Distribution and Presence of Polymers in Porous Media
Abstract
:1. Introduction
2. Materials and Method
2.1. Experimental Materials
2.2. Methods
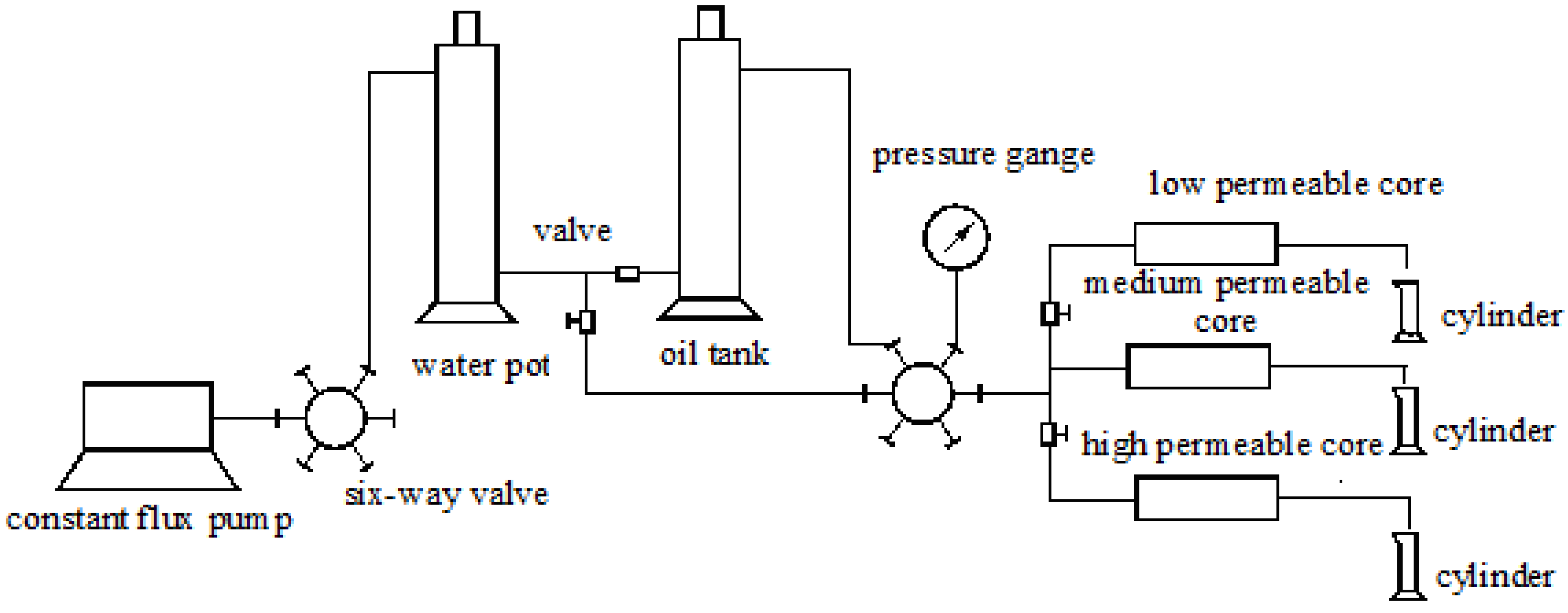
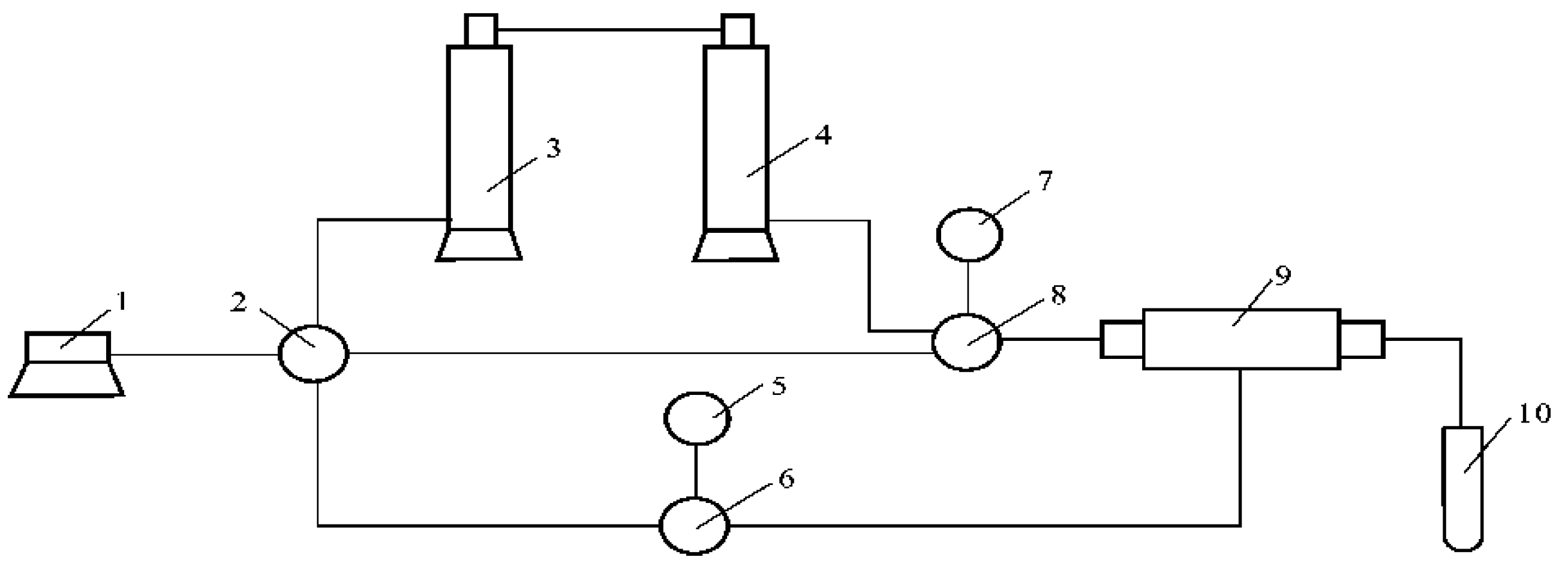
2.2.1. Three Parallel Core Experiments
2.2.2. Three-Dimensional Slab Model Experiment
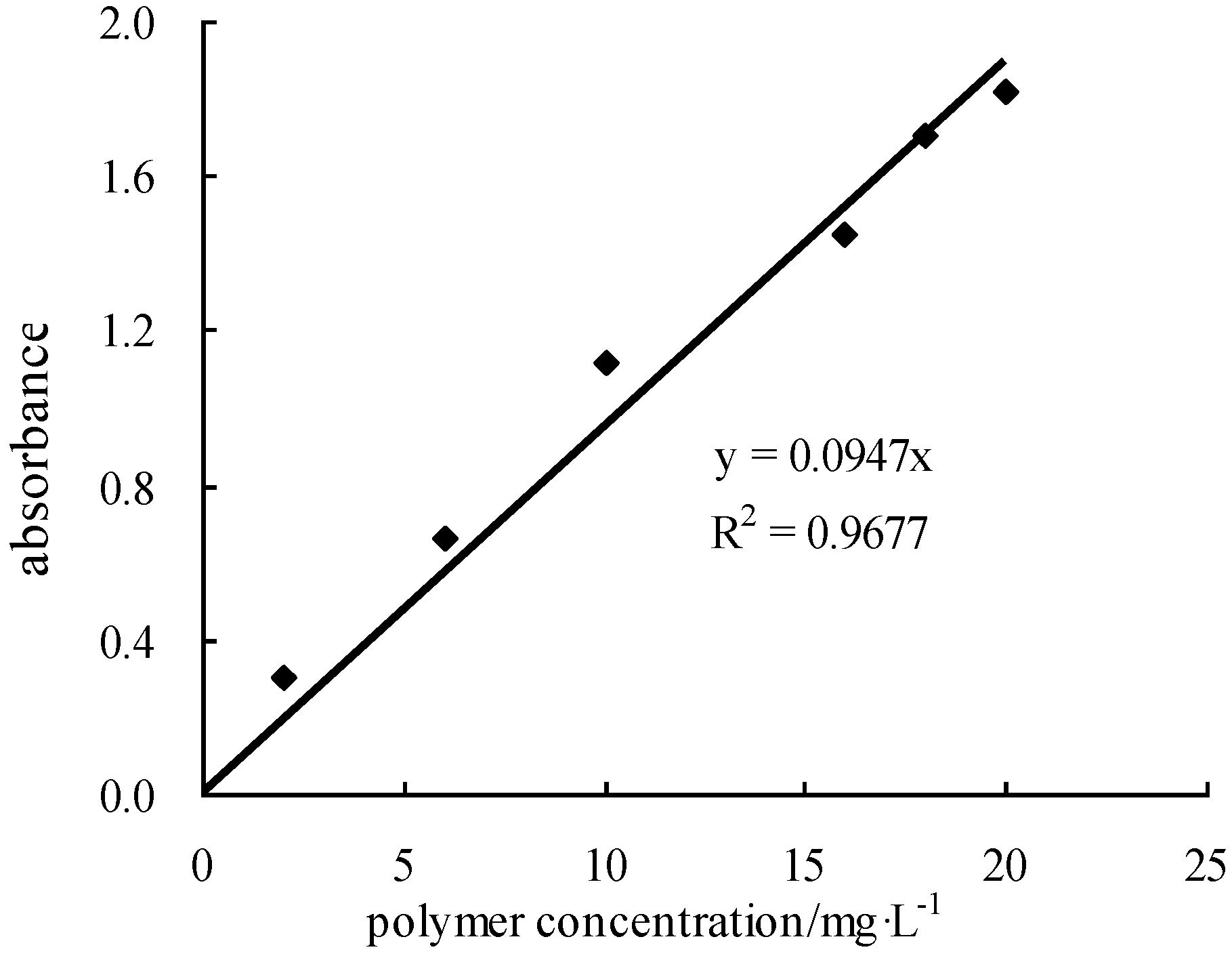
2.2.3. Polymer Concentration Determination Method
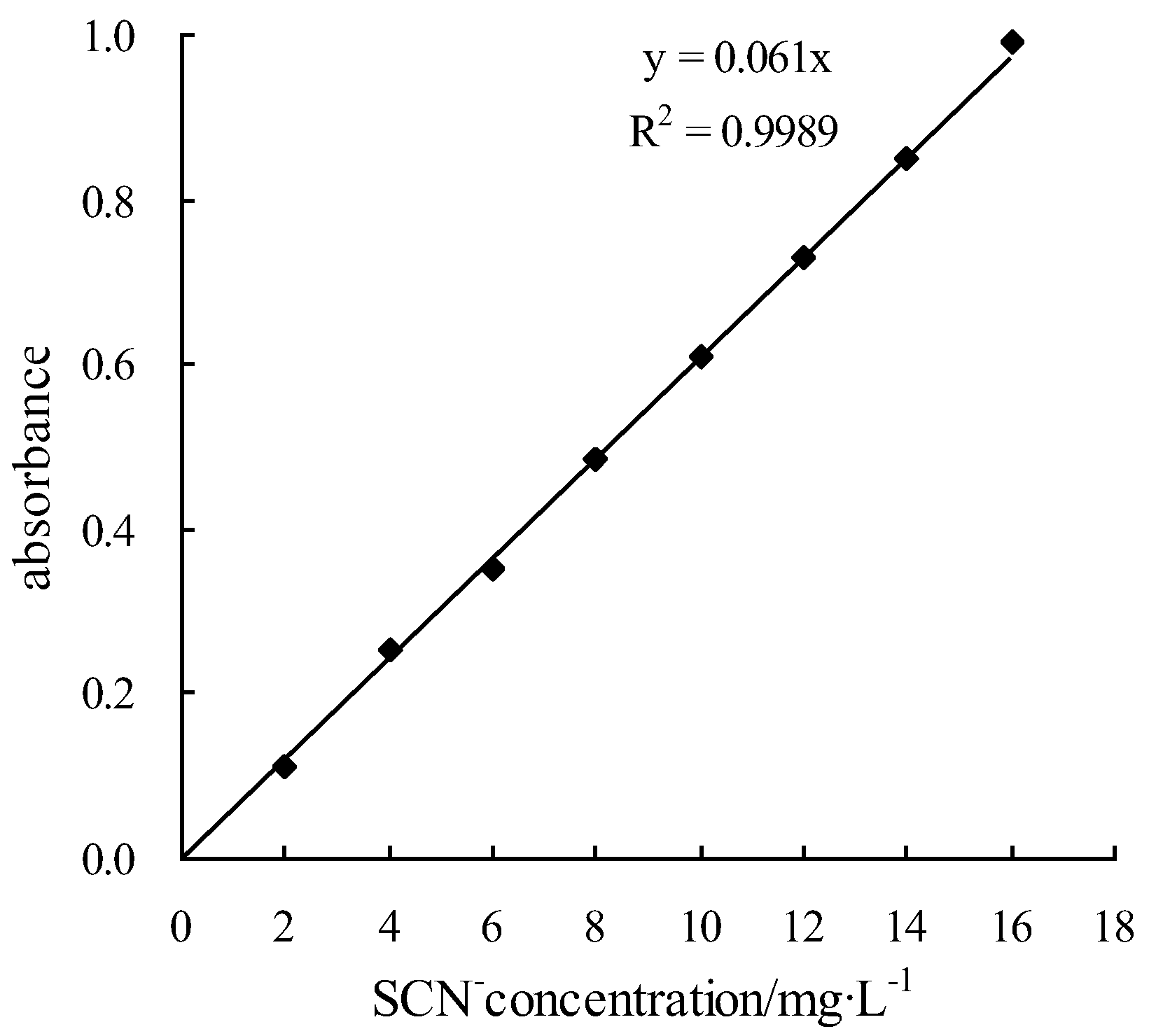
2.2.4. Potassium Thiocyanate Tracer Determination Method
2.2.5. Static-Equilibrium Adsorption
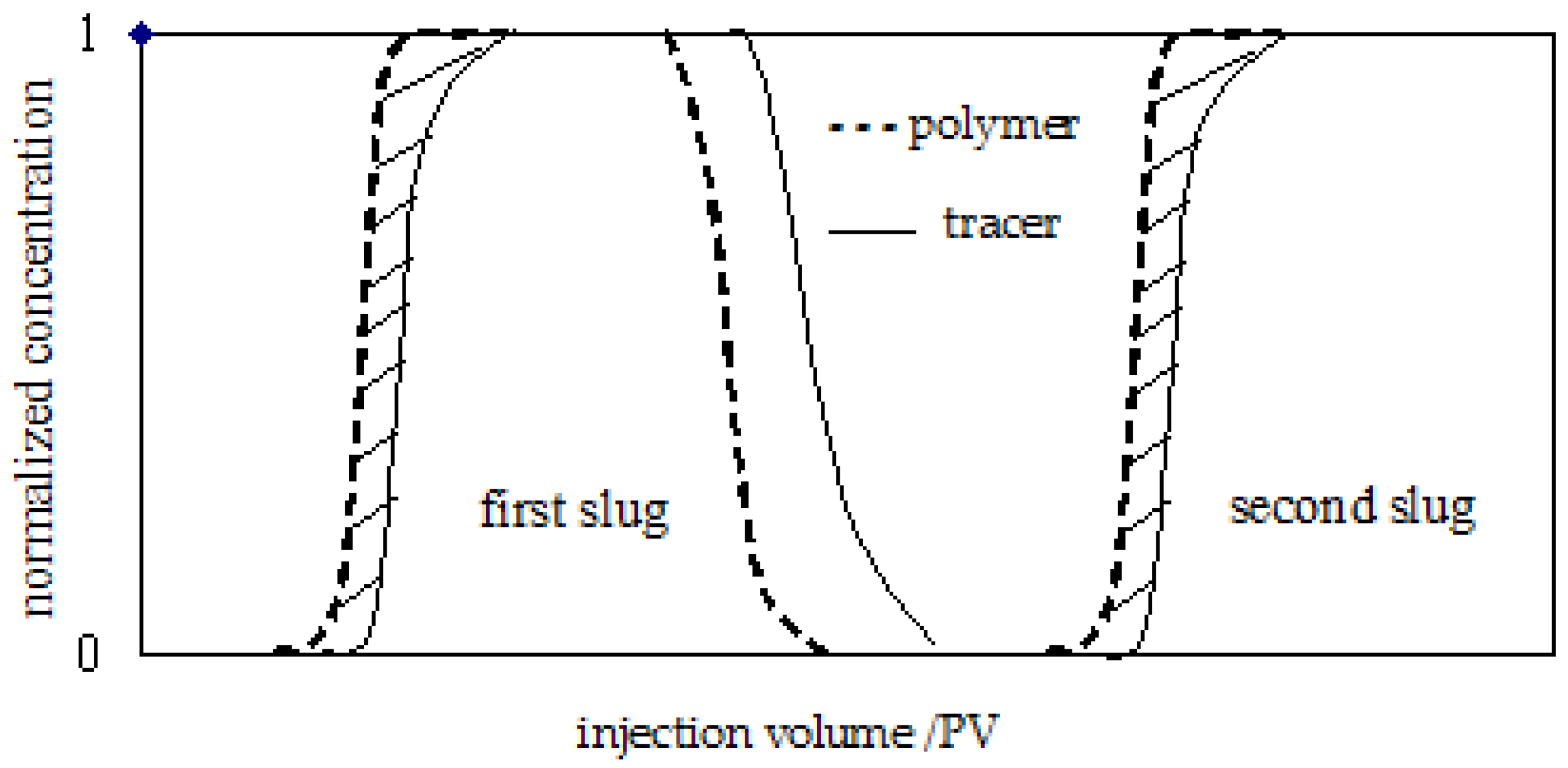
2.2.6. Double-Slug Experiments
3. Results and Discussion
3.1. Distribution of Polymer
3.1.1. Vertical Distribution
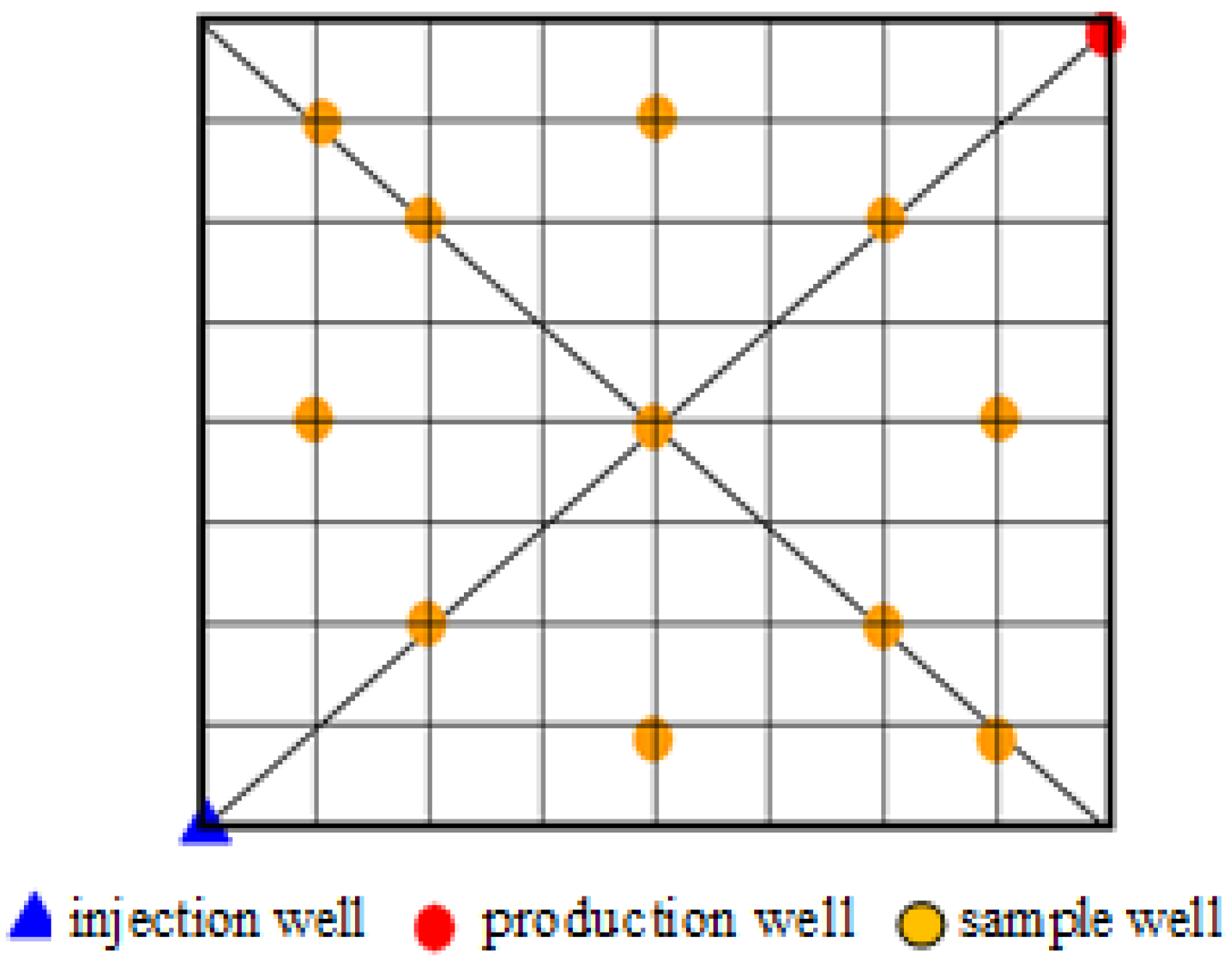
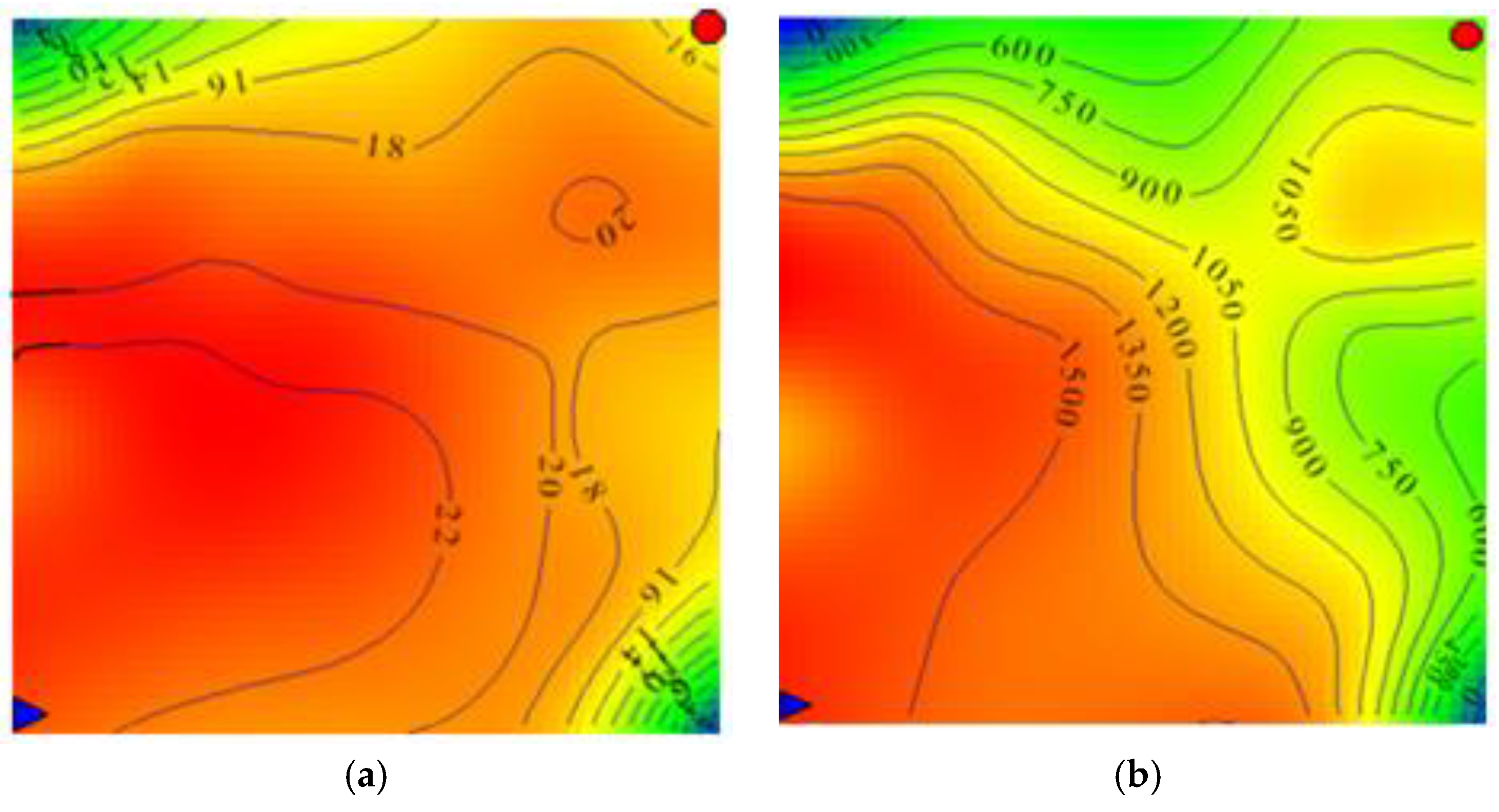
3.1.2. Plane Distribution
 represents the injection well and
represents the injection well and  represents the production well.
represents the production well. 3.2. Present State of Polymers
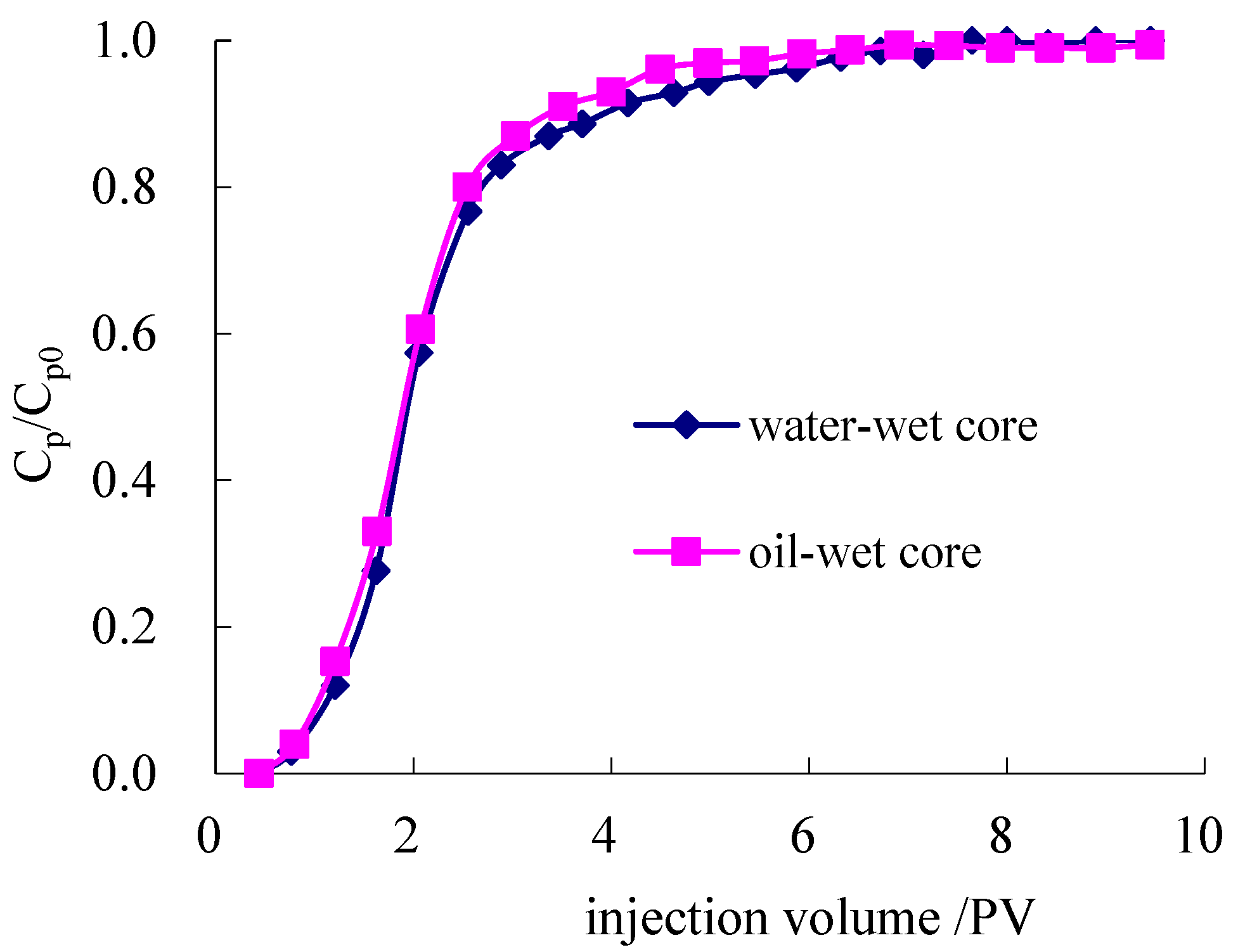
3.2.1. Determination of Inaccessible Pore Volume
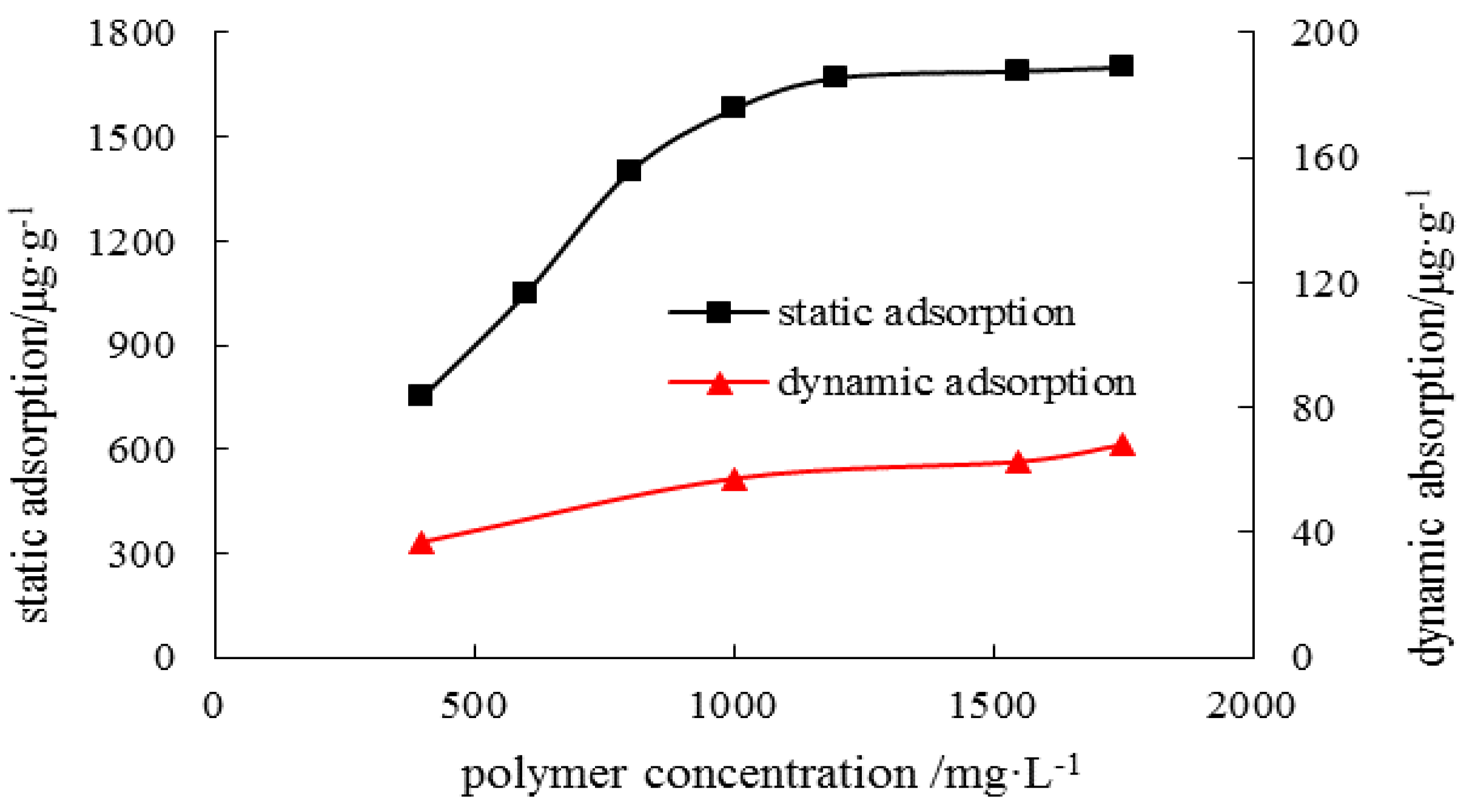
3.2.2. Dynamic Retention Characteristics
Dynamic Retention of the Polymer
Dynamic Adsorption of the Polymer
Dynamic Trapping of the Polymer
Dissolution Amount of Polymer
