Abstract
In this work, the characteristics of waste pig fat degradation using supercritical alcohols have been studied. Comparative analysis of the influence of supercritical methanol and supercritical ethanol as solvents on the transesterification was the primary focus of this research. The experiments were carried out with waste pig fat to alcohol weight ratios of 1:1.5 (molar ratio: 1:40.5 for methanol and 1:28 for ethanol), 1:2.0 (molar ratio: 1:54 for methanol and 1:37.5 for ethanol) and 1:2.5 (molar ratio: 1:67.5 for methanol and 1:47 for ethanol) at transesterification temperatures 250, 270 and 290 °C for holding time 0, 15, 30, 45 and 60 min. Increase in the transesterification and holding time increased the conversion while increase in alcohol amount from 1:1.5 to 1:2.0 and 1:2.5 had minimal effect on the conversion. Further, majority of the ester composition in using SCM as solvent falls in the carbon range of C17:0, C19:1 and C19:2 while that for SCE falls in the carbon range of C18:0, C20:1 and C20:2. Glycerol was only present while using SCM as solvent.
1. Introduction
Biodiesel, as an alternative fuel, has many merits. It is derived from a renewable, domestic resource, thereby relieving reliance on petroleum fuel imports. Biodiesel is superior to conventional diesel in terms of its sulphur content, aromatic content and flash point. Basically it is sulphur free and non-aromatic while conventional diesel can contain up to 500 ppm SO2 and 20–40 wt % aromatic compounds [1]. In addition, it is biodegradable and non-toxic. Compared to petroleum-based diesel, biodiesel has more favorable combustion emission profile, such as low emissions of carbon monoxide, particulate matter and unburned hydrocarbons.
In general, biodiesel consists of fatty acid methyl esters (FAMEs) and is produced from renewable sources such as vegetable oils or animal fats. Carbon dioxide produced by combustion of biodiesel from plant source can be recycled by photosynthesis, thereby minimizing its impact on greenhouse effect [2,3,4]. Commercially, the use of expensive refined vegetable oils as a feedstock is not economically viable because nearly 70% of the cost is attributed to the raw materials [1,5]. Consequently, the utilization of a low quality, inexpensive and abundant feedstock has gained attention for significant reduction in the processing cost [6]. Inexpensive triglycerides sources such as waste or nonedible oils and animal fats have the potential not only to decrease production cost but also to make biodiesel profitable without government subsidies [7]. Waste cooking oils and animal fats are intriguing feedstock because they are two to three times cheaper than refined vegetable oils and are abundantly available to fulfill the market demand for biodiesel production [8]. Although carbon dioxide generated from the combustion of biodiesel from pig fat cannot be recycled by photosynthesis, the reduction of cost is one of the major factors in selecting it as the raw material.The necessity of using low-cost feedstock has been anticipated by several research groups that have conducted studies on biodiesel production from mutton tallow [9], bovine fat [10], lard [11] and waste cooking oils [12,13], either by the base- or acid-catalyzed methods. As pork is one of the major food item in Korea, the amount of waste pig fat generated is enormously high, the reason why pig fat has been used as raw material in this study.
Generally, there are two common methods for transesterification of biological source to biodiesel namely catalyzed transesterification method and supercritical fluid method. A large amount of waste water is generated during neutralization and washing of the product in catalyzed transesterification. In addition, a complicated purification and separation process is required, which leads to a low yield of biodiesel and high processing costs [6]. The transesterification for biodiesel production via supercritical alcohols has been suggested to overcome the drawbacks related to the homogeneous catalytic process [14,15,16,17].The comparison of the two methods shows that the supercritical method is much simpler and more environmentally friendly. Furthermore, the presence of water and FFA does not affect the yield of biodiesel as transesterification of triglyceride and esterification of FFA take place simultaneously [18]. Economic analyses performed on the supercritical transesterification process are very encouraging due to the possibility of scaling up the process to industrial levels [19,20,21,22,23]. Hence, this technology is expected to be appropriate for the production of biodiesel from waste pig fat without any pre-treatment.
