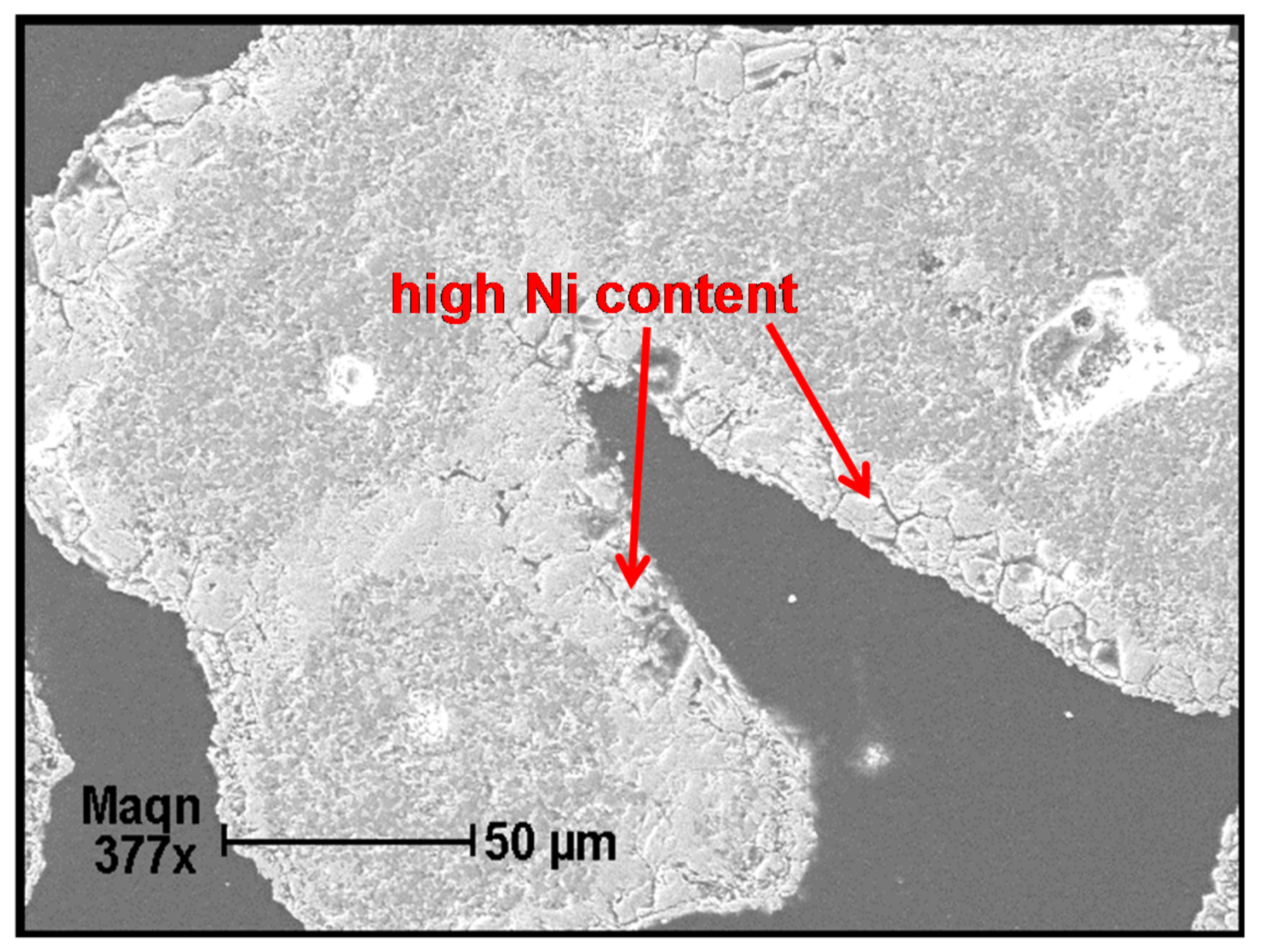
2.4. Sample Characterizations
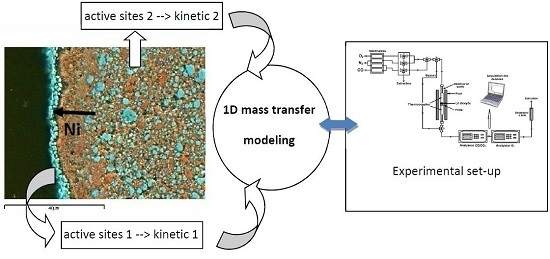
3. Numerical Part
3.1. Kinetic Models
3.2. 1D-Unsteady Simulation of the Reaction in the Fixed Bed Reactor
4. Results and Discussion
4.1. Experiments in Fixed Bed Reactor
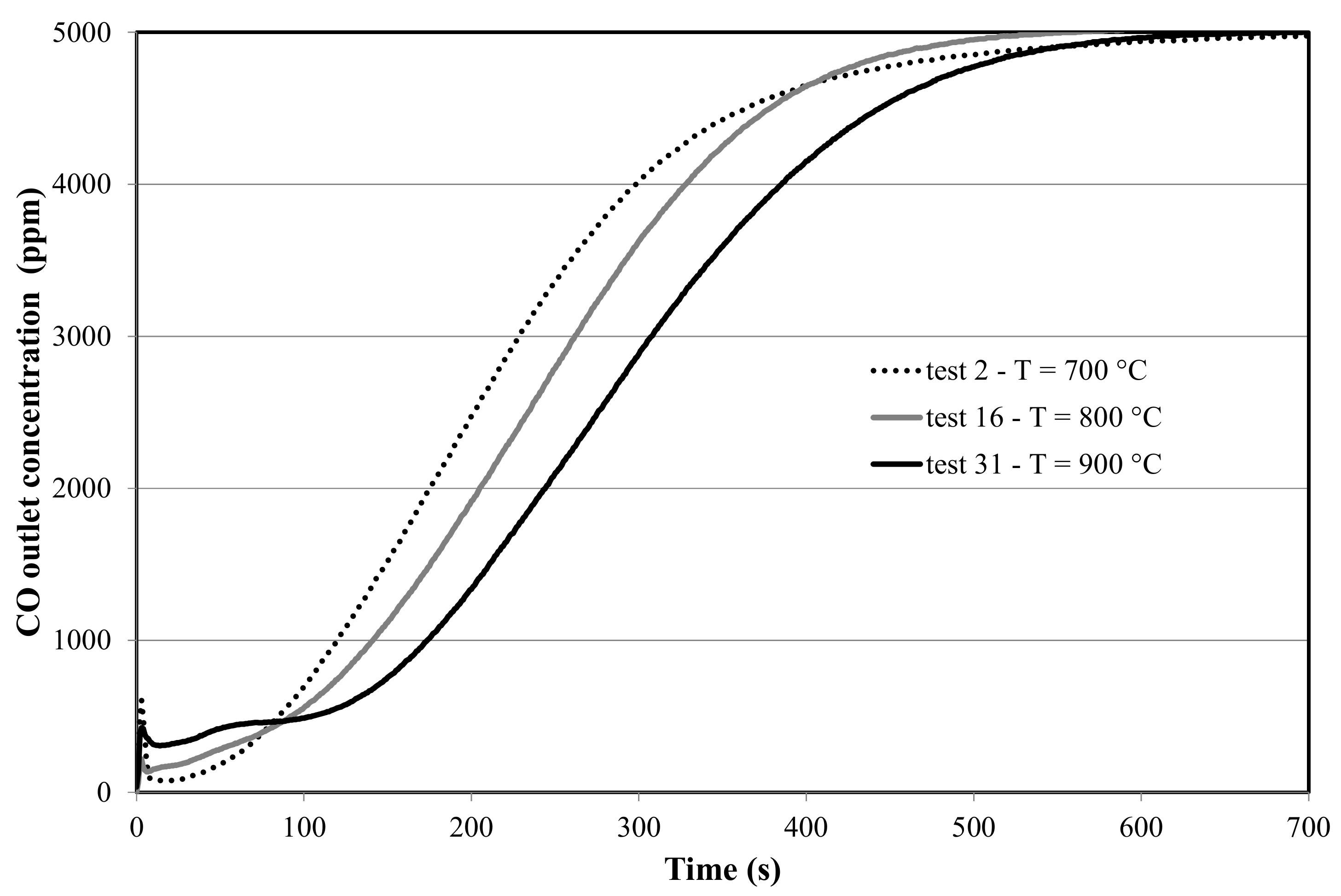
4.1.1. Effect of Temperature
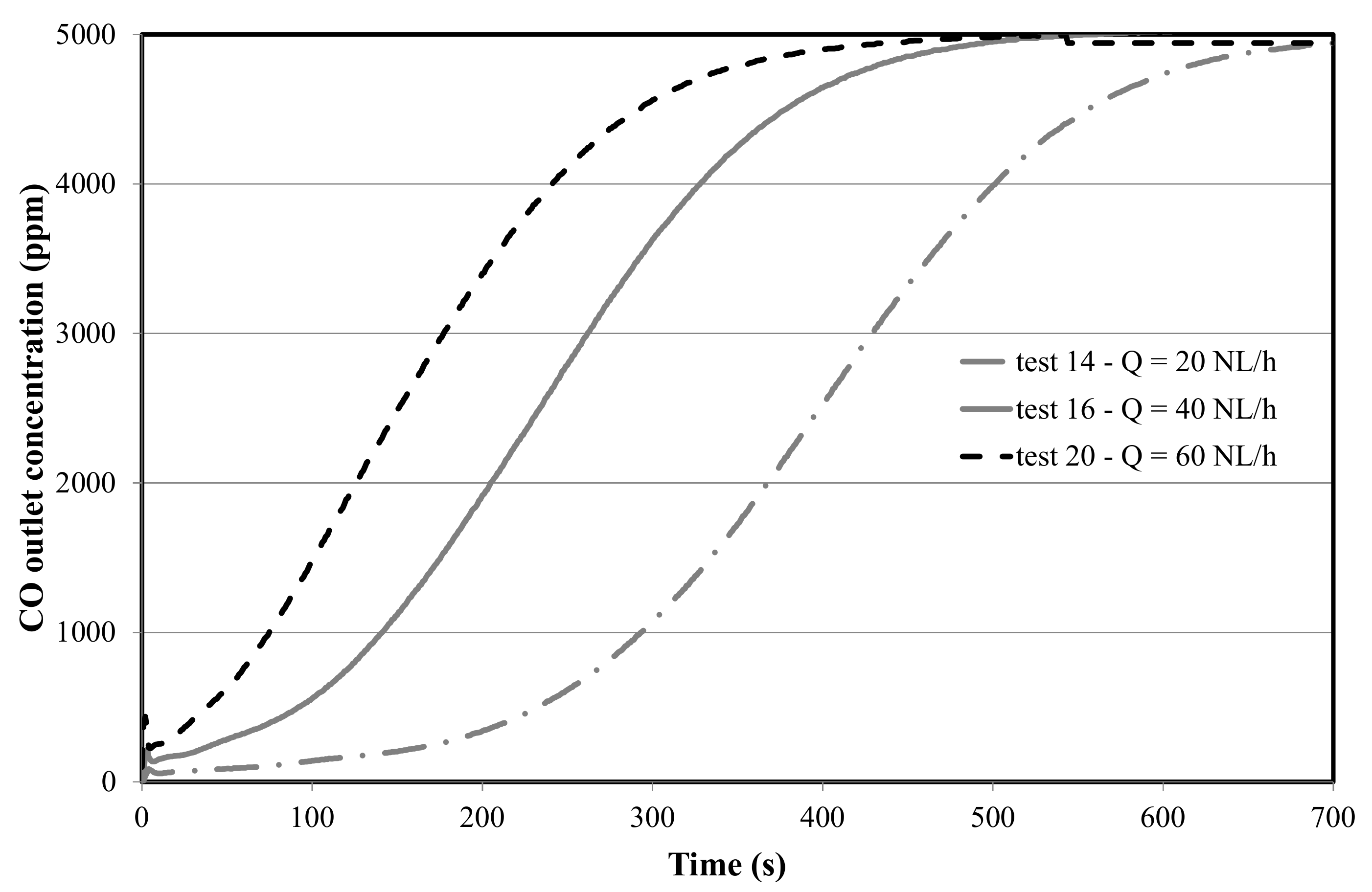
4.1.2. Effect of Flow Rate
4.1.3. Effect of CO/NiO Ratio
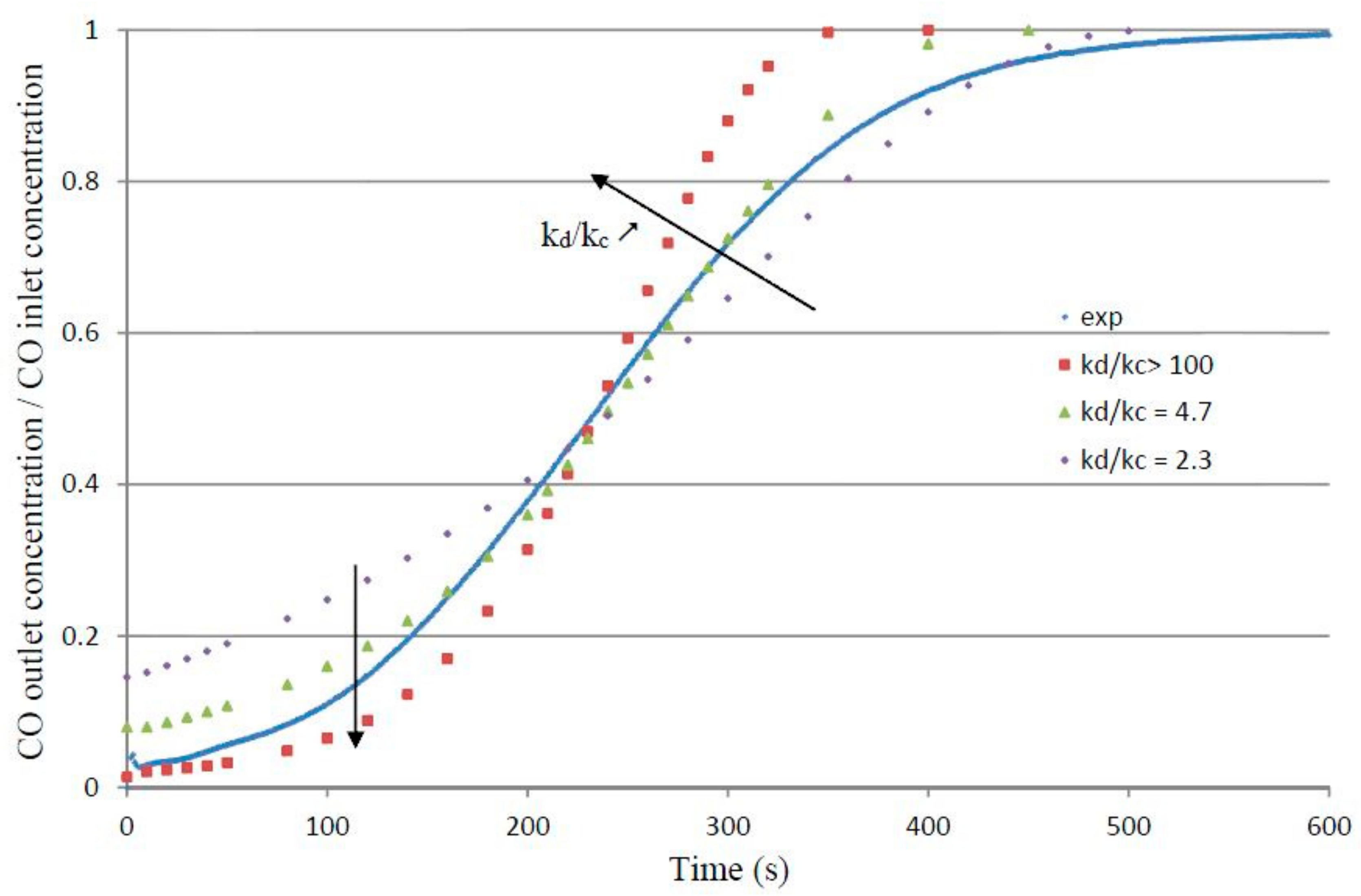
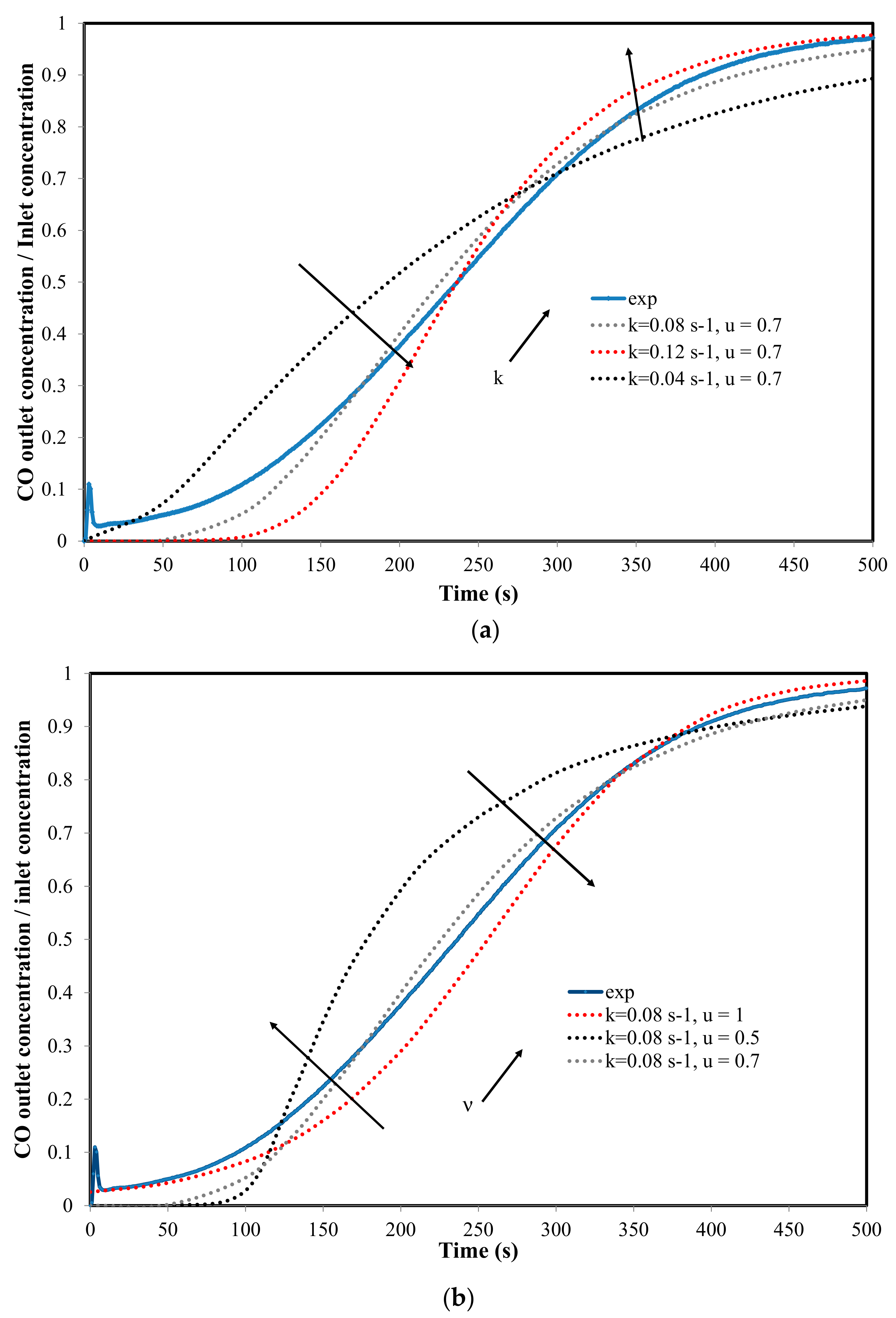
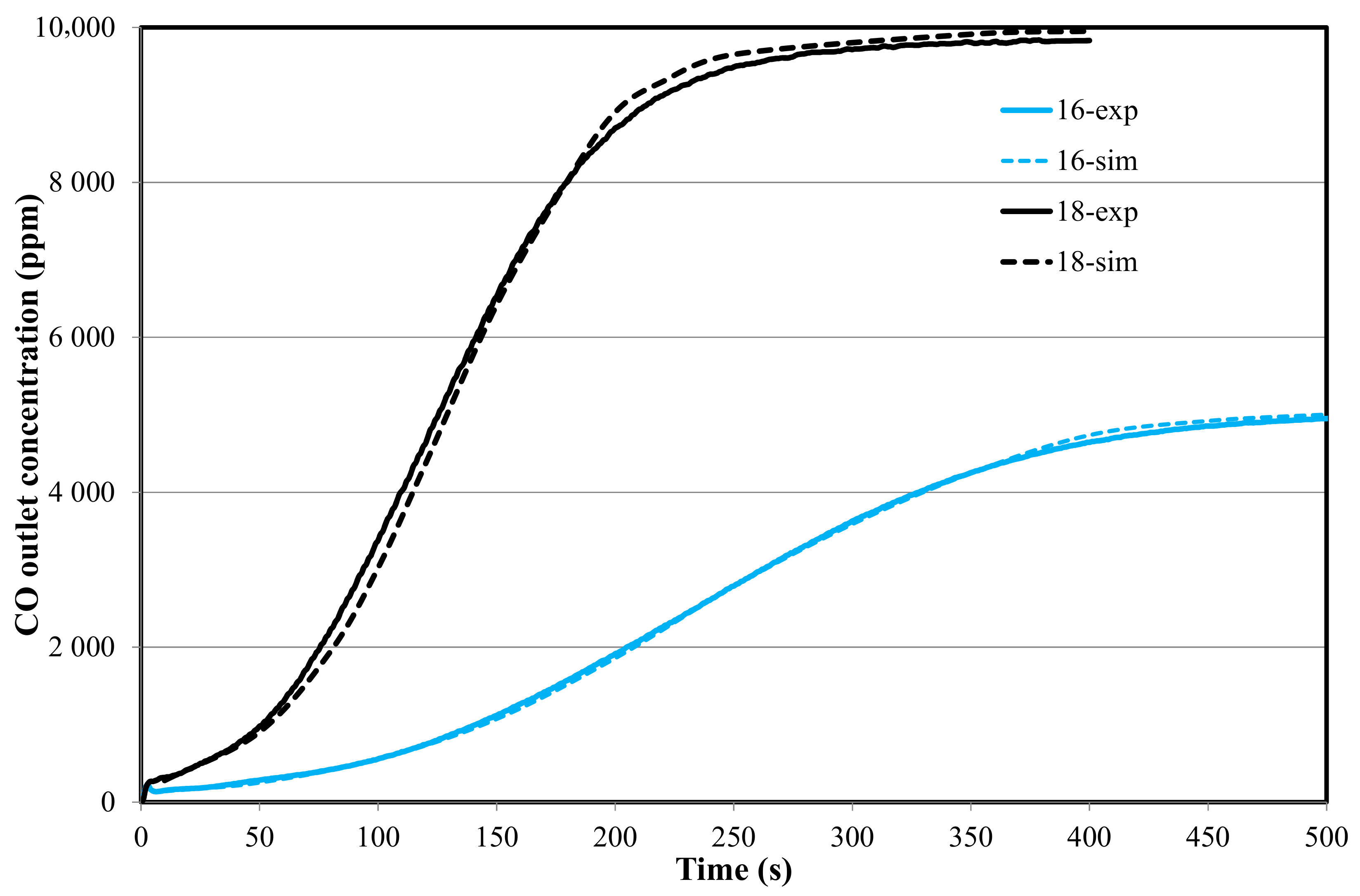
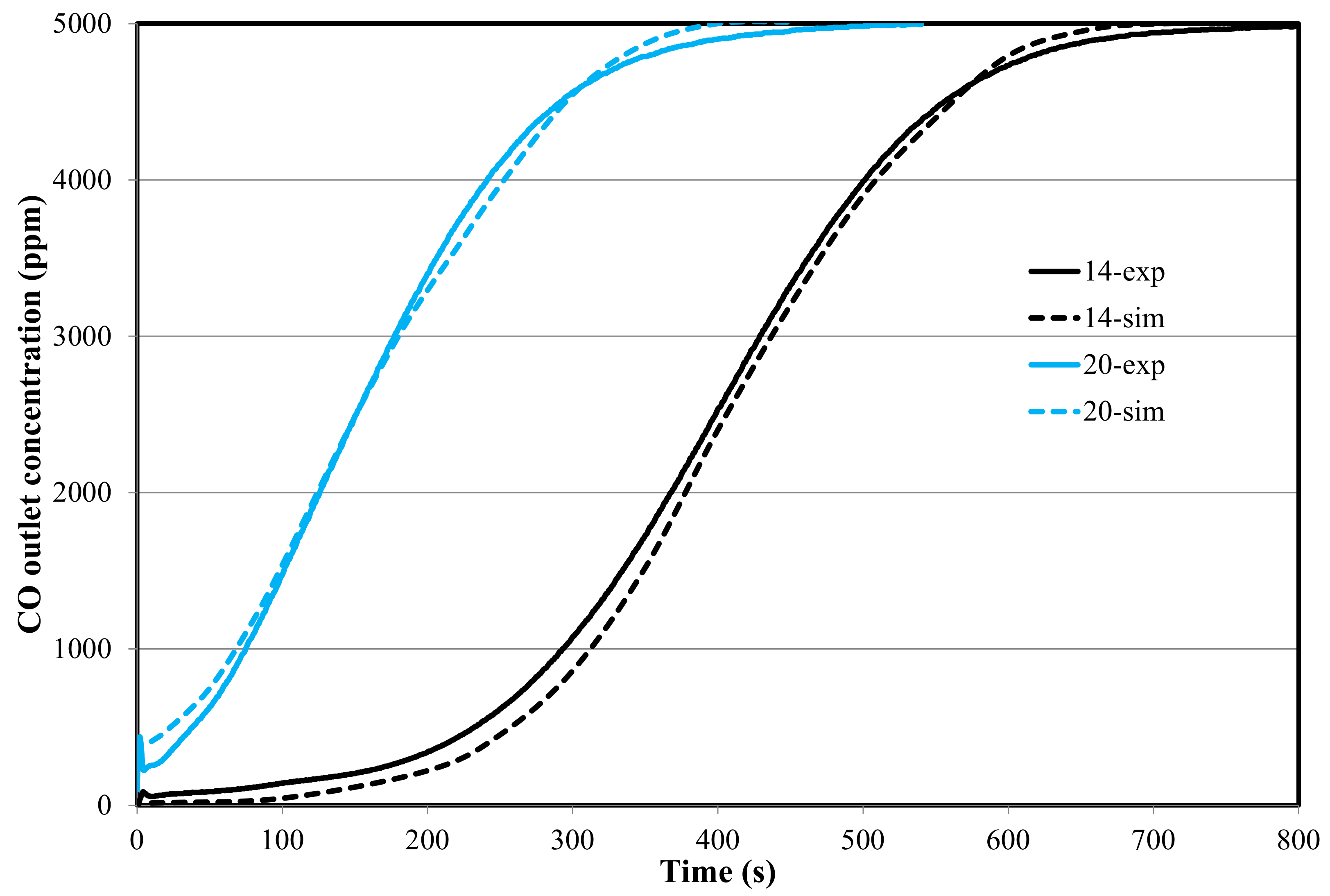
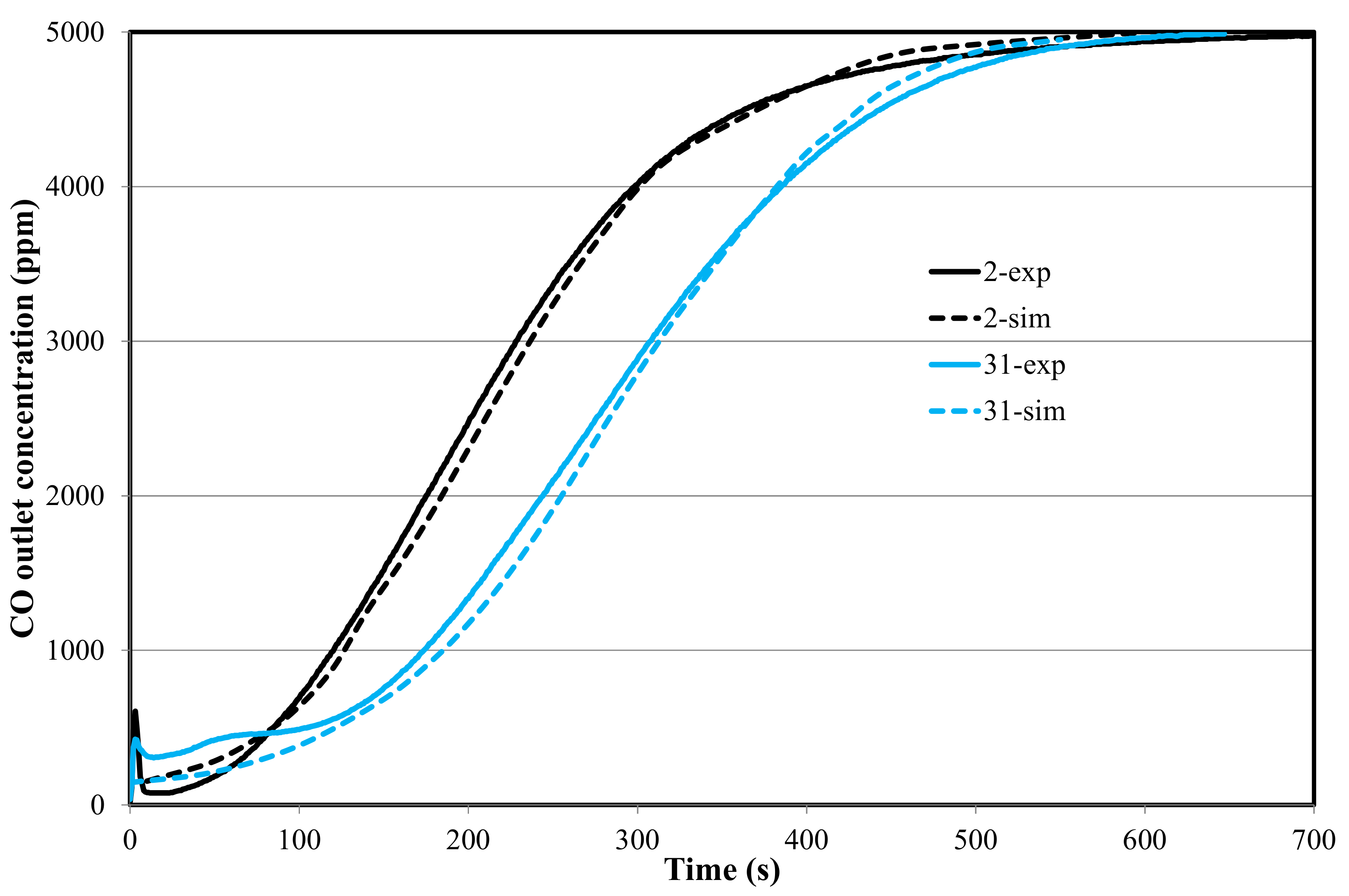
4.2. Reaction Modeling in the Fixed Bed (1D-Unsteady Approach)
5. Conclusions
Author Contributions
Conflicts of Interest
Nomenclature
| Latin letters | |
| C | CO concentration (mol·m−3) |
| D | Diffusion coefficient (m2·s−1) |
| k | Kinetic constant ((mol·m−3)−n·s−1) |
| n | Reaction order |
| nO | Available mole of oxygen in OC (mol) |
| Q | Flow rate (NL h−1) |
| r(x,t) | Reaction rate (mol m−3 s−1) |
| T | Temperature (°C) |
| t | Time (s) |
| u | Average velocity (m s−1) |
| V(x) | Discretized volume of the bed (m3) |
| x | Coordinate (m) |
| X | Conversion yield |
| Greek letters | |
| ν | Avrami coefficient |
| Subscript | |
| 0 | inlet reactor |
| 1 | site 1 |
| 2 | site 2 |
| c | chemical reaction |
| d | diffusion |
| ∝ | end of experiment |
References
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis, Firth Assessment Report; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Liu, C.; Li, X. Carbon storage and sequestration by urban forests in Shenyang, China. Urban For. Urban Green. 2012, 11, 121–128. [Google Scholar] [CrossRef]