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Lee, S.; Kim, D.H.; Huh, C.; Pope, G.A. Development of a Comprehensive Rheological Property Database for EOR Polymers. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009. [Google Scholar]
- Seright, R.S. How Much Polymer Should Be Injected during a Polymer Flood? Review of Previous and Current Practices. SPE J. 2016, 1–18. [Google Scholar]
- Huang, B.; Zhang, W.; Xu, R.; Shi, Z.; Fu, C.; Wang, Y.; Song, K. A Study on the Matching Relationship of Polymer Molecular Weight and Reservoir Permeability in ASP Flooding for Duanxi Reservoirs in Daqing Oil Field. Energies 2017, 10, 951. [Google Scholar] [CrossRef]
- Alsofi, A.M.; Blunt, M.J. Polymer flooding design and optimization under economic uncertainty. J. Pet. Sci. Eng. 2014, 124, 46–59. [Google Scholar] [CrossRef]
- Sheng, J.J.; Leonhardt, B.; Azri, N. Optimization of polymer flooding in reservoirs under bottom-water condition to maximize economic profit. J. Can. Pet. Technol. 2015, 54, 116–126. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.; Dai, C.; Ren, S.; Jiao, C. Techniques of enhanced oil recovery after polymer flooding. J. China Univ. Pet. 2006, 30, 86–89. [Google Scholar]
- Fu, X.; Zeng, J.; Gao, W.; Tian, C.; Lin, J. Overview of further improvement of oil recovery technology after polymer flooding. Pet. Geol. Eng. 2010, 24, 88–90. [Google Scholar]