Water offers a remarkable solvent tenability with its wide range of dielectric constants; its applications and potential as a new reaction medium in chemistry has been reviewed [24]. The fact that the critical temperatures of alcohols (Tc = 512.6, 513.9 K; Pc = 80.9, 61.4 bar; ρc = 0.272, 0.276 g/cm3 for methanol and ethanol, respectively) are quite low compared to that of water (Tc = 647.1 K; Pc = 220.6 bar; ρc = 0.322 g/cm3) suggests that hydrogen bonding is weaker in alcohols than water [25]. Alcohols may be an alternative to water as supercritical solvents considering their less corrosive and aggressive chemical nature, the lower critical temperatures and pressures, and their reasonably high dielectric constants. As these alcohols have lower critical temperatures and pressures, they offer milder conditions for reaction. In addition, these alcohols are expected to readily dissolve relatively high molecular weight products from cellulose, hemicelluloses, and lignin because of their low dielectric constants when compared with that of water [26].
In this study, one-step transesterification of waste pig fat in supercritical alcohol (SCA) was performed to obtain the liquid products. To date no research have been conducted to study the comparative analysis of the liquid obtained from transesterification of waste pig fat using different alcohols although some researches have been done using methanol [6]. Nevertheless, detail product analysis has not been done so far using pig fat as the raw material and ethanol and methanol as the solvents. Therefore, this study attempts to compare the effect of two different alcohols in the product obtained from the transesterification of waste pig fat.
2. Experimental Section
2.1. Materials and Apparatus
The chemical composition for the waste pig fat sample was referred from literature [27,28]. The Fatty Acid (FA) composition of waste pig fat is shown in Table 1. As solvents, methanol of 99.5% purity manufactured by Ducksan Chemical Co. (Ansan, Korea), and ethanol of 99.9% purity produced by OCI Company Ltd. (Seoul, Korea) were used. Figure 1 illustrates the schematic diagram of the batch-type reactor used in this work manufactured by Parr Instrument Co. (Moline, IL, USA) with volume of 25 mL. The permissible reactor conditions are 500 °C and 55 MPa. The temperature of the reactant was measured by a K-type thermocouple, whereas the pressure inside the vessel was monitored by a digital pressure gauge.

Table 1.
Fatty acid (FA) composition of waste pig fat.
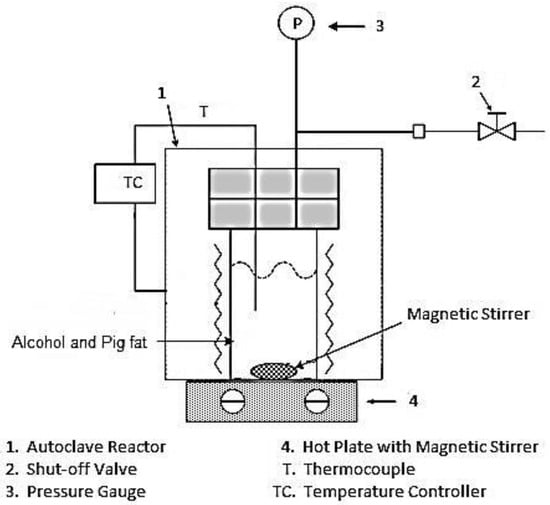
Figure 1.
Schematic diagram of the experimental apparatus.