- IPCC Guidelines. Chapter 5: Carbon dioxide transport, injection and geological storage. In 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Ishida, M.; Jin, H. A new advanced power-generation system using chemical-looping combustion. Energy 1994, 19, 415–422. [Google Scholar] [CrossRef]
- Ishida, M.; Zheng, D.; Akehata, T. Evaluation of a chemical-looping-combustion power-generation system by graphic exergy analysis. Energy 1987, 12, 147–154. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Leckner, B.; Mattisson, T. A fluidized-bed combustion process with inherent CO2 separation; application of chemical-looping combustion. Chem. Eng. Sci. 2001, 56, 3101–3113. [Google Scholar] [CrossRef]
- Lyngfelt, A. Chemical-looping combustion of solide fuels–Status of development. Appl. Energy 2014, 113, 1869–1873. [Google Scholar] [CrossRef]
- Wang, P.; Means, N.; Shekhawat, D.; Berry, D.; Massoudi, M. Chemical-Looping Combustion and gasification of coals and oxygen carrier development: A brief review. Energies 2015, 8, 10605–10635. [Google Scholar] [CrossRef]
- Bhave, A.; Taylor, H.S.; Fennell, P.; Livingston, W.R.; Shah, N.; Mac Dowell, N.; Dennis, J.; Kraft, M.; Pourkashanian, M.; Insa, M.; et al. Screening and techno-economic assessment of biomass-based power generation with CCS technologies to meet 2050 CO2 targets. Appl. Energy 2017, 190, 481–489. [Google Scholar] [CrossRef]
- Noorman, S.; van Sint Annaland, M.; Kuipers, J.A.M. Experimental validation of packed bed chemical-looping combustion. Chem. Eng. Sci. 2010, 65, 92–97. [Google Scholar] [CrossRef]