- Feng, Q.; Shi, S.; Fu, X.; Dai, C.; Tang, E. Numerical simulation of the residual polymer reutilization. Acta Pet. Sin. 2010, 31, 801–805. [Google Scholar]
- Zhu, Y.; Cao, X.; Song, X.; Zhang, Z.; Han, Y. Research on polymer distributing law by the three-dimensional anisotropic model. Oil Drill. Prod. Technol. 2006, 28, 39–41. [Google Scholar]
- Xu, J.; Wang, L.; Zhu, K. Concentration distribution and variation in a polymer-flooding reservoir. Tsinghua Univ. Sci. Tech. 2002, 42, 455–457. [Google Scholar]
- Lu, X.; Gao, Z.H.; Zhao, X.J.; Yan, W.H. Distribution Regularities of Remaining Oil in Heterogeneous Reserviors after polymer flooding. Acta Pet. Sin. 1996, 17, 55–58. [Google Scholar]
- Liu, L. The Roles of residual oil Distribution and Measurement of Further Exploitation after Polymer Flooding. Master’s Thesis, Daqing Petroleum Institute, Daqing, China, 2005. [Google Scholar]
- Miao, J.S.; Zhao, F.L.; Li, S.; Yu, Y.H.; Chen, M. Presence and distribution of residual polymer in formation. Spec. Oil Gas Reserv. 2005, 12, 88–90. [Google Scholar]
- Zhang, J.; Lu, S.; Li, J.; Zhang, P.; Xue, H.; Zhao, X.; Xie, L. Adsorption Properties of Hydrocarbons (n-Decane, Methyl Cyclohexane and Toluene) on Clay Minerals: An Experimental Study. Energies 2017, 10, 1577. [Google Scholar] [CrossRef]
