A Critical Review of Spinel Structured Iron Cobalt Oxides Based Materials for Electrochemical Energy Storage and Conversion
Abstract