- Blas, L.; Dutournié, P.; Dorge, S.; Josien, L.; Kehrli, D.; Lambert, A. Thermal stability study of NiAl2O4 binders for Chemical-Looping Combustion application. Fuel 2016, 182, 50–56. [Google Scholar] [CrossRef]
- Dueso, C.; Abad, A.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Adánez, J.; Lyngfelt, A. Reactivity of a NiO/Al2O3 oxygen carrier prepared by impregnation for chemical-looping combustion. Fuel 2010, 89, 3399–3409. [Google Scholar] [CrossRef]
- Gayán, P.; de Diego, L.F.; García-Labiano, F.; Adánez, J.; Abad, A.; Dueso, C. Effect of support on reactivity and selectivity of Ni-based oxygen carriers for chemical-looping combustion. Fuel 2008, 87, 2641–2650. [Google Scholar] [CrossRef]
- Blas, L.; Dorge, S.; Michelin, L.; Dutournié, P.; Lambert, A.; Chiche, D.; Bertholin, S. Influence of the regeneration conditions on the performances and the microstructure modifications of NiO/NiAl2O4 for chemical looping combustion. Fuel 2015, 153, 284–293. [Google Scholar] [CrossRef]
- Hossain, M.M.; de Lasa, H.I. Chemical-looping combustion (CLC) for inherent separations—A review. Chem. Eng. Sci. 2008, 63, 4433–4451. [Google Scholar] [CrossRef]
- Ohlemüller, P.; Alobaid, F.; Gunnarson, A.; Ströhle, J.; Epple, B. Development of a porous model for coal chemical looping combustion and validation against 100 kWth tests. Appl. Energy 2015, 157, 433–448. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Zhang, Y.; Liu, X. Three dimensional modelling of a coal-fired chemical looping combustion process in the circulating fluidized bed fuel reactor. Energy Fuels 2013, 27, 2173–2184. [Google Scholar] [CrossRef]