- Wan, H.; Seright, R.S. Is Polymer Retention Different under Anaerobic vs. Aerobic Conditions? In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar]
- Zhang, G.; Seright, R.S. Hydrodynamic Retention and Rheology of EOR Polymers in Porous Media. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 13–15 April 2015. [Google Scholar]
- Fulin, Z. Oilfield Chemistry; China University of Petroleum Press: Dongying, China, 2000; pp. 105–149. [Google Scholar]
- Bhupendra, N.; Lawrence, G.; Willhite, P.; Green, D.W. The effect of inaccessible pore volume on the flow of polymer and solvent through porous media. In Proceedings of the SPE Annual Fall Technical Conference and Exhibition, Houston, TX, USA, 1–3 October 1978. [Google Scholar]
- Hu, B. Polymer Flooding Petroleum Production Engineering; Petroleum Industry Press: Beijing, China, 1997; pp. 27–28. [Google Scholar]
- Li, J.; Liu, Y.J.; Guo, S.L.; Li, B.-H. A method for calculating inaccessible pore volume of polymer. Pet. Geol. Oilfield Dev. Daqing 2008, 27, 114–117. [Google Scholar]
- Tang, E.; Zhang, X.; Yang, J. The effect of inaccessible pore volume on the flow of polymer solution in porous media. Pet. Geol. Recovery Effic. 2009, 16, 80–82. [Google Scholar]
- Cheng, T.; Pu, W. Polymer Evaluation Methods; Petroleum Industry Press: Beijing, China, 1997; p. 107. [Google Scholar]
