Microcosmic Mechanism Investigation on Lightning Arc Damage of Wind Turbine Blades Based on Molecular Reaction Dynamics and Impact Current Experiment
Abstract
:1. Introduction
2. Molecular Simulation of Material Arc Damage
2.1. Modeling and Simulation of Balsa Wood and PVC
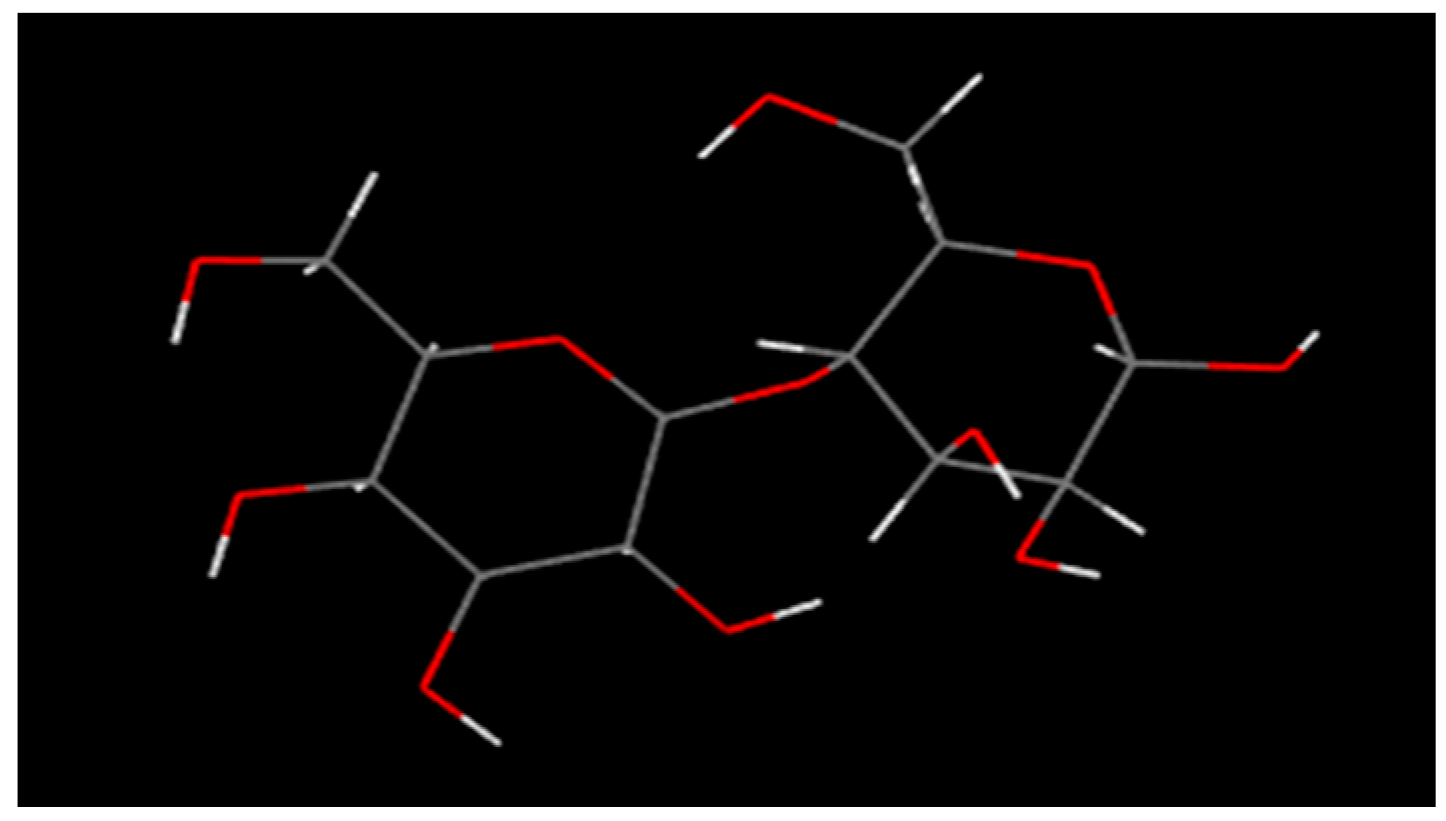
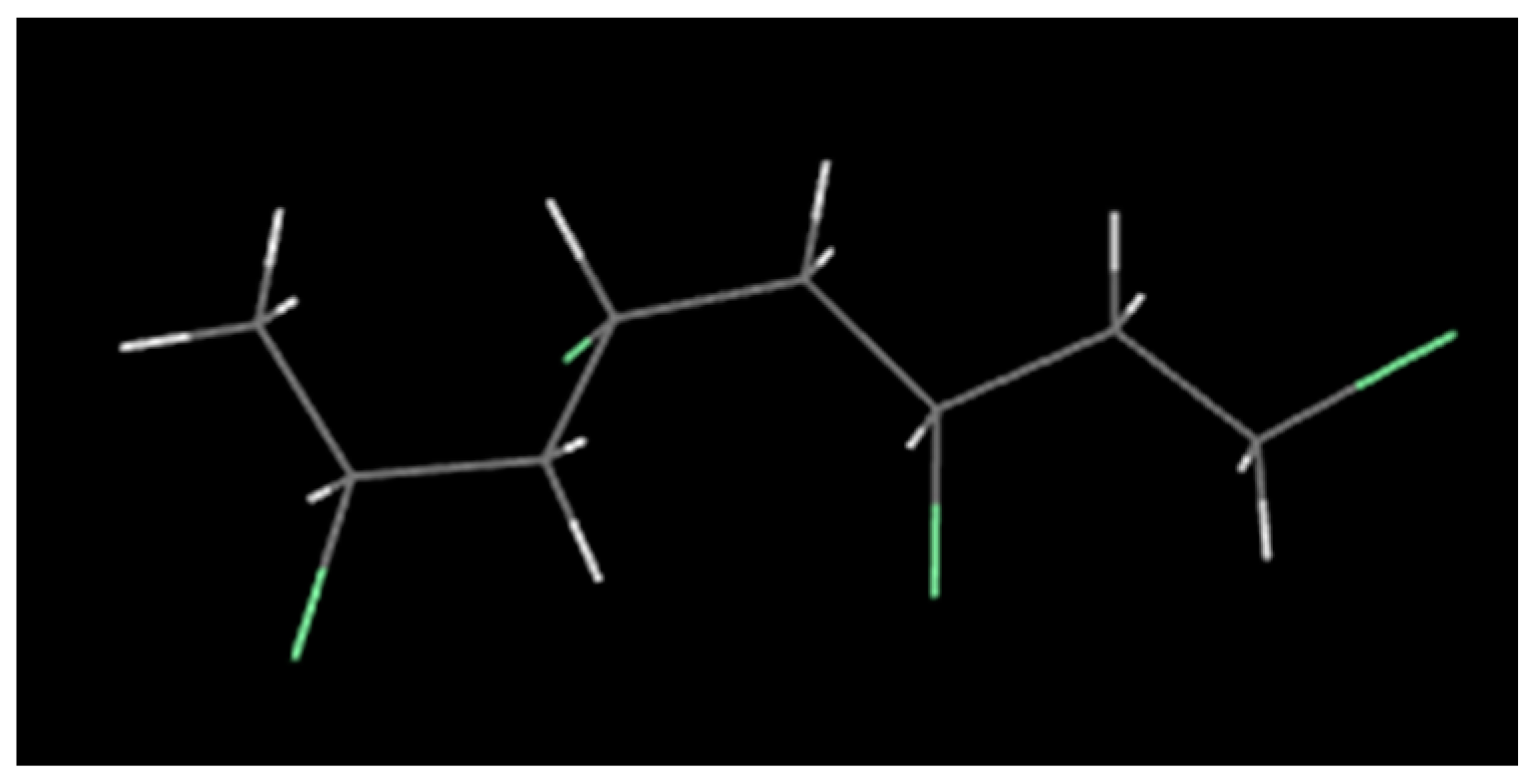
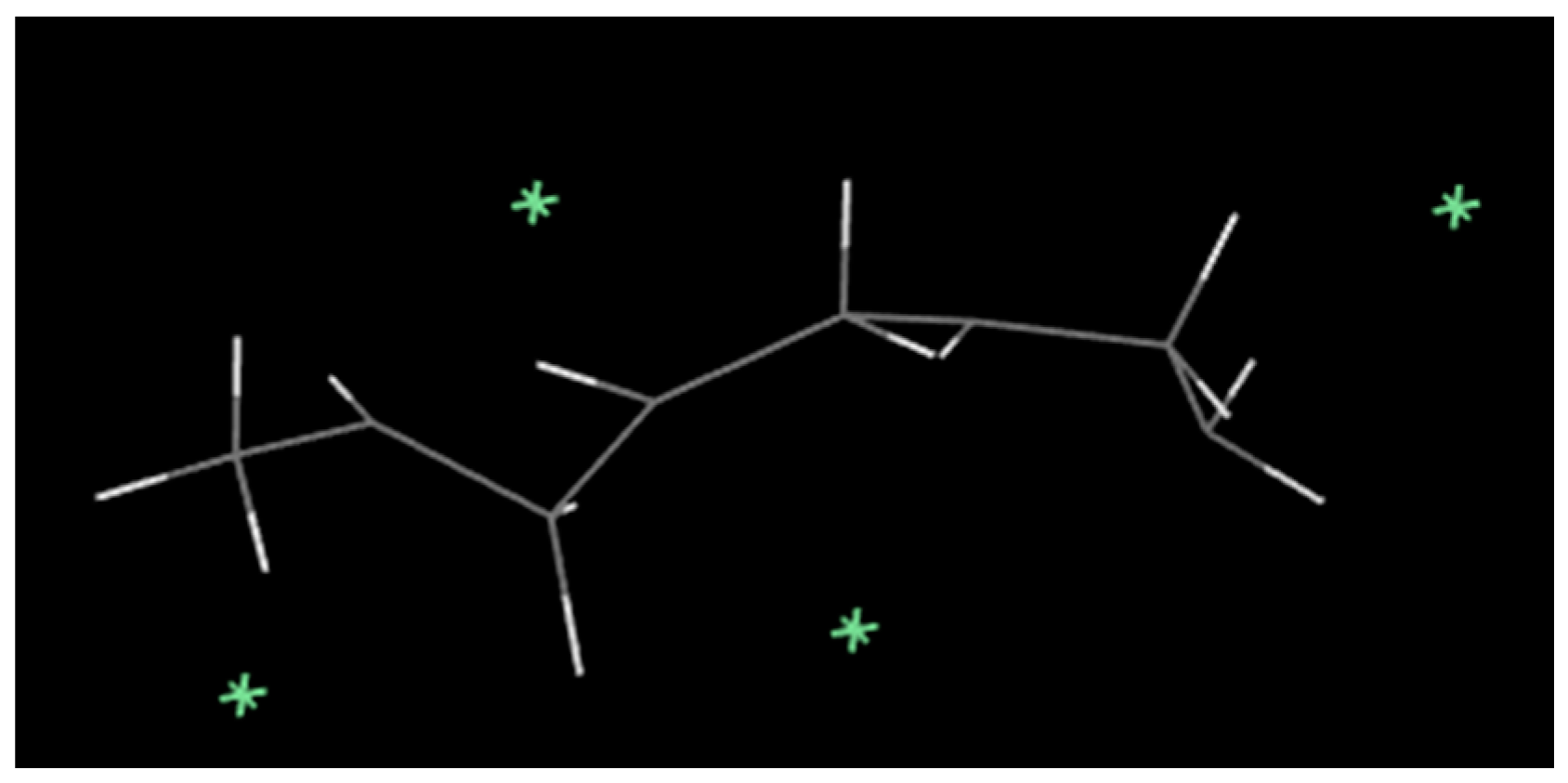
2.1.1. Molecular Model of PVC and Balsa Wood
2.1.2. Simulation Process
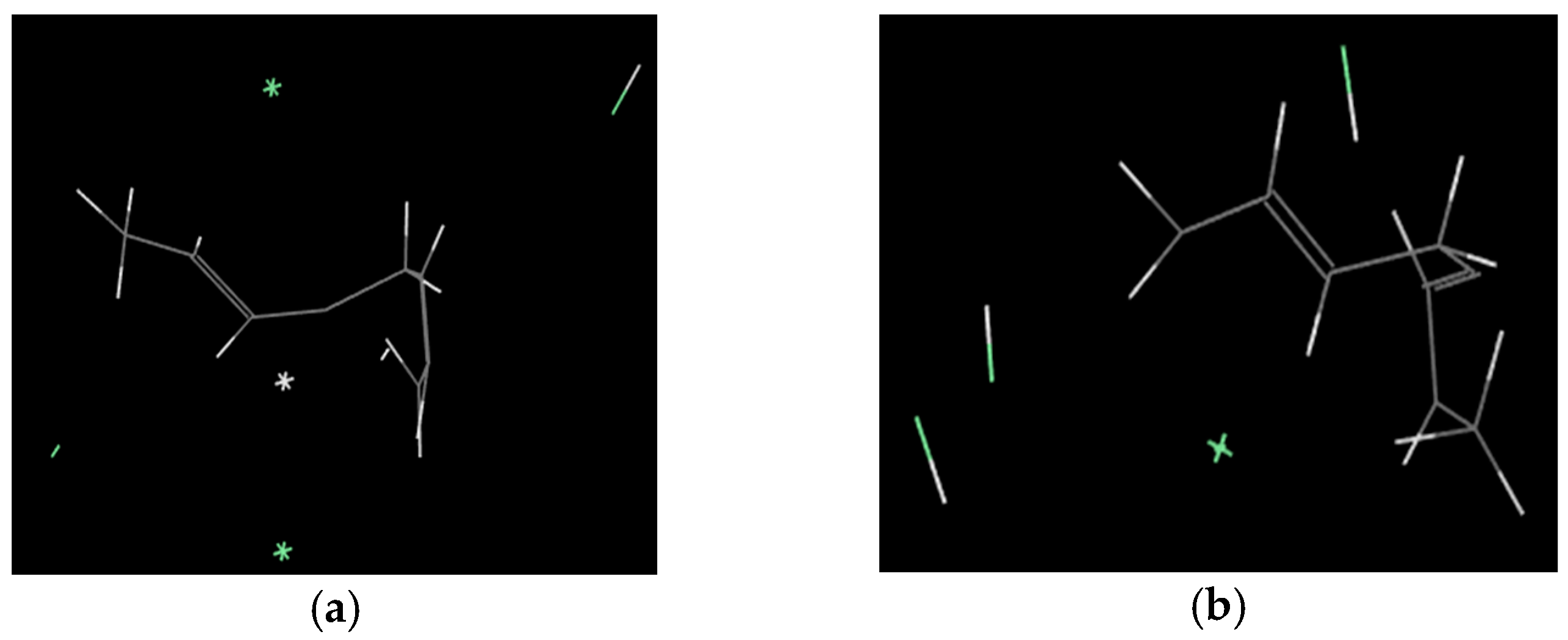
2.2. Simulation Results
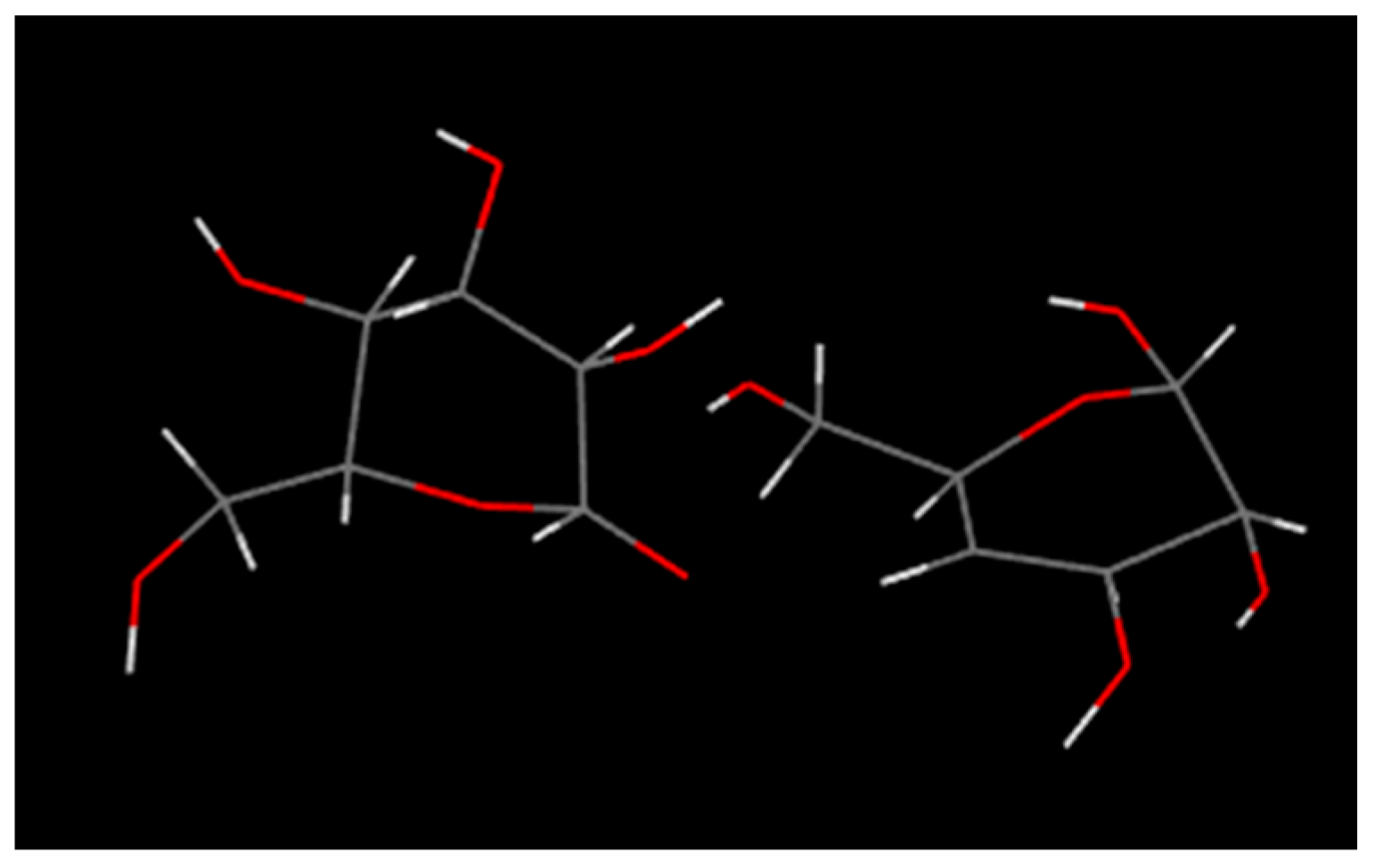
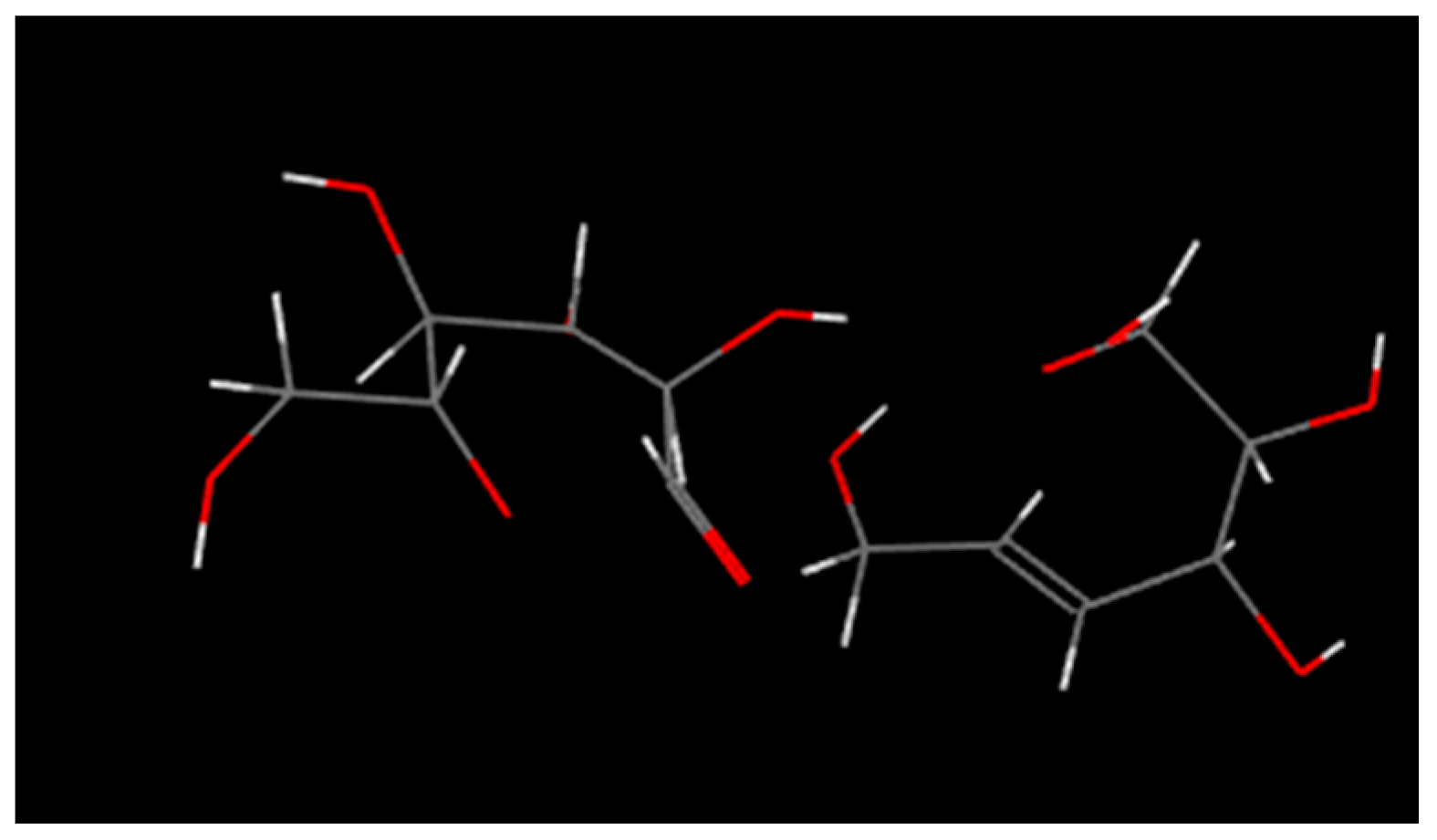
2.2.1. The Mechanism of Pyrolysis Process
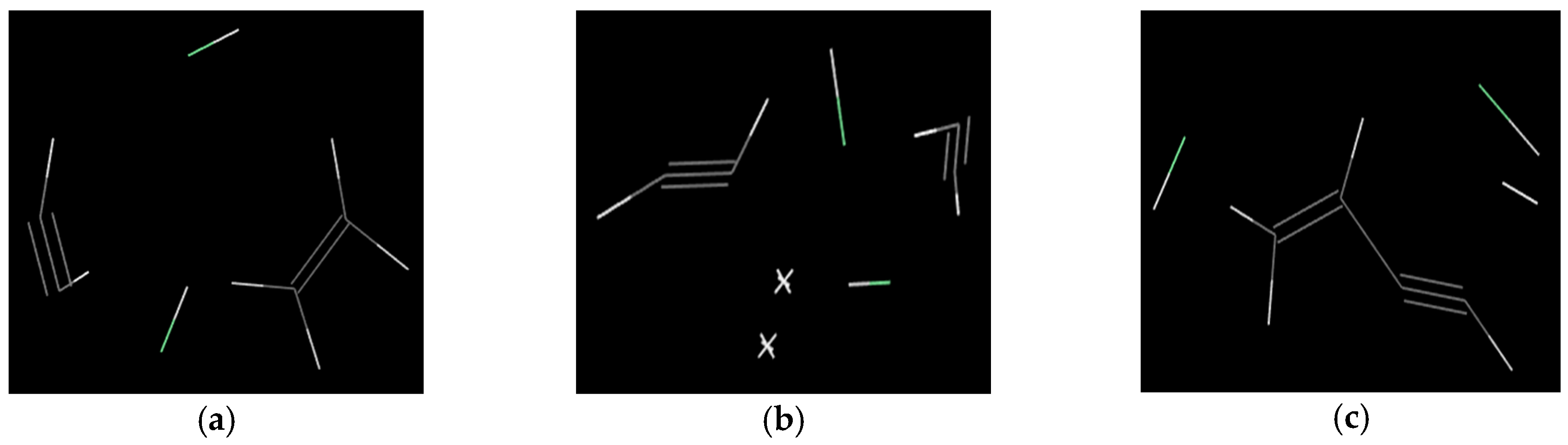