- Alobaid, F.; Ohlemüller, P.; Ströhle, J.; Epple, B. Extended Euler-Euler model for the simulation of a 1 MWth chemical-looping pilot plant. Energy 2015, 93, 2395–2405. [Google Scholar] [CrossRef]
- Han, L.; Bollas, G.M. Chemical-looping combustion in a reverse flow fixed bed reactor. Energy 2016, 102, 669–681. [Google Scholar] [CrossRef]
- Diglio, G.; Bareschino, P.; Mancusi, E.; Pepe, F. Novel quasi authermal hydrogen production process in a fixed-bed reactor using a chemical looping approach: A numerical study. Int. J. Hydrog. Energy 2017, 42, 15010–15023. [Google Scholar] [CrossRef]
- Liang, Z.; Qin, W.; Dong, C. Experimental and Theoretical Study of the Interactions between Fe2O3/Al2O3 and CO. Energies 2017, 10, 598. [Google Scholar] [CrossRef]
- Blas, L.; Dorge, S.; Dutournié, P.; Lambert, A.; Chiche, D.; Bertholin, S.; Josien, L. Study of the performances of an oxygen carrier: Experimental investigation of the binder’s contribution and characterization of its structural modifications. Comptes Rendus Chim. 2015, 18, 45–55. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, L.; Bollas, G.M. Kinetics of NiO reduction by H2 and Ni oxidation at conditions relevant to chemical-looping combustion and reforming. Int. J. Hydrog. Energy 2014, 39, 8535–8556. [Google Scholar] [CrossRef]
- Iliuta, I.; Tahoces, R.; Patience, G.S.; Rifflart, S.; Luck, F. Chemical-looping combustion process: Kinetics and mathematical modelling. AIChE J. 2010, 56, 1063–1079. [Google Scholar] [CrossRef]
- Han, L.; Zhou, Z.; Bollas, G.M. Model-based analysis of chemical-looping combustion experiments. Part II: Optimal design of CH4-NIO reduction experiments. Chem. Eng. Sci. 2014, 113, 116–128. [Google Scholar] [CrossRef]