- Manichand, R.N.; Seright, R. Field vs. Laboratory Polymer Retention Values for a Polymer Flood in the Tambaredjo Field. SPE Reserv. Eval. Eng. 2014, 17, 314–325. [Google Scholar] [CrossRef]
- Di, Q.; Shen, C.; Wang, Z.H.; Gu, C.; Shi, L.; Fanf, H. Experimental research on drag reduction of flow in micro channels of rocks using nano-particle adsorption method. Acta Pet. Sin. 2009, 30, 125–128. [Google Scholar]
- Zhang, G.; Seright, R.S. Effect of Concentration on HPAM Retention in Porous Media. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–2 October 2014. [Google Scholar]
- Shu, L.; Liu, J.; Lv, X.; Guo, Y. Measurement optimization of polymer concentration of polyacrymide by the starch-cadmium iodine method. Appl. Chem. Ind. 2010, 39, 1766–1782. [Google Scholar]
- Jing, G.-L.; Li, Y.-Q.; Yang, J. Spectrophotometric determination of concentration of ammonium. Thiocyanate Tracer Oilfield Prod. Water 2000, 17, 191–192. [Google Scholar]
- Li, H.-B. Characteristic Static Adsorption of Hydrophobically Associating Polymer AP from Aqueous Solution. Oilfield Chem. 2006, 23, 349–351. [Google Scholar]
- Broseta, D.; Medjahed, F.; Lecourtier, J.; Robin, M. Polymer adsorption/retention in porous media: Effects of core wettability on residual oil. SPE Adv. Technol. Ser. 1995, 3, 103–112. [Google Scholar] [CrossRef]