2.2. Procedure
The waste pig fat sample was stored in vacuum for more than 24 h before the experiment ensuring minimum moisture capture during the pre-setup works. The experiments were carried out with waste pig fat to alcohol weight ratios of 1:1.5 (Molar ratio: 1:40.5 for methanol and 1:28 for ethanol), 1:2.0 (Molar ratio: 1:54 for methanol and 1:37.5 for ethanol) and 1:2.5 (Molar ratio: 1:67.5 for methanol and 1:47 for ethanol) at transesterification temperatures 250, 270 and 290 °C for holding time 0, 15, 30, 45 and 60 min. At room temperature, the waste pig fat with alcohol at definite weight ratio was loaded into the autoclave reactor. The reaction pressure was controlled by varying the volume of alcohol and waste pig fat fed into the reactor. The total amount of waste pig fat and alcohol accommodated into the reactor was determined to ensure that supercritical pressure was reached. The pressure range obtained for supercritical methanol (SCM) and supercritical ethanol (SCE) are 114–187 and 98–145 bars, respectively. During the reaction, a magnetic stirrer was used for rigorous stirring at a rate of 500 rpm in order to mix the waste pig fat and alcohol solution homogeneously. Maintaining a constant heating rate was difficult therefore the set point of the temperature controller was set to a higher temperature than the required experimental temperature accordingly for this experiment. When the reaction temperature reached the required experimental condition, the heating of the vessel was immediately stopped and the vessel was cooled in an ice bath to stop the reaction instantly. The analysis of the characteristics of the liquid products on varying reaction temperature, time, and waste pig fat to alcohol ratios has been performed through Gas Chromatography Mass Spectrometry (GC-MS) (Agilent GC-6890 with MSD-5975 detector, Santa Clara, CA, USA). Table 2 indicates the operation conditions of GC-MS used in this work. The area percentage method was used to estimate the amount of alkyl esters in the product.

Table 2.
Operation conditions of gas chromatography mass spectrometry (GC-MS) analysis used in this work.
3. Results and Discussion
The qualitative analysis of the product obtained during transesterification of waste pig fat in SCA is absolutely necessary to understand biodiesel formation from waste pig fat. The product obtained from transesterification of waste pig fat is significantly affected by the experimental conditions. Figure 2, Figure 3 and Figure 4 shows the conversion during transesterification of waste pig fat at various reaction temperatures of 250, 270 and 290 °C for SCM and SCE on varying the holding time for waste pig fat to alcohol ratio of 1:1.5, 1:2.0 and 1:2.5, respectively. Temperature plays a crucial role in supercritical alcohol transesterification reaction for biodiesel production. As the critical temperature of methanol and ethanol are 239 and 243 °C, respectively, the reaction temperature must be higher than these critical values. The difference in the conversion obtained at higher temperature for SCM and SCE is small although the conversion obtained by using SCM is comparatively higher than that while using SCE for lower holding time. Increasing reaction holding time and temperature, both had favorable influence on the conversion. Although the conversion was low for lower holding time, the conversion improved significantly with increase in the holding time. Compared to conventional catalytic reactions [14,29,30] which required enormous amount of reaction time, supercritical alcohol reaction can be completed in a substantially lower duration. As shown in Figure 2, Figure 3 and Figure 4, the conversion increased steadily with the increment of holding time for both SCM and SCE. At the holding time of 60 min, the conversions were above 99% for both SCM and SCE. This is a significant advantage of supercritical technology compared to catalytic reactions that requires hours of reaction time to achieve the same amount of conversion.
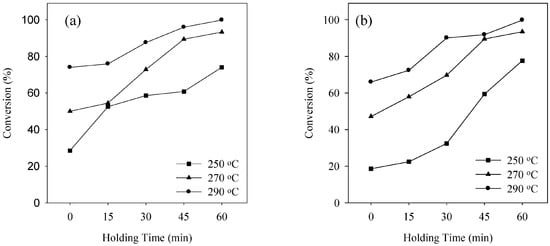
Figure 2.
Conversion at various reaction temperature using (a) SCM and (b) SCE for waste pig fat to alcohol ratio of 1:1.5.
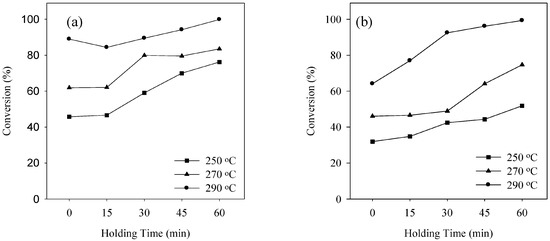
Figure 3.
Conversion at various reaction temperature using (a) SCM and (b) SCE for waste pig fat to alcohol ratio of 1:2.0.