- Ishida, M.; Jin, H.; Okamoto, T. A fundamental study of a new kind of medium material for chemical looping combustion. Energy Fuels 1996, 10, 958–963. [Google Scholar] [CrossRef]
- Utigard, T.A.; Wu, M.; Plascencia, G.; Marin, T. Reduction kinetics of goro nickel oxide using hydrogen. Chem. Eng. Sci. 2005, 60, 2061–2068. [Google Scholar] [CrossRef]
- Dueso, C.; Ortiz, M.; Abad, A.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Adánez, J. Reduction and oxidation kinetics of nickel-based oxygen-carriers for chemical-looping combustion and chemical-looping reforming. Chem. Eng. J. 2012, 188, 142–154. [Google Scholar] [CrossRef]
- Ryu, H.J.; Bae, D.H.; Han, K.H.; Lee, S.Y.; Jin, G.T.; Choi, J.H. Oxidation and reduction characteristics of oxygen carrier particles and reaction kinetics by unreacted core model. Korean J. Chem. Eng. 2001, 18, 831–837. [Google Scholar] [CrossRef]
- Hossain, M.M.; de Lasa, H. Reduction and oxidation kinetics of Co-Ni/Al2O3 oxygen carrier involved in a chemical-looping combustion cycles. Chem. Eng. Sci. 2010, 65, 98–106. [Google Scholar] [CrossRef]
- Garcia-labiano, F.; de Diego, L.F.; Adanez, J.; Abad, A.; Gayan, P. Reduction and oxidation kinetics of a copper-based oxygen carrier prepared by impregnation for chemical looping combustion. Ind. Eng. Chem. Res. 2004, 43, 8168–8177. [Google Scholar] [CrossRef]
- Hossain, M.M.; de Lasa, H. Reactivity and stability of Co-Ni/Al2O3 oxygen carrier in multicycle chemical-looping combustion. AIChE J. 2007, 53, 1817–1829. [Google Scholar] [CrossRef]
- Kruggel-Emden, H.; Rickelt, S.; Stepanek, F.; Munjiza, A. Development and testing of an interconnected multiphase CFD-model for chemical looping combustion. Chem. Eng. Sci. 2010, 65, 4732–4745. [Google Scholar] [CrossRef]