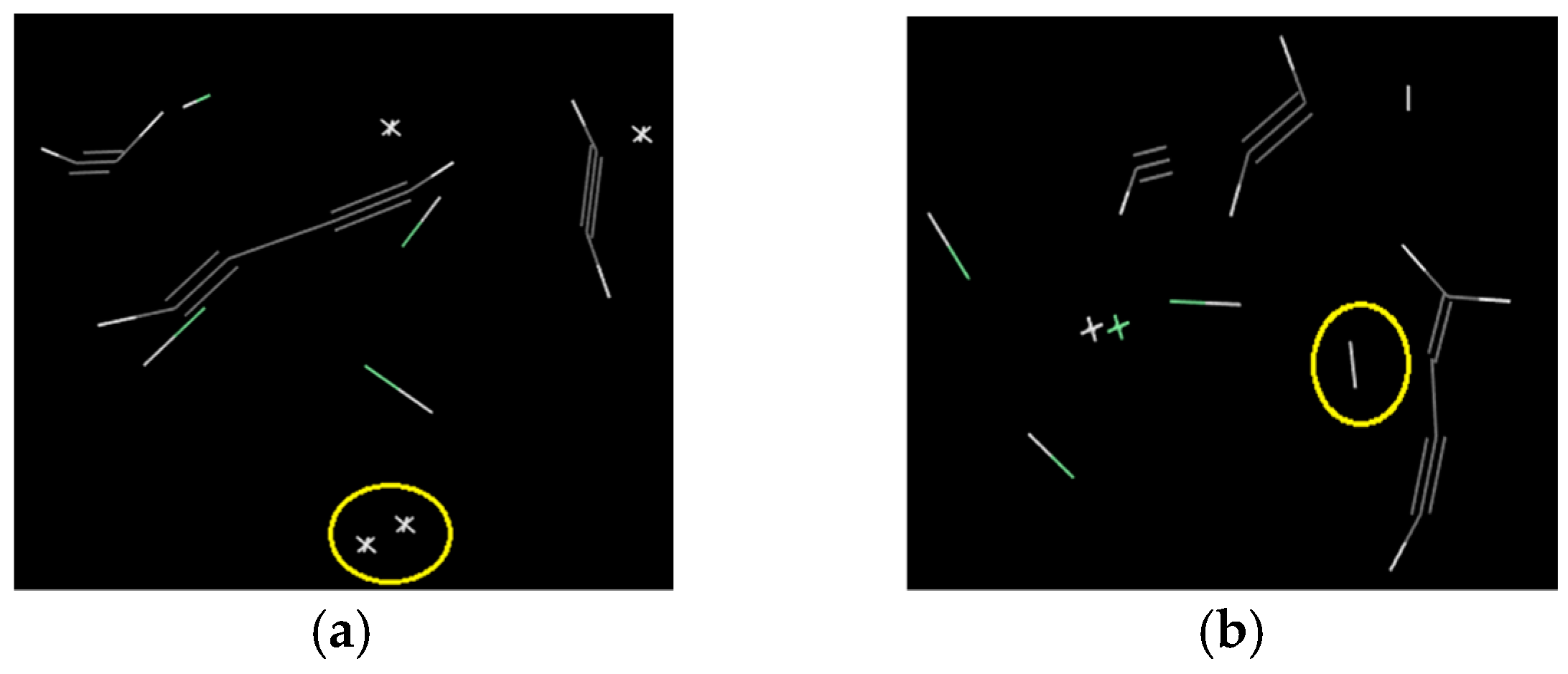
2.2.2. The Main Pyrolysis Gas Products and the Generation Mechanism
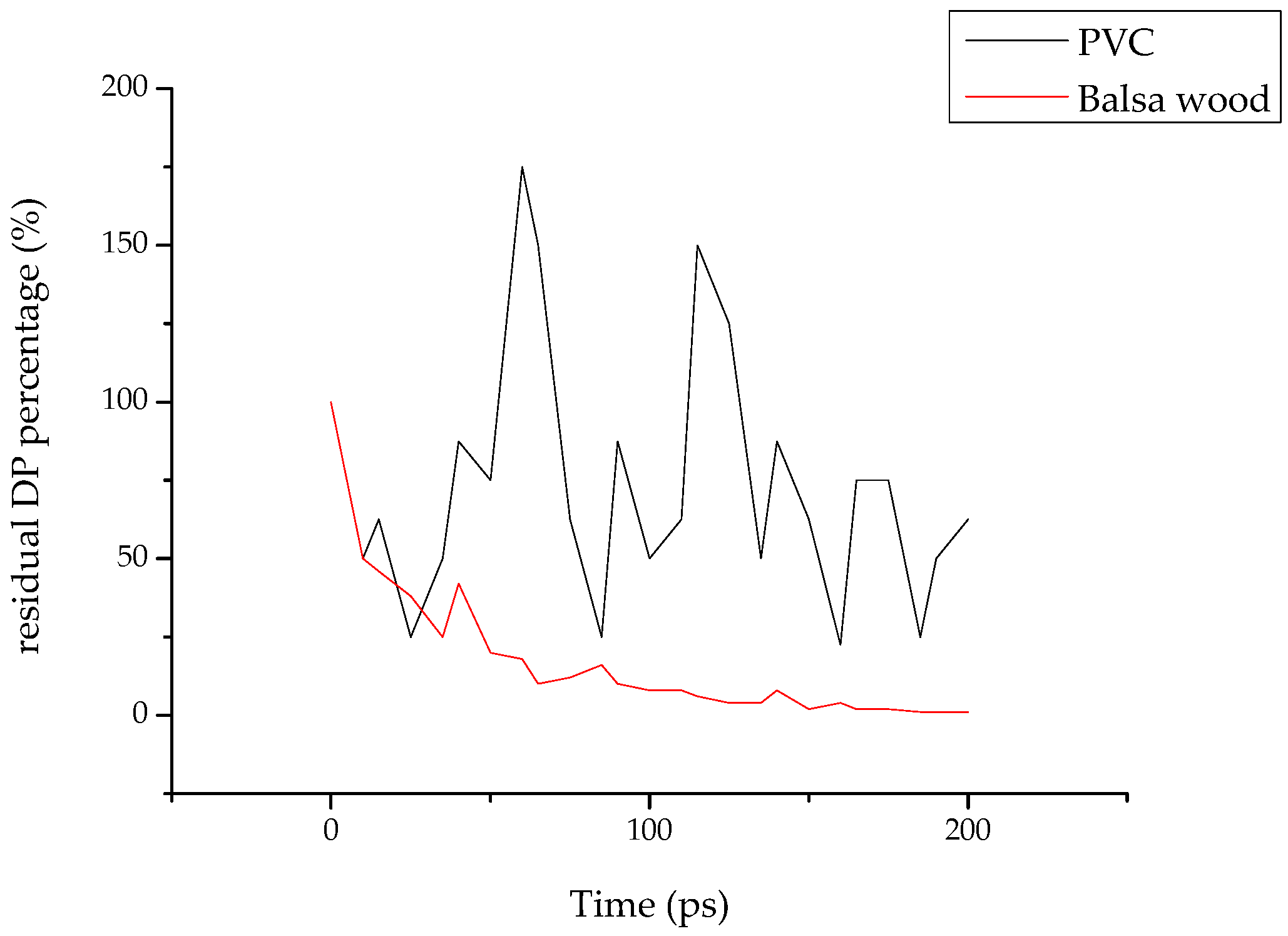
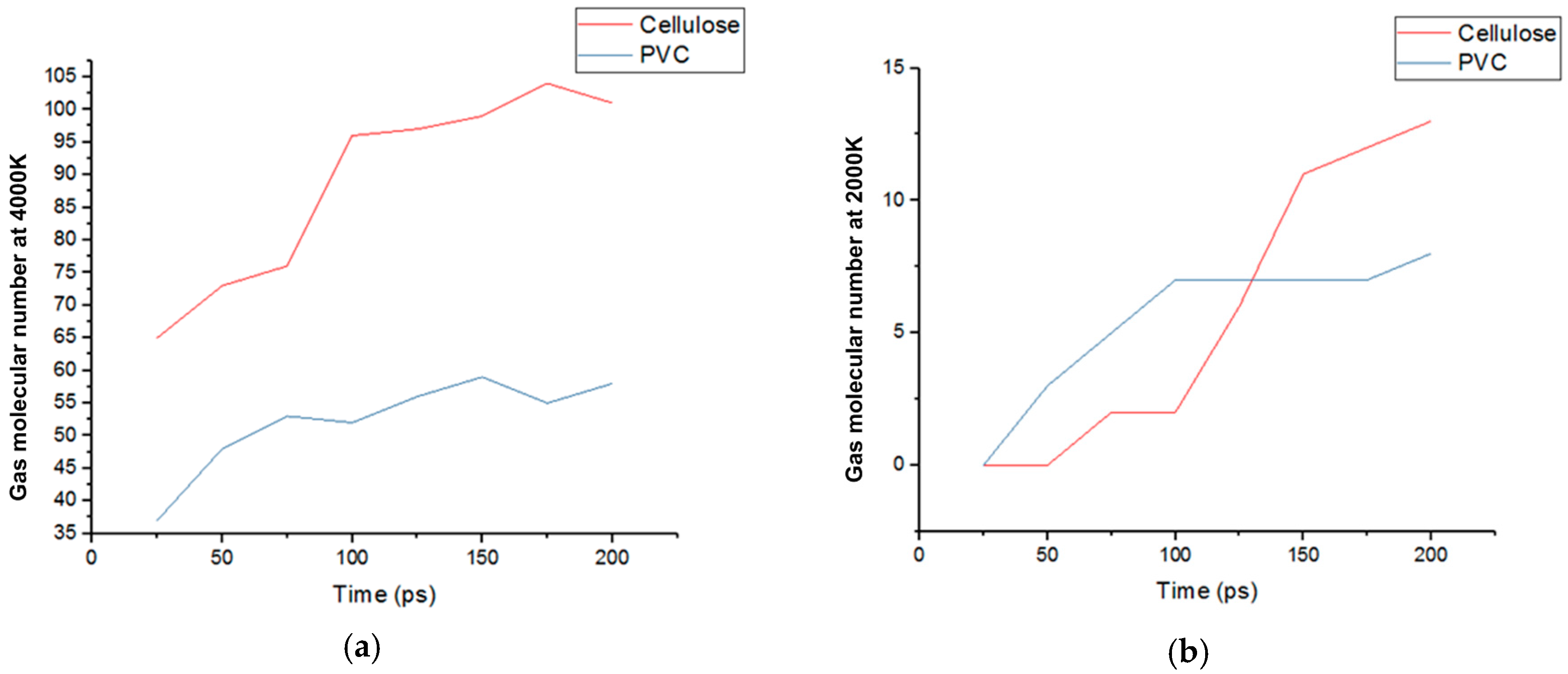
2.2.3. Quantitative Comparison of the Gas Products of Cellulose and PVC
3. Impact Current Experiments
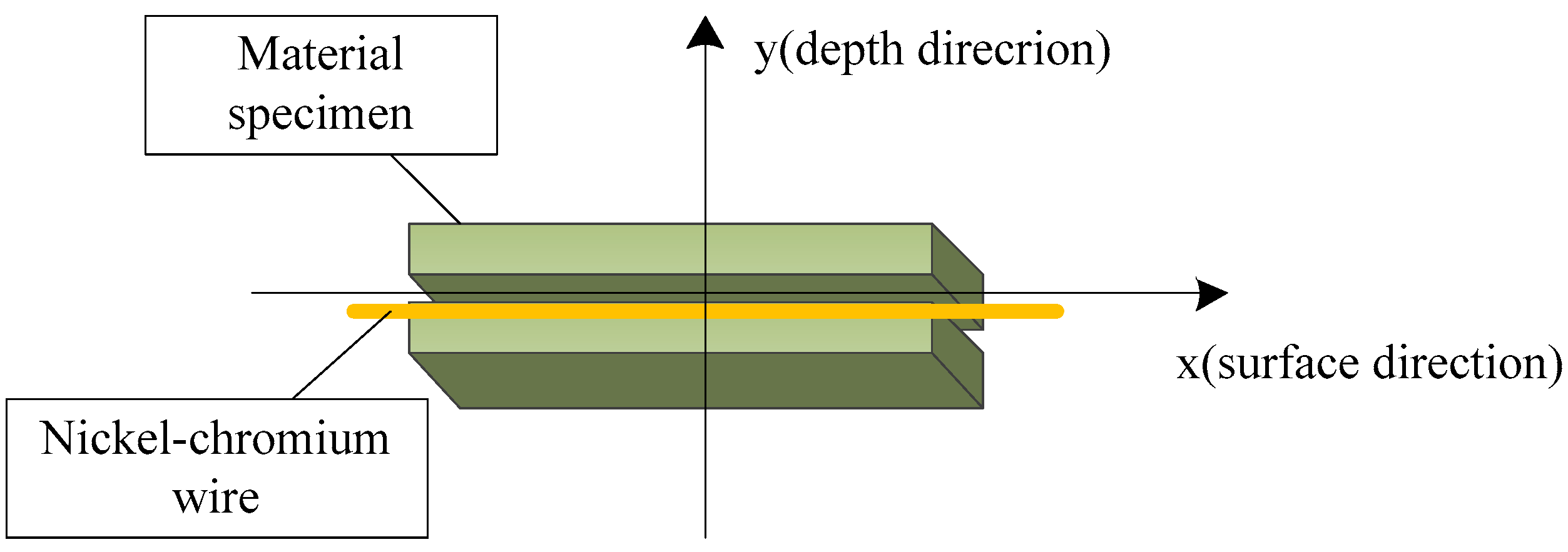
3.1. Experimental Methods
3.2. Experimental Results
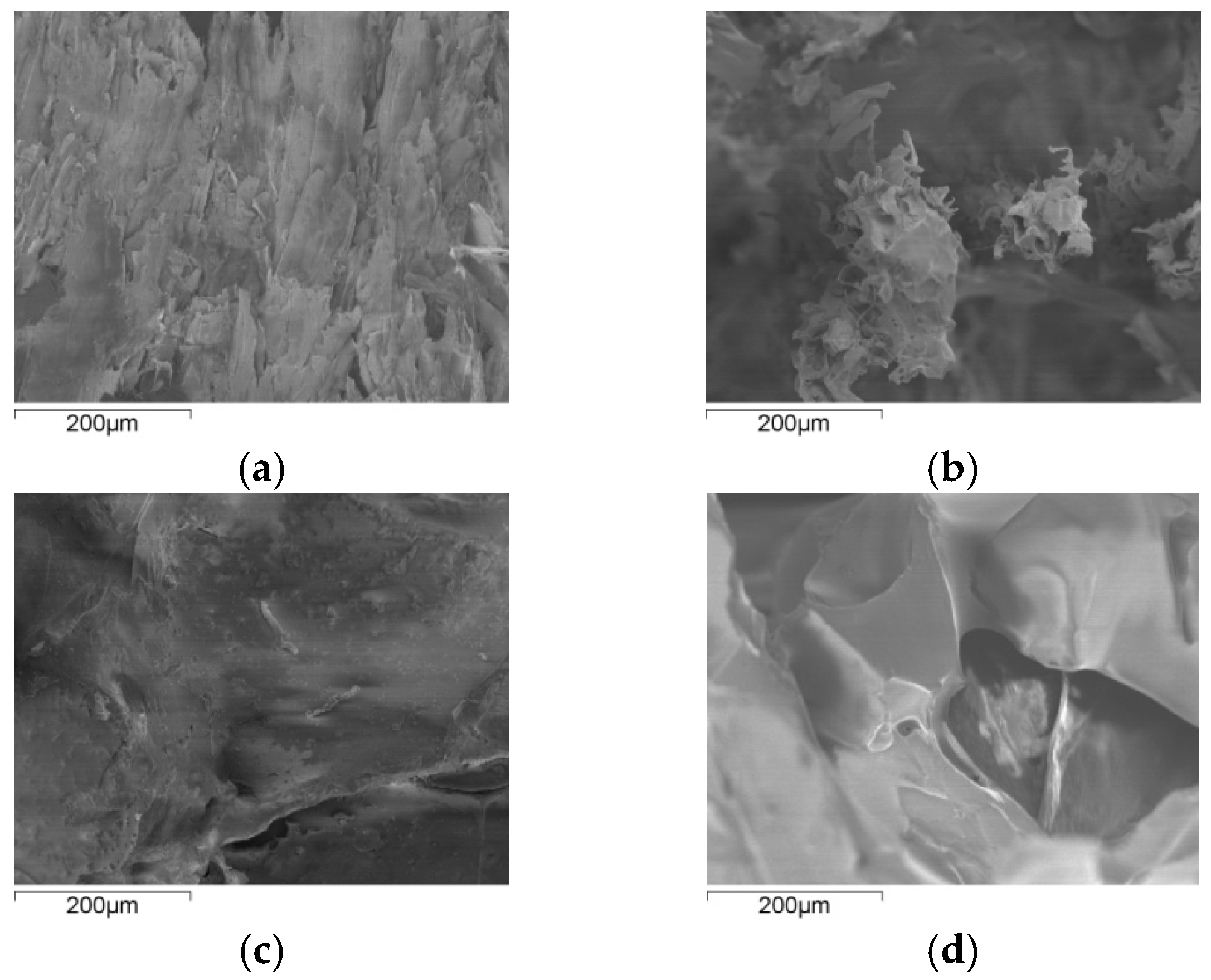
3.2.1. Damage Morphology of the Two Materials
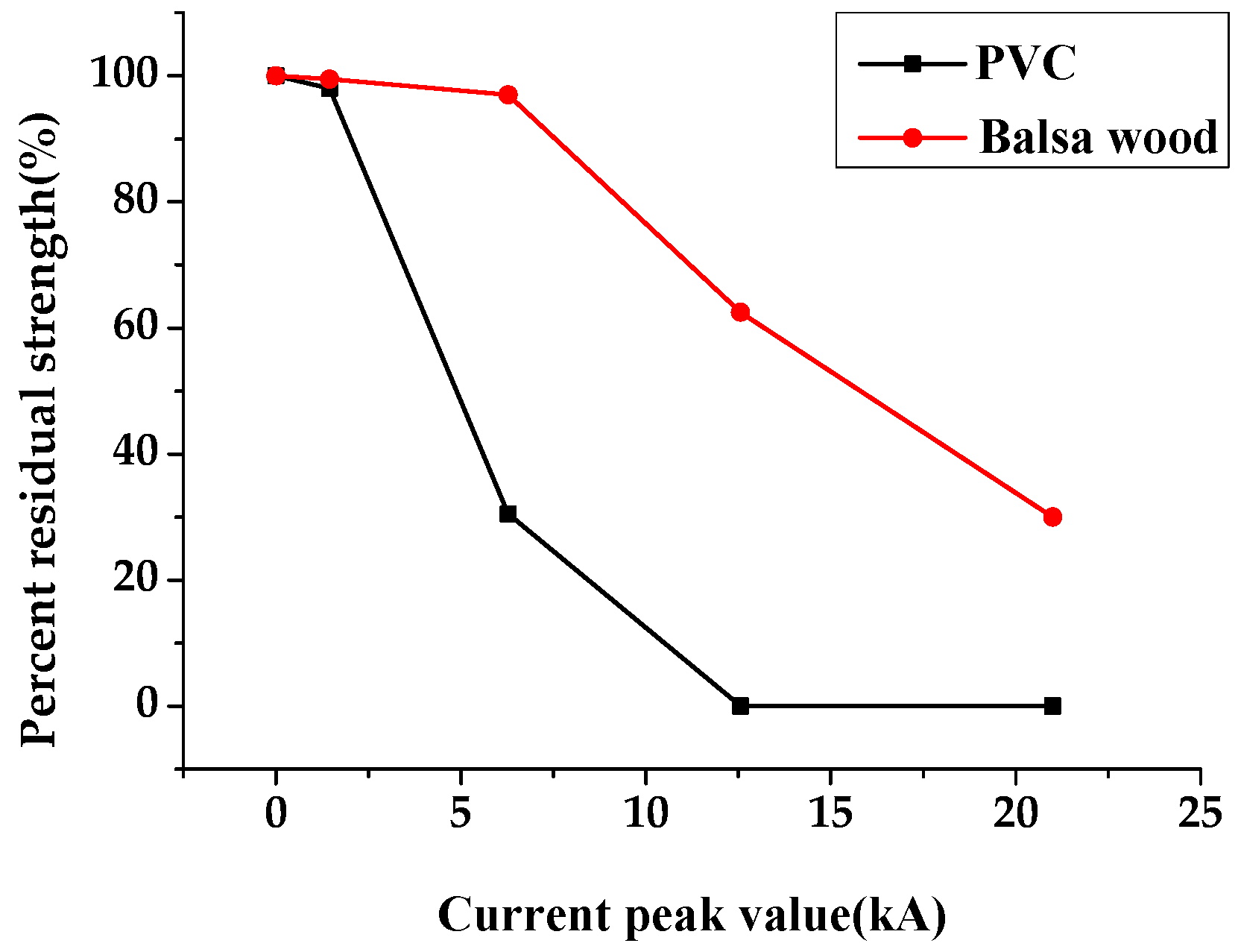
3.2.2. Damage Degree of the Two Materials
4. The Comparison of Balsa Wood and PVC