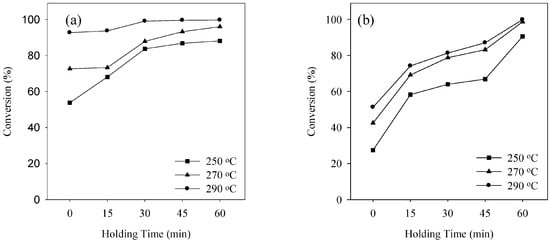
Figure 4.
Conversion at various reaction temperature using (a) SCM and (b) SCE for waste pig fat to alcohol ratio of 1:2.5.
The waste pig fat to alcohol molar ratios is also one of the most important variables affecting the yield of methyl esters. Higher weight ratios result in greater ester production in a shorter time [31]. In a supercritical alcohol reaction, an excessive amount of methanol is employed in order to shift the equilibrium towards producing more biodiesel [32]. In this study the molar ratio of alcohol to waste pig fat was varied by varying the weight ratio as shown in Figure 2, Figure 3 and Figure 4. From Figure 2, Figure 3 and Figure 4, it can be inferred that the effect of varying the waste pig fat to alcohol ratio from 1:1.5 to 1:2.5 was not significant on the conversion percentage. The optimum molar ratios as described by Tan et al. [33] for SCM and SCE during transesterification of palm oil are 1:40 and 1:33, respectively. The molar ratio obtained from the weight basis in this study falls in the optimum range. Although the lower weight ratio for SCE, i.e., 1:1.5 is slightly lower than the optimum condition described above, but is enough to carry the conversion which can be observed from the similarity of the conversion obtained. Although enormous amounts of alcohol can enhance the reaction rate, excessive concentration of alcohol in the reaction mixture can inhibit transesterification reaction. Moreover, the purification process of biodiesel becomes energy intensive due to extreme amount of alcohol in the product mixture [34]. Hence, the molar ratio of waste pig fat to alcohol should be kept at optimum in supercritical alcohol transesterification reaction.
In fact, it has been argued that one of the attractive characteristics of biodiesel supercritical method is the low reaction time [14]. For instance, Kusdiana and Saka [29] obtained a conversion in methyl esters as high as 95 wt % in about 240 s of reaction in a batch reactor. Minami and Saka [35] reported conversions of around 90 wt % in 30 min reaction for methyl esterification of oleic acid in continuous mode. 99.8% conversion was obtained for SCM and SCE at holding time of 60 min for this study. Similar conversion can be obtained at lower holding time by increasing the transesterification temperature or also by increasing the voltage of the electrical heater.
The evolution of components in terms of carbon number for SCM and SCE are shown in Table 3, Table 4, Table 5 and Table 6. The product has been classified as glycerol, non-esters and esters. Glycerol is usually the byproduct of the transesterification. In spite of several applications proposed [36], it has become an economic issue in the biodiesel cost along with various environmental issues [37]. It can be inferred that the presence of glycerol is significant in using SCM while glycerol is almost absent while using SCE as solvent. The absence of glycerol in SCE results into higher amount of products in the biodiesel range. The presence of glycerol in solution helps drive the equilibrium towards reactant, lowering the yield of esters [38]. Methanol and ethanol are not miscible with triglycerides at ambient temperature, and the reaction mixtures are usually mechanically stirred to enhance mass transfer. During this course of reaction, emulsions usually form. In the case of methanolysis, these emulsions quickly and easily break down to form a lower glycerol rich layer and upper methyl ester rich layer. In ethanolysis, these emulsions are more stable and severely complicate the separation and purification of esters [39]. The absence of glycerol in using SCE will enhance its usability compared to SCM resulting into increased ester content. Further, it can be observed that majority of the ester composition on using SCM as solvent falls in the carbon range of C17:0, C19:1 and C19:2 while that for SCE falls in the carbon range of C18:0, C20:1 and C20:2. This clearly shows the effect of addition of one extra carbon from ethanol to the product. From Table 3 and Table 5, it can be observed that with some exceptions, the further decomposition of esters occur with increase in the reaction holding time for all transesterification temperatures. From Table 4 and Table 6, it can be inferred that no significant variation in ester composition occurred on varying the waste pig fat to alcohol ratio. The non-ester component is less than 5% for both SCM and SCE. Although, the results obtained in this study are still not enough to conclude which alcohol is better comparatively, the ester yield in ethanol is slightly higher which is due to the absence of glycerol in SCE. In general glycerol is generated during transesterification. However, the absence of glycerol during transesterification of waste pig fat using SCE is an interesting deviation from the regularly observed trend for further investigation.