| VIP | Polymer Breakthrough Time/Month | Recovery When Water Cut at 95%/% | The Additional Recovery/% |
|---|---|---|---|
| 0 | 79.7 | 44.55 | 20.35 |
| 0.15 | 67.1 | 37.83 | 13.63 |
| 0.25 | 58.3 | 33.34 | 9.14 |
| 0.35 | 50.5 | 28.85 | 4.65 |
| 0.45 | 42.3 | 24.37 | 0.17 |
| Composition | Na+, K+ | Ca2+ | Mg2+ | CO32− | HCO3− | SO42− | Cl− | Total Salinity |
|---|---|---|---|---|---|---|---|---|
| content/(mg/L) | 3091.96 | 276.17 | 158.68 | 14.21 | 311.48 | 85.29 | 5436.34 | 9374.12 |
| Experiment | Artificial Core | Chemicals and Materials |
|---|---|---|
| Three Parallel Core Experiments | Three cylindrical cores (sectional area, 4.9 cm2; length, 9.8 cm). The water permeability of cores is 300 × 10−3 μm2, 900 × 10−3 μm2 and 2700 × 10−3 μm2 | polymer, constant flux pump, core holder, Brookfield DV-II rotational viscometer, pressure sensor(Haian County Petroleum Science and Technology Co., Ltd., Haian, China), precise pressure gauge, graduate, cylinder, oil tank, et al. |
| Three-Dimensional Slab Model Experiment | The slab model (the model parameter is shown in Table 4. | polymer, constant flux pump, core holder, Brookfield DV-II rotational viscometer (DV-II+, Brokfield, WI, USA), pressure sensor, precise pressure gauge, graduate, et al. |