- Richardson, J.T.; Scates, R.M.; Twigg, M.V. X-ray diffraction of hydrogen reduction of NiO: α-Al2O3 steam reforming catalysts. Appl. Catal. A 2004, 267, 35–46. [Google Scholar] [CrossRef]
- Gomez-Barea, A.; Ollero, P. An approximate method for solving gas-solid non-catalytic reactions. Chem. Eng. Sci. 2006, 61, 3725–3735. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Ligthfoot, E.N. Transport Phenomena, 2nd ed.; J. Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Annamalai, K.; Puri, I.K. Combustion Science and Engineering; CRC Press: New York, NY, USA, 2007. [Google Scholar]
- Diglio, G.; Bareschino, P.; Mancusi, E.; Pepe, F. Simulation of hydrogen production through chemical looping reforming process in a packed-bed reactor. Chem. Eng. Res. Des. 2016, 105, 137–151. [Google Scholar] [CrossRef]
- Noorman, S.; Gallucci, F.; Annaland, M.V.; Kuipers, J.A.M. A theoretical investigation of CLC in packed beds. Part 2: Reactor model. Chem. Eng. J. 2011, 167, 369–376. [Google Scholar] [CrossRef]
- Valencia, P.; Espinosa, K.; Ceballos, A.; Pinto, M.; Almonacid, S. Novel modeling methodology for the characterization of enzymatic hydrolysis of proteins. Proc. Biochem. 2015, 50, 589–597. [Google Scholar] [CrossRef]
- Dutournié, P.; Salagnac, P.; Glouannec, P. Optimization of radiant-convective Drying of a porous medium by design of experiment methodology. Dry. Technol. 2006, 24, 953–963. [Google Scholar] [CrossRef]