- The pyrolysis temperature of PVC was found lower than that of balsa wood. According to the pyrolysis simulation of the two materials at different temperatures, the release of HCl in PVC occurred earlier than glycosidic bond breaks in balsa wood at the same temperature, so that the heat resistance of balsa wood was better than that of PVC. The impact experiments also showed that the PVC was damaged at a lower current than balsa wood. In addition, PVC softened easily at high temperature because its glass transition temperature is low (78–81 °C), and the ultraviolet light and oxygen could also cause photooxidation of PVC, causing the flexibility of PVC to decrease, thus in the strong light and high temperature areas balsa wood resulted better than PVC as blade material.
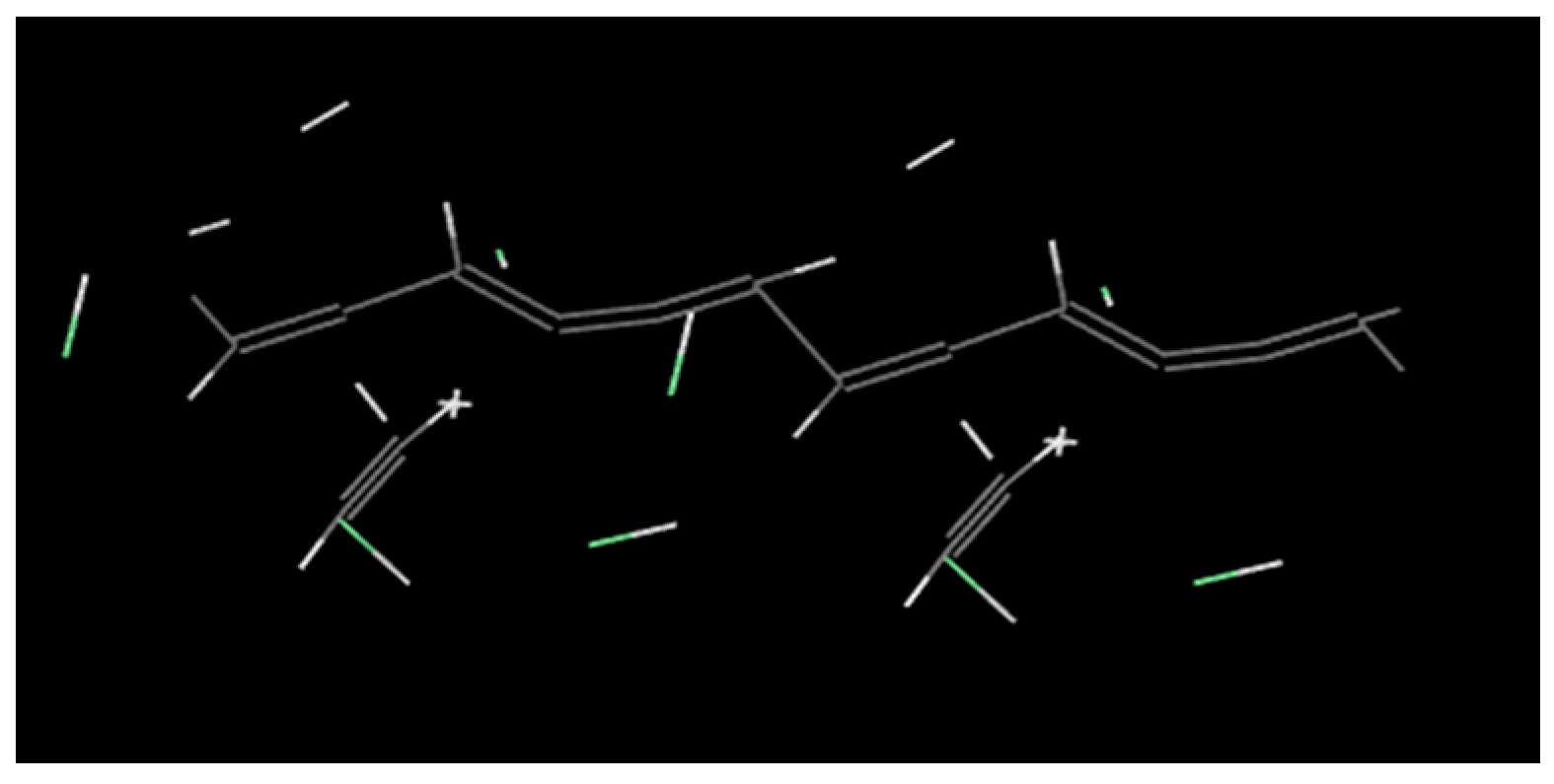
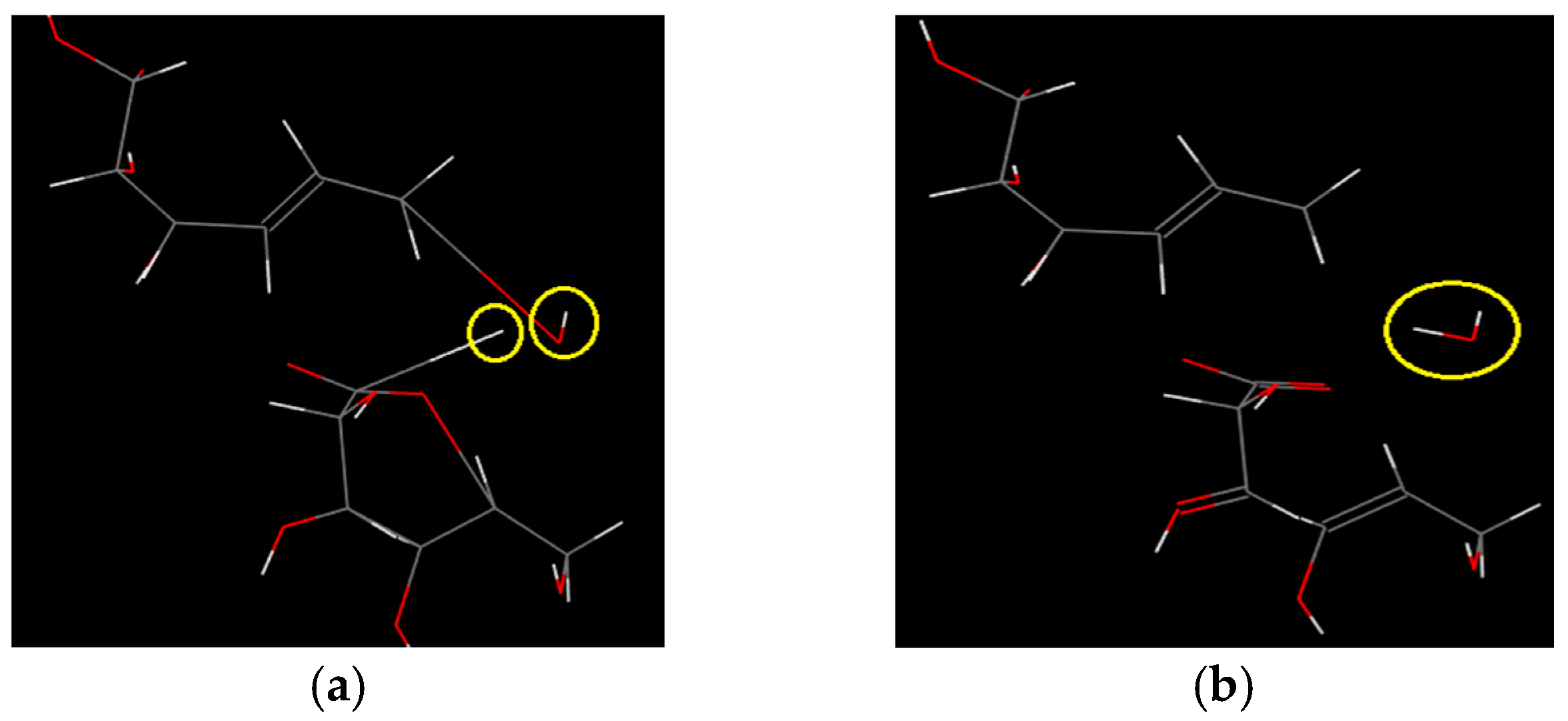
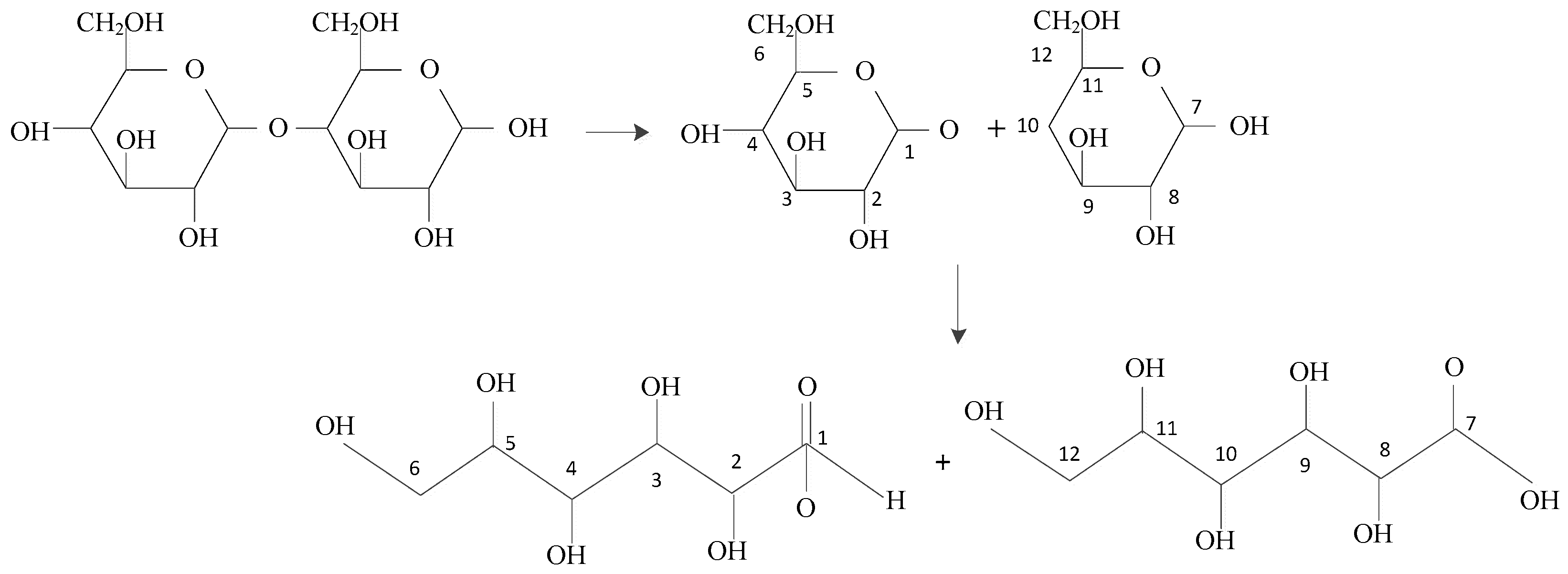
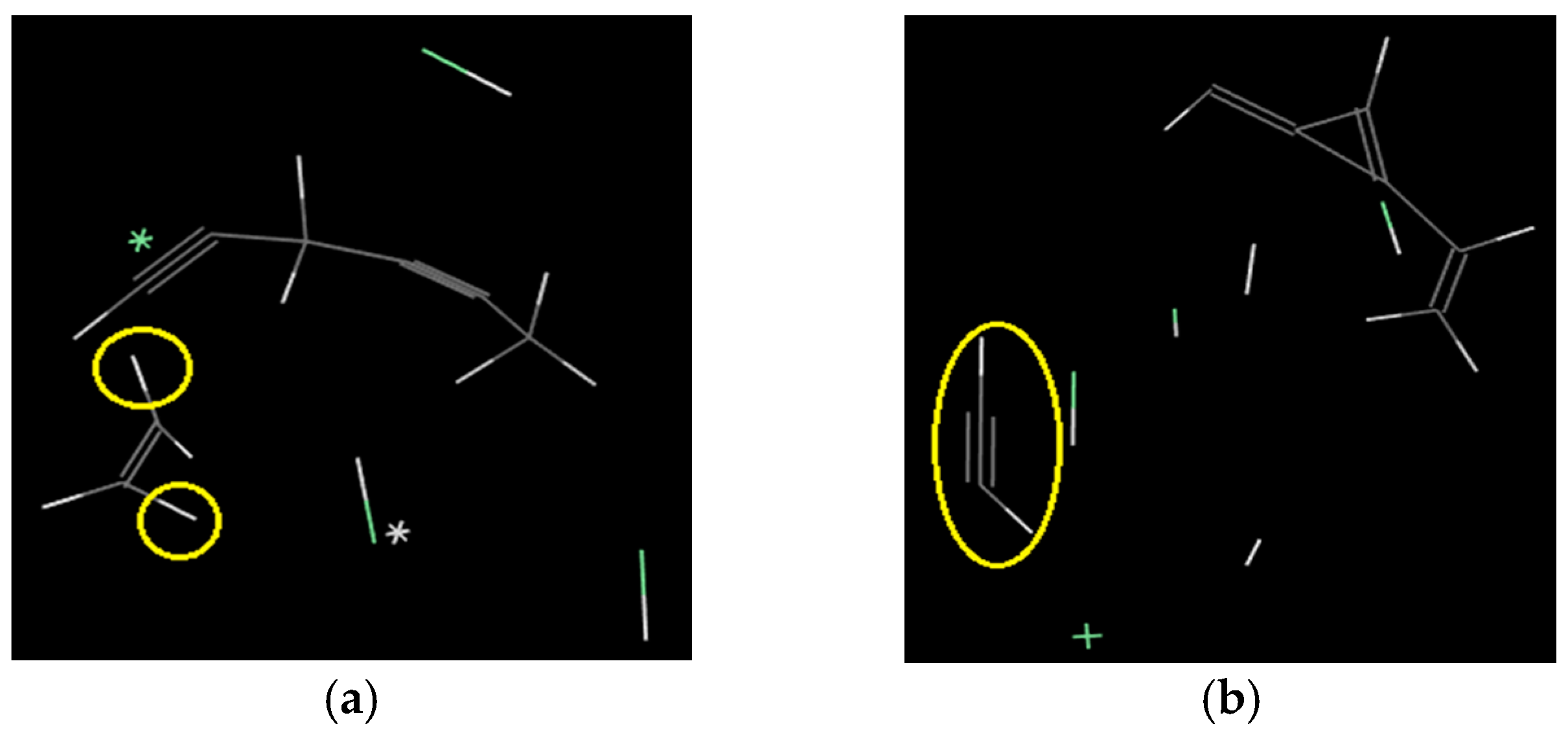
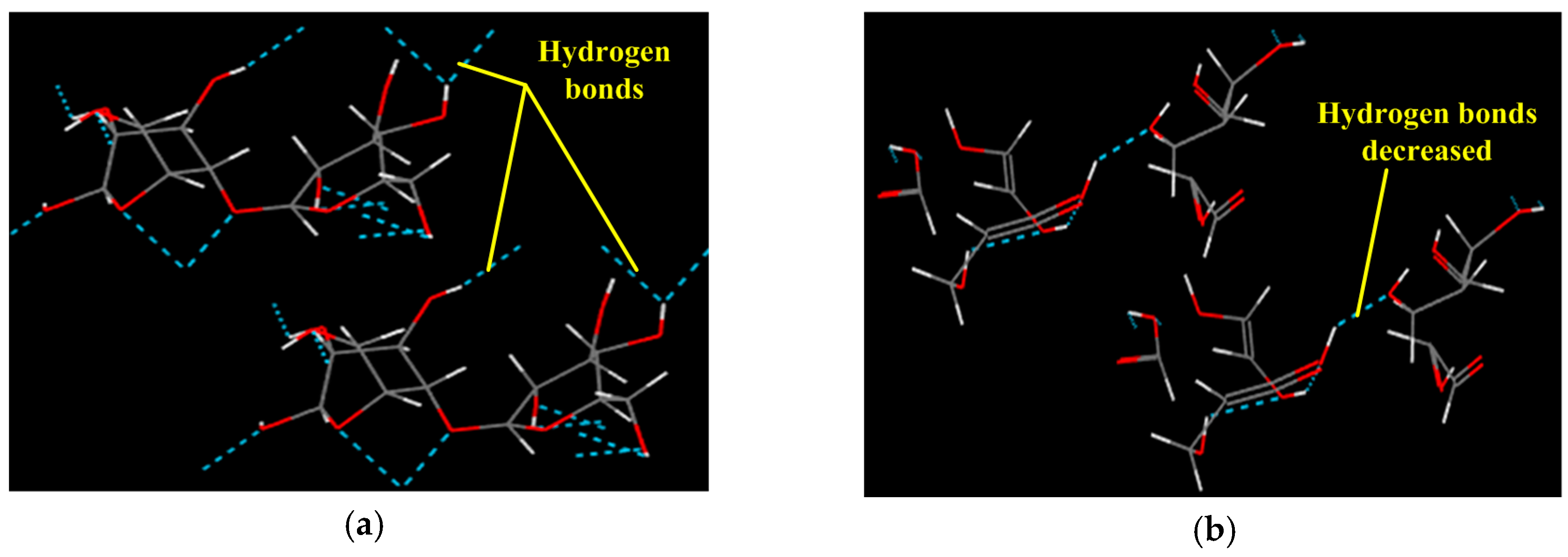
- The rigidity of cellulose decreased greatly during pyrolysis. The cellulose has stronger rigidity compared with PVC. Firstly the molecular polarity of cellulose made the interaction between the molecular chains stronger; secondly the hexagonal pyranoid rings in the molecule made the chain rotation difficult; thirdly there were a lot of hydrogen bonds in the molecules or between molecules, especially intramolecular hydrogen bonds, making