| Polymer Concentration Determination Method | polymer, sodium acetate, cadmium iodide, hydrated aluminum sulfate, saturated bromine water, soluble starch, acetic acid, sodium formate, UV-2100 spectrophotometer, BS423S millesimal balance (BS423S, Shanghai Precision Scientific Instruments Co., Ltd., Shanghai, China), et al. | |
| Potassium Thiocyanate Tracer Determination Method | tracer (potassium thiocyanate), sodium formate, nitric acid, ammonium ferrous sulfate, UV-2100 spectrophotometer (UV-2100, unico Shanghai, China), BS423S millesimal balance, et al. | |
| Static-Equilibrium Adsorption | polymer, sand, temperature controlled bath, conical flask, the chemicals and materials used in Polymer Concentration Determination Method et al. | |
| Double-Slug Experiments | Two cylindrical core (sectional area, 4.9 cm2; and length,9.8 cm), and their gas permeability is 2500 × 10−3 μm2 | polymer, tracer (potassium thiocyanate), the chemicals and materials used in Polymer Concentration Determination Method and Potassium Thiocyanate Tracer Determination Method. constant flux pump, core holder, BS423S millesimal balance, Brookfield DV-II rotational viscometer, pressure sensor, precise pressure gauge, graduate, et al. |
| Gas Permeability/×10−3 μm2 | Size/cm | Porosity/% | Soii/% |