Table 3.
The weight percentage of compounds according to the carbon number of liquid products obtained at different transesterification temperature for different holding times for waste pig fat to methanol ratio of 1:1.5.

Table 4.
The weight percentage of compounds according to the carbon number of liquid products obtained at different holding times for different waste pig fat to methanol ratios at transesterification temperature of 290 °C.

Table 5.
The weight percentage of compounds according to the carbon number of liquid products obtained at different transesterification temperature for different holding times for waste pig fat to ethanol ratio of 1:1.5.

Table 6.
The weight percentage of compounds according to the carbon number of liquid products obtained at different holding times for different waste pig fat to ethanol ratios at transesterification temperature of 290 °C.
There are numerous studies which show that either SCM or SCE is better for biodiesel generation. Comparatively, SCM has shown to be more suitably used in biodiesel production compared to SCE reaction as reported by others [16,30,40]. However, studies by Madras et al. [41] and Poudel and Oh [42] discuss the better suitability of SCE than SCM in biodiesel production. There are no studies that focus on the comparative analysis of transesterification of waste pig fat using SCM and SCE. Shin et al. [6] studied the biodiesel production from waste lard using supercritical methanol. But, from an environmental point of view, the requirement of methanol makes the current biodiesel product not totally 100% renewable as methanol is derived from fossil-based products. Ethanol, on the other hand, can be produced from agricultural biomass via fermentation technology and is easily available in the market at a high purity [43]. Comparative analysis of SCM and SCE in biodiesel production is indispensable considering the similarity in their properties and the merits they can provide compared to the supercritical water and supercritical carbon dioxide [26].
4. Conclusions
In this study, the transesterification of waste pig fat using supercritical methanol and supercritical ethanol has been studied with waste pig fat to alcohol ratios of 1:1.5, 1:2.0 and 1:2.5 for transesterification temperature of 250, 270 and 290 °C for holding time 0, 15, 30, 45 and 60 min. The qualitative analysis of the product obtained during the transesterification of waste pig fat in SCA is indispensable to understand the biodiesel formation. It was concluded that increasing the transesterification temperature and holding time had a favorable influence on the conversion. However the increase in the waste pig fat to alcohol ratio from 1:1.5 to 1:2.0 and 1:2.5 had minimal effect on the conversion. This is possible from the fact that although enormous amounts of alcohol can enhance the reaction rate, excessive concentration of alcohol in the reaction mixture can inhibit transesterification reaction. Hence, the molar ratio of waste pig fat to alcohol should be kept at optimum in supercritical transesterification reaction. The majority of the ester composition in using SCM as solvent falls in the carbon range of C17:0, C19:1 and C19:2 while that for SCE falls in the carbon range of C18:0, C20:1 and C20:2. The glycerol was only present while using SCM as solvent.
Acknowledgments
This work was supported by a Grant from the Human Resources Development Program (No. 20154030200940) of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) funded by the Ministry of Trade, Industry and Energy of the Korean government.