the glycosidic bonds unable to rotate, so the rigidity of balsa wood is greatly increased. However, it could be seen from the pyrolysis of cellulose that the glycosidic bonds broke during the pyrolysis process, the DP decreased sharply, the pyranoid ring structure was broken, and the intramolecular hydrogen bonds were changed, as shown in Figure 21, so the molecular chain structure was destroyed, and thus the rigidity of the cellulose was greatly reduced.
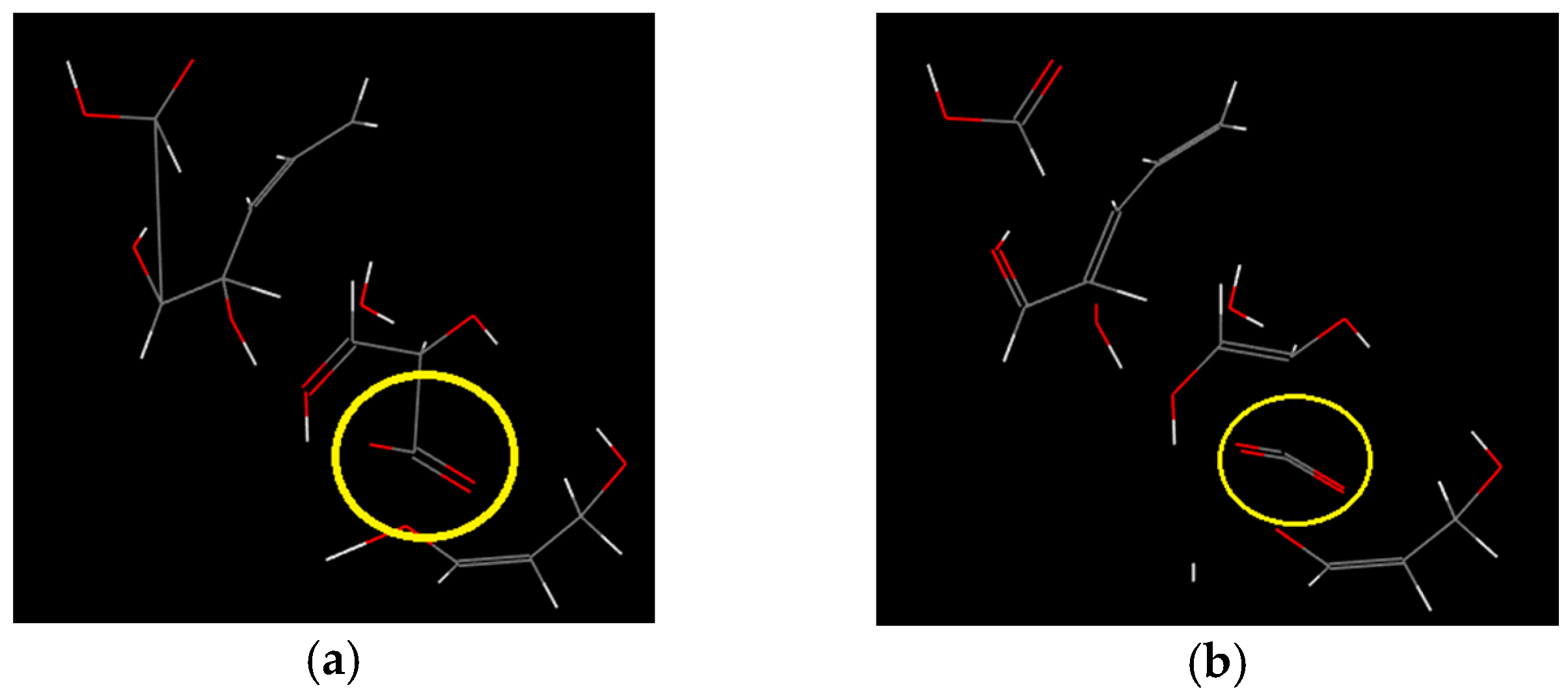
- The decomposition of cellulose was mainly due to the breakage of the chains, the decrease of DP, and formation of a lot of gaseous products, leading to macroscopic fiber