- Lam, J.; Carmichael, S.T.; Lowry, W.E.; Segura, T. Hydrogel design of experiments methodology to optimize hydrogel for iPSC-NPC culture. Adv. Healthc. Mat. 2015, 4, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Hinkelmann, K.; Kempthorne, O. Design and Analysis of Experiments, Introduction to Experimental Design, 2nd ed.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]


| Cycle | T (°C) | Q (NL·h−1) | [CO] (vol %) | Ratio (C0/NiO) | CO Oxidized (mol) | Time (t) = C0/2 (s) |
|---|---|---|---|---|---|---|
| 2 | 700 | 40 | 0.51 | 2.55 | 5.47 × 10−4 | 200 |
| 3 | 700 | 40 | 0.51 | 2.55 | 5.40 × 10−4 | 200 |
| 5 | 739 | 28.1 | 0.22 | 1.1 | 5.87 × 10−4 | 735 |
| 6 | 739 | 28.1 | 0.22 | 1.1 | 5.82 × 10−4 | 724 |
| 7 | 739 | 28.1 | 0.80 | 4 | 5.84 × 10−4 | 204 |
| 8 | 739 | 28.1 | 0.80 | 4 | 5.79 × 10−4 | 202 |
| 9 | 739 | 51.9 | 0.22 | 1.1 | 5.61 × 10−4 | 373 |
| 10 | 739 | 51.9 | 0.22 | 1.1 | 5.43 × 10−4 | 359 |
| 12 | 739 | 51.9 | 0.80 | 4 | 5.40 × 10−4 | 95 |
| 13 | 739 | 51.9 | 0.80 | 4 | 5.59 × 10−4 | 101 |
| 14 | 800 | 20.0 | 0.51 | 2.55 | 5.78 × 10−4 | 397 |
| 15 | 800 | 20.0 | 0.51 | 2.55 | 5.88 × 10−4 | 408 |
| 16 | 800 | 40.0 | 0.51 | 2.55 | 5.96 × 10−4 | 233 |
| 17 | 800 | 40.0 | 0.51 | 2.55 | 5.94 × 10−4 | 233 |
| 18 | 800 | 40.0 | 1.00 | 5 | 6.18 × 10−4 | 123 |
| 19 | 800 | 40.0 | 1.00 | 5 | 6.30 × 10−4 | 125 |
| 20 | 800 | 60.0 | 0.51 | 2.55 | 5.93 × 10−4 | 149 |
| 21 | 800 | 60.0 | 0.51 | 2.55 | 5.92 × 10−4 | 148 |
| 23 | 861 | 28.1 | 0.80 | 4 | 7.15 × 10−4 | 259 |
| 24 | 861 | 28.1 | 0.80 | 4 | 7.11 × 10−4 | 255 |
| 25 | 861 | 28.1 | 0.22 | 1.1 | 7.12 × 10−4 | 913 |
| 26 | 861 | 28.1 | 0.22 | 1.1 | 7.04 × 10−4 | 898 |
| 27 | 861 | 51.9 | 0.22 | 1.1 | 6.62 × 10−4 | 457 |
| 28 | 861 | 51.9 | 0.22 | 1.1 | 6.50 × 10−4 | 451 |
| 29 | 861 | 51.9 | 0.80 | 4 | 6.66 × 10−4 | 126 |
| 30 | 861 | 51.9 | 0.80 | 4 | 6.70 × 10−4 | 128 |