Authors Contributions
Jeeban Poudel and Malesh Shah produced the experimental data, prepared figures and table; Jeeban Poudel, Malesh Shah and Sujeeta Karki wrote the draft of the paper; Sujeeta Karki was responsible for final editing and proofreading; Sea Cheon Oh supervised the research and finalized the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Phan, A.N.; Phan, T.M. Biodiesel production from waste cooking oils. Fuel 2008, 87, 3490–3496. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, M.; McLean, D.; Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [PubMed]
- Körbitz, W. Biodiesel production in Europe and North America, an encouraging prospect. Renew. Energy 1999, 16, 1078–1083. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Das, L. Biodiesel development and characterization for use as a fuel in compression ignition engines. J. Eng. Gas Turbines Power 2001, 123, 440–447. [Google Scholar] [CrossRef]
- Meng, X.; Chen, G.; Wang, Y. Biodiesel production from waste cooking oil via alkali catalyst and its engine test. Fuel Process. Technol. 2008, 89, 851–857. [Google Scholar] [CrossRef]
- Shin, H.; Lee, S.; Ryu, J.; Bae, S. Biodiesel production from waste lard using supercritical methanol. J. Supercrit. Fluids 2012, 61, 134–138. [Google Scholar] [CrossRef]
- Marulanda, V.F.; Anitescu, G.; Tavlarides, L.L. Investigations on supercritical transesterification of chicken fat for biodiesel production from low-cost lipid feedstocks. J. Supercrit. Fluids 2010, 54, 53–60. [Google Scholar] [CrossRef]
- Tan, K.; Lee, K.; Mohamed, A. Potential of waste palm cooking oil for catalyst-free biodiesel production. Energy 2011, 36, 2085–2088. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Hanif, M.A.; Qasim, M. Biodiesel production from waste tallow. Fuel 2008, 87, 2961–2966. [Google Scholar] [CrossRef]
- Moraes, M.S.A.; Krause, L.C.; da Cunha, M.E.; Faccini, C.S.; de Menezes, E.W.; Veses, R.C.; Rodrigues, M.R.A.; Caramão, E.B. Tallow biodiesel: Properties evaluation and consumption tests in a diesel engine. Energy Fuels 2008, 22, 1949–1954. [Google Scholar] [CrossRef]
- Jeong, G.; Yang, H.; Park, D. Optimization of transesterification of animal fat ester using response surface methodology. Bioresour. Technol. 2009, 100, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, A.B.; Watts, K.C.; Islam, M.R. Waste cooking oil as an alternate feedstock for biodiesel production. Energies 2008, 1, 3–18. [Google Scholar] [CrossRef]
- Cetinkaya, M.; Karaosmanoglu, F. Optimization of base-catalyzed transesterification reaction of used cooking oil. Energy Fuels 2004, 18, 1888–1895. [Google Scholar] [CrossRef]
- He, H.; Wang, T.; Zhu, S. Continuous production of biodiesel fuel from vegetable oil using supercritical methanol process. Fuel 2007, 86, 442–447. [Google Scholar] [CrossRef]
- Poudel, J.; Oh, S.C. Degradation characteristics of wood using supercritical alcohols. Energies 2012, 5, 5038–5052. [Google Scholar] [CrossRef]
- Sanjel, N.; Gu, J.H.; Oh, S.C. Transesterification Kinetics of Waste Vegetable Oil in Supercritical Alcohols. Energies 2014, 7, 2095–2106. [Google Scholar] [CrossRef]
- Demirbaş, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energy Convers. Manag. 2003, 44, 2093–2109. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Methyl Esterification of Free Fatty Acids of Rapeseed Oil as Treated in Supercritical Methanol. J. Chem. Eng. Jpn. 2001, 34, 383–387. [Google Scholar] [CrossRef]
- Van Kasteren, J.; Nisworo, A. A process model to estimate the cost of industrial scale biodiesel production from waste cooking oil by supercritical transesterification. Resour. Conserv. Recycl. 2007, 50, 442–458. [Google Scholar] [CrossRef]
- Pinnarat, T.; Savage, P.E. Assessment of noncatalytic biodiesel synthesis using supercritical reaction conditions. Ind. Eng. Chem. Res. 2008, 47, 6801–6808. [Google Scholar] [CrossRef]
- Anitescu, G.; Deshpande, A.; Tavlarides, L.L. Integrated technology for supercritical biodiesel production and power cogeneration. Energy Fuels 2008, 22, 1391–1399. [Google Scholar] [CrossRef]