fractures and missing materials. The decomposition of PVC was mainly shown in terms of release of HCl gas, and other small gas molecules would be generated in further reaction, and the molecular chains broke and reconnected constantly. Considering its low softening temperature, the damage of PVC was mainly shown as pore extension on a macroscopic level.
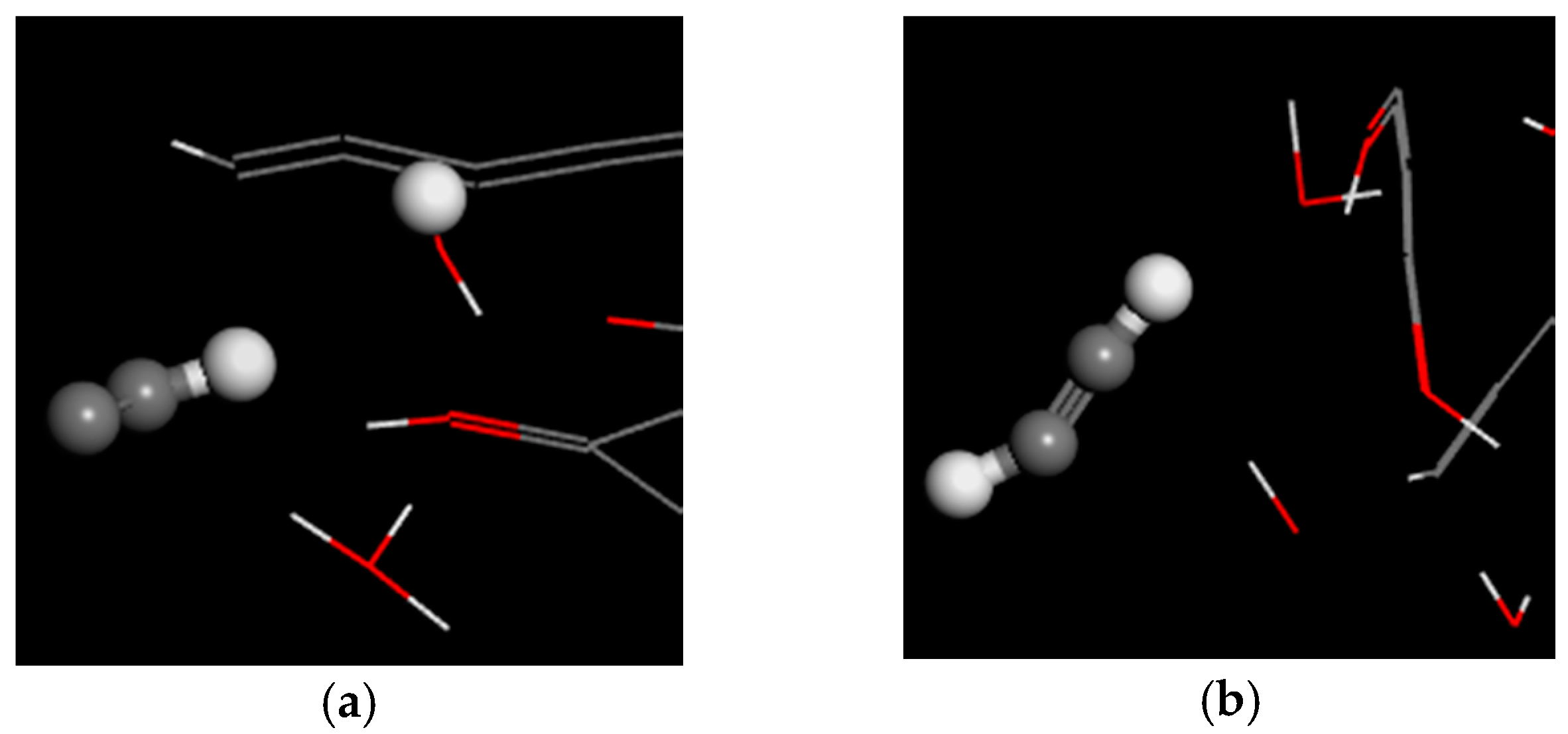
- The main air products of balsa wood in the simulation were CO, H2O, H2 and C2H2. Those of PVC were HCl, H2, and C2H2. The gas product expansion accelerated the fiber fracture in balsa wood and the pore extension in PVC. According to the simulation results, the gas products of balsa wood were much more plentiful than those of PVC, so the material damage caused by gas products was more serious in balsa wood. In addition, because the cellulose in the balsa wood can generate hydrogen bonds with the water molecules in the surrounding environment, the moisture content of balsa wood is much higher than that of PVC. On the one hand, the water molecules could promote the pyrolysis of the cellulose; on the other hand, the combined water would evaporate at high temperature, which could also exacerbate balsa wood damage, so PVC was better than balsa wood as a turbine blade material in humid areas (such as those where offshore wind turbines are installed).