| 31 | 900 | 40.0 | 0.51 | 2.55 | 6.92 × 10−4 | 275 |
| 32 | 900 | 40.0 | 0.51 | 2.55 | 7.13 × 10−4 | 284 |
| 33 | 800 | 40.0 | 0.1 | 0.1 | 7.08 × 10−3 | 4643 |
| 34 | 800 | 40.0 | 0.1 | 0.1 | 7.35 × 10−3 | 4800 |
| Reactor and Oxygen Carrier | Operating Conditions |
|---|---|
| Bed length = 5 mm | Fuel = carbon monoxide in nitrogen |
| Oxygen carrier = NiO/NiAl2O4 (60/40 wt %.) | Inlet CO concentration = 0.1 to 1 vol %. |
| Dilution OC (ratio = 1 to 5 with SiC) | 20 ≤ Q ≤ 60 NL·h−1 |
| Available mole of oxygen (nO (t = 0)) = experimental one | 700 ≤ T ≤ 900 °C |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blas, L.; Dutournié, P.; Jeguirim, M.; Josien, L.; Chiche, D.; Bertholin, S.; Lambert, A. Numerical Modeling of Oxygen Carrier Performances (NiO/NiAl2O4) for Chemical-Looping Combustion. Energies 2017, 10, 864. https://doi.org/10.3390/en10070864
Blas L, Dutournié P, Jeguirim M, Josien L, Chiche D, Bertholin S, Lambert A. Numerical Modeling of Oxygen Carrier Performances (NiO/NiAl2O4) for Chemical-Looping Combustion. Energies. 2017; 10(7):864. https://doi.org/10.3390/en10070864
Chicago/Turabian StyleBlas, Lucia, Patrick Dutournié, Mejdi Jeguirim, Ludovic Josien, David Chiche, Stephane Bertholin, and Arnold Lambert. 2017. "Numerical Modeling of Oxygen Carrier Performances (NiO/NiAl2O4) for Chemical-Looping Combustion" Energies 10, no. 7: 864. https://doi.org/10.3390/en10070864
APA StyleBlas, L., Dutournié, P., Jeguirim, M., Josien, L., Chiche, D., Bertholin, S., & Lambert, A. (2017). Numerical Modeling of Oxygen Carrier Performances (NiO/NiAl2O4) for Chemical-Looping Combustion. Energies, 10(7), 864. https://doi.org/10.3390/en10070864