- Deshpande, A.; Anitescu, G.; Rice, P.; Tavlarides, L. Supercritical biodiesel production and power cogeneration: Technical and economic feasibilities. Bioresour. Technol. 2010, 101, 1834–1843. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Lee, H.; Lee, Y.; Han, C. Design and economic analysis of the process for biodiesel fuel production from transesterificated rapeseed oil using supercritical methanol. Ind. Eng. Chem. Res. 2009, 48, 5370–5378. [Google Scholar] [CrossRef]
- Shaw, R.W.; Brill, T.B.; Clifford, A.A.; Eckert, C.A.; Franck, E.U. Supercritical water: A medium for chemistry. Chem. Eng. News 1991, 69, 26–39. [Google Scholar]
- Reid, R.C.; Prausnitz, J.M.; Poling, B.E. The Properties of Gases and Liquids; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar]
- Yamazaki, J.; Minami, E.; Saka, S. Liquefaction of beech wood in various supercritical alcohols. J. Wood Sci. 2006, 52, 527–532. [Google Scholar] [CrossRef]
- Rohman, A.; Triyana, K.; Erwanto, Y. Differentiation of lard and other animal fats based on triacylglycerols composition and principal component analysis. Int. Food Res. J. 2012, 19, 475–479. [Google Scholar]
- Indrasti, D.; Man, Y.B.C.; Mustafa, S.; Hashim, D.M. Lard detection based on fatty acids profile using comprehensive gas chromatography hyphenated with time-of-flight mass spectrometry. Food Chem. 2010, 122, 1273–1277. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from waste cooking oil via base-catalytic and supercritical methanol transesterification. Energy Convers. Manag. 2009, 50, 923–927. [Google Scholar] [CrossRef]
- Han, H.; Cao, W.; Zhang, J. Preparation of biodiesel from soybean oil using supercritical methanol and CO 2 as co-solvent. Process Biochem. 2005, 40, 3148–3151. [Google Scholar] [CrossRef]
- Tan, K.T.; Gui, M.M.; Lee, K.T.; Mohamed, A.R. An optimized study of methanol and ethanol in supercritical alcohol technology for biodiesel production. J. Supercrit. Fluids 2010, 53, 82–87. [Google Scholar] [CrossRef]
- Tan, K.T.; Gui, M.M.; Lee, K.T.; Mohamed, A.R. Supercritical alcohol technology in biodiesel production: A comparative study between methanol and ethanol. Energy Sources A Recovery Util. Environ. Eff. 2010, 33, 156–163. [Google Scholar] [CrossRef]
- Saka, S.; Kusdiana, D. Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Minami, E.; Saka, S. Kinetics of hydrolysis and methyl esterification for biodiesel production in two-step supercritical methanol process. Fuel 2006, 85, 2479–2483. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sust. Energy Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Huber, M.L.; Lemmon, E.W.; Kazakov, A.; Ott, L.S.; Bruno, T.J. Model for the thermodynamic properties of a biodiesel fuel. Energy Fuels 2009, 23, 3790–3797. [Google Scholar] [CrossRef]
- Demirbas, A. Studies on cottonseed oil biodiesel prepared in non-catalytic SCF conditions. Bioresour. Technol. 2008, 99, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Tomasevic, A.; Siler-Marinkovic, S. Methanolysis of used frying oil. Fuel Process. Technol. 2003, 81, 1–6. [Google Scholar] [CrossRef]
- Zhou, W.; Konar, S.K.; Boocock, D.G. Ethyl esters from the single-phase base-catalyzed ethanolysis of vegetable oils. J. Am. Oil Chem. Soc. 2003, 80, 367–371. [Google Scholar] [CrossRef]
- Warabi, Y.; Kusdiana, D.; Saka, S. Reactivity of triglycerides and fatty acids of rapeseed oil in supercritical alcohols. Bioresour. Technol. 2004, 91, 283–287. [Google Scholar] [CrossRef]
- Madras, G.; Kolluru, C.; Kumar, R. Synthesis of biodiesel in supercritical fluids. Fuel 2004, 83, 2029–2033. [Google Scholar] [CrossRef]
- Poudel, J.; Oh, S.C. A kinetic analysis of wood degradation in supercritical alcohols. Ind. Eng. Chem. Res. 2012, 51, 4509–4514. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.T.; Bhatia, S. Supercritical ethanol technology for the production of biodiesel: Process optimization studies. J. Supercrit. Fluids 2009, 49, 286–292. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).