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rachidi, F.; Rubinstein, M.; Montanya, J.; Bermudez, J.L.; Sola, R.R.; Sola, G.; Korovkin, N. A Review of Current Issues in Lightning Protection of New Generation. IEEE Trans. Ind. Electron. 2008, 55, 2489–2496. [Google Scholar] [CrossRef]
- Cotton, I.; Jenkins, N.; Pandiaraj, K. Lightning protection for wind turbine blades and bearings. Wind. Energy 2001, 4, 23–37. [Google Scholar] [CrossRef]
- Piwko, R.; Miller, N.; Sanchez-Gasca, J.; Yuan, X.; Dai, R.; Lyons, J. Integrating large wind farms into weak power grids with long transmission lines. In Proceedings of the CES/IEEE 5th International Power Electronics and Motion Control Conference (IPEMC 2006), Shanghai, China, 14–16 August 2006; IEEE: Piscataway, NJ, USA, 2006; pp. 1–7. [Google Scholar]
- Tsai, K.C.; Pan, C.T.; Cooperman, A.; Johnson, S.; van Dam, C. An Innovative Design of a Microtab Deployment Mechanism for Active Aerodynamic Load Control. Energies 2015, 8, 5885–5897. [Google Scholar] [CrossRef]
- Fernandez-Gamiz, U.; Zulueta, E.; Boyano, A.; Ramos-Hernanz, J.A.; Lopez-Guede, J.M. Microtab Design and Implementation on a 5 MW Wind Turbine. Appl. Sci. 2017, 7, 536. [Google Scholar] [CrossRef]
- Toshio, O.; Yoshiyasu, H.; Akinori, Y. Coupled thermal–electrical analysis for carbon fiber epoxy composites exposed. Compos. Part A 2010, 41, 973–981. [Google Scholar]
- Vasa, N. J.; Naka, T.; Yokoyama, S.; Wada, A.; Asakawa, A.; Arinaga, S. Experimental Study on Lightning Attachment Manner considering Various Types of Lightning Protection Measures on Wind Tuine Blades. In Proceedings of the International Conference on Lightning Protection, Kanazawa, Japan, 17–21 September 2006; pp. 1483–1487. [Google Scholar]
- Feraboli, P.; Miller, M. Damage resistance and tolerance of carbon epoxy composite coupons subjected. Compos. Part A 2009, 40, 954–967. [Google Scholar] [CrossRef]
- Kithil, R. Case Study of Lightning Damage to Wind Turbine Blade; National Lightning Safety Institute: Louisville, CO, USA, 2008. [Google Scholar]
- Adri, C.T.; Siddharth, D.; Francois, L.; William, A.G. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar]
- Kimberly, C.; Adri, C.T.; William, A.G. ReaxFF Reactive Force Field for Molecular Dynamics Simulations of Hydrocarbon Oxidation. J. Phys. Chem. A 2008, 112, 1040–1053. [Google Scholar]
- Adri, C.T.; Yehuda, Z.; Faina, D.; Ronnie, K. Atomistic-Scale Simulations of the Initial Chemical Events in the Thermal Initiation of Triacetonetriperoxide. J. Am. Chem. Soc. 2005, 127, 11053–11062. [Google Scholar]
- Ken-ichi, N.; Rajiv, K.K.; Aiichiro, N.; Priya, V.; van Duin, A.C.T.; Goddard, W.A., III. Dynamic Transition in the Structure of an Energetic Crystal during Chemical Reactions at Shock Front Prior to Detonation. Phys. Rev. Lett. 2007, 99, 148303. [Google Scholar] [CrossRef]
- Akira, T.; Akihisa, I. Calculations of Mixing Enthalpy and Mismatch Entropy for Ternary Amorphous Alloys. Mater. Trans. JIM 2007, 41, 1372–1378. [Google Scholar]
- Miller, J.A.; Pilling, M.J.; Troe, J. Unraveling combustion mechanisms through a quantitative understanding of elementary reactions. Proc. Combust. Inst. 2005, 30, 43–88. [Google Scholar] [CrossRef]
- Goddard, W.A., III; Mueller, J.E.; Chenoweth, K.; van Duin, A.C.T. ReaxFF Monte Carlo reactive dynamics: Application to resolving the partial occupations of the M1 phase of the MoVNbTeO catalyst. Catal. Today 2010, 157, 71–76. [Google Scholar] [CrossRef]
- Goddard, W.A.; Adri, C.T.; Chenoweth, K.; van Duin, A.; Chenoweth, K.; Cheng, M.J.; Pudar, S.; Oxgaard, J.; Merinov, B.; Jang, Y.H.; et al. Development of the ReaxFF reactive force field for mechanistic studies of catalytic selective oxidation processes on BiMoOx. Top. Catal. 2006, 38, 93–103. [Google Scholar] [CrossRef]
- Chenoweth, K.; Adri, C.T.; Goddard, W.A. The ReaxFF Monte Carlo Reactive Dynamics Method for Predicting Atomistic Structures of Disordered Ceramics: Application to the Mo3VOx Catalyst. Angew. Chem. Int. Ed. 2009, 121, 7766–7770. [Google Scholar] [CrossRef]
- Nielson, K.D.; van Duin, A.C.T.; Oxgaard, J.; Deng, W.Q.; Goddard, W.A. Development of the ReaxFF Reactive Force Field for Describing Transition Metal Catalyzed Reactions, with Application to the Initial Stages of the Catalytic Formation of Carbon Nanotubes. J. Phys. Chem. A 2005, 109, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Jing, X.; Wang, S.; Jia, Q.X. Behavior investigation of phenolic hydroxyl groups during the pyrolysis of cured phenolic resin via molecular dynamics simulation. Polym. Degrad. Stab. 2016, 125, 97–104. [Google Scholar] [CrossRef]
- Zeng, F.; Peng, C.; Liu, Y.; Qu, J. Reactive Molecular Dynamics Simulations on the Disintegration of PVDF, FP-POSS, and Their Composite during Atomic Oxygen Impact. J. Phys. Chem. A 2015, 119, 8359–8368. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ren, D.; Cheng, X. The theoretical investigation of the β-crystobalite structure under the effect of electric field. Comput. Mater. Sci. 2015, 96, 306–311. [Google Scholar] [CrossRef]
- Diao, Z.; Zhao, Y.; Chen, B.; Duan, C.; Song, S. ReaxFF reactive force field for molecular dynamics simulations of epoxy resin thermal decomposition with model compound. J. Anal. Appl. Pyrolysis 2013, 104, 618–624. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, S.; Liao, Y.; Cen, K. Mechanism Study of Cellulose Rapid Pyrolysis. Ind. Eng. Chem. Res. 2004, 43, 5605–5610. [Google Scholar] [CrossRef]
| Material Type | Peak Value of Impulse Current (kA) | |||
|---|---|---|---|---|
| 1.45 | 6.28 | 12.56 | 21 | |
| Balsa wood |  |  |  |  |
| PVC |  |  |  | / |
| Peak Value of Current | 1.45 kA | 6.28 kA | 12.56 kA | 21 kA |
|---|---|---|---|---|
| Width size (cm) | 0.4 | 0.7 | 3 | 4.5 |
| Depth size (cm) | 0.1 | 0.2 | 2 | 2 |
| Peak Value of Current | 1.45 kA | 6.28 kA | 12.56 kA |
|---|---|---|---|
| Width size (cm) | 0.35 | 1 | 2.5 |
| Depth size (cm) | 0.1 | 2 | 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Jiang, L.; Zhao, T.; Zou, L. Microcosmic Mechanism Investigation on Lightning Arc Damage of Wind Turbine Blades Based on Molecular Reaction Dynamics and Impact Current Experiment. Energies 2017, 10, 2010. https://doi.org/10.3390/en10122010
Zhang L, Jiang L, Zhao T, Zou L. Microcosmic Mechanism Investigation on Lightning Arc Damage of Wind Turbine Blades Based on Molecular Reaction Dynamics and Impact Current Experiment. Energies. 2017; 10(12):2010. https://doi.org/10.3390/en10122010
Chicago/Turabian StyleZhang, Li, Liyang Jiang, Tong Zhao, and Liang Zou. 2017. "Microcosmic Mechanism Investigation on Lightning Arc Damage of Wind Turbine Blades Based on Molecular Reaction Dynamics and Impact Current Experiment" Energies 10, no. 12: 2010. https://doi.org/10.3390/en10122010
APA StyleZhang, L., Jiang, L., Zhao, T., & Zou, L. (2017). Microcosmic Mechanism Investigation on Lightning Arc Damage of Wind Turbine Blades Based on Molecular Reaction Dynamics and Impact Current Experiment. Energies, 10(12), 2010. https://doi.org/10.3390/en10122010