|---|---|---|---|
| 2500 | 59.8 × 59.2 × 4.58 | 30.4 | 73.1 |
| Core | K/×10−3 μm2 | PV/mL | φ/% | Polymer Injection Volume/mL | Injection Volume Percentage/% |
|---|---|---|---|---|---|
| high permeable core | 2700 | 15.4 | 32.3 | 17.2 | 68.3 |
| medium permeable core | 900 | 14.8 | 30.8 | 6.6 | 26 |
| low permeable core | 300 | 14.1 | 29.4 | 1.5 | 5.7 |
| Length/cm | Cross-Sectional Area/cm2 | Gas Permeability/10−3 μm2 | Water Permeability/10−3 μm2 | Dry Weight/g | Wet Weight/g | Pore Volume/mL | Porosity/% |
|---|---|---|---|---|---|---|---|
| 9.45 | 4.52 | 2484 | 1600 | 71.48 | 83.98 | 12.5 | 29.23 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Fan, H.; You, Q.; Jia, Y. Distribution and Presence of Polymers in Porous Media. Energies 2017, 10, 2118. https://doi.org/10.3390/en10122118
Zhao J, Fan H, You Q, Jia Y. Distribution and Presence of Polymers in Porous Media. Energies. 2017; 10(12):2118. https://doi.org/10.3390/en10122118
Chicago/Turabian StyleZhao, Juan, Hongfu Fan, Qing You, and Yi Jia. 2017. "Distribution and Presence of Polymers in Porous Media" Energies 10, no. 12: 2118. https://doi.org/10.3390/en10122118
APA StyleZhao, J., Fan, H., You, Q., & Jia, Y. (2017). Distribution and Presence of Polymers in Porous Media. Energies, 10(12), 2118. https://doi.org/10.3390/en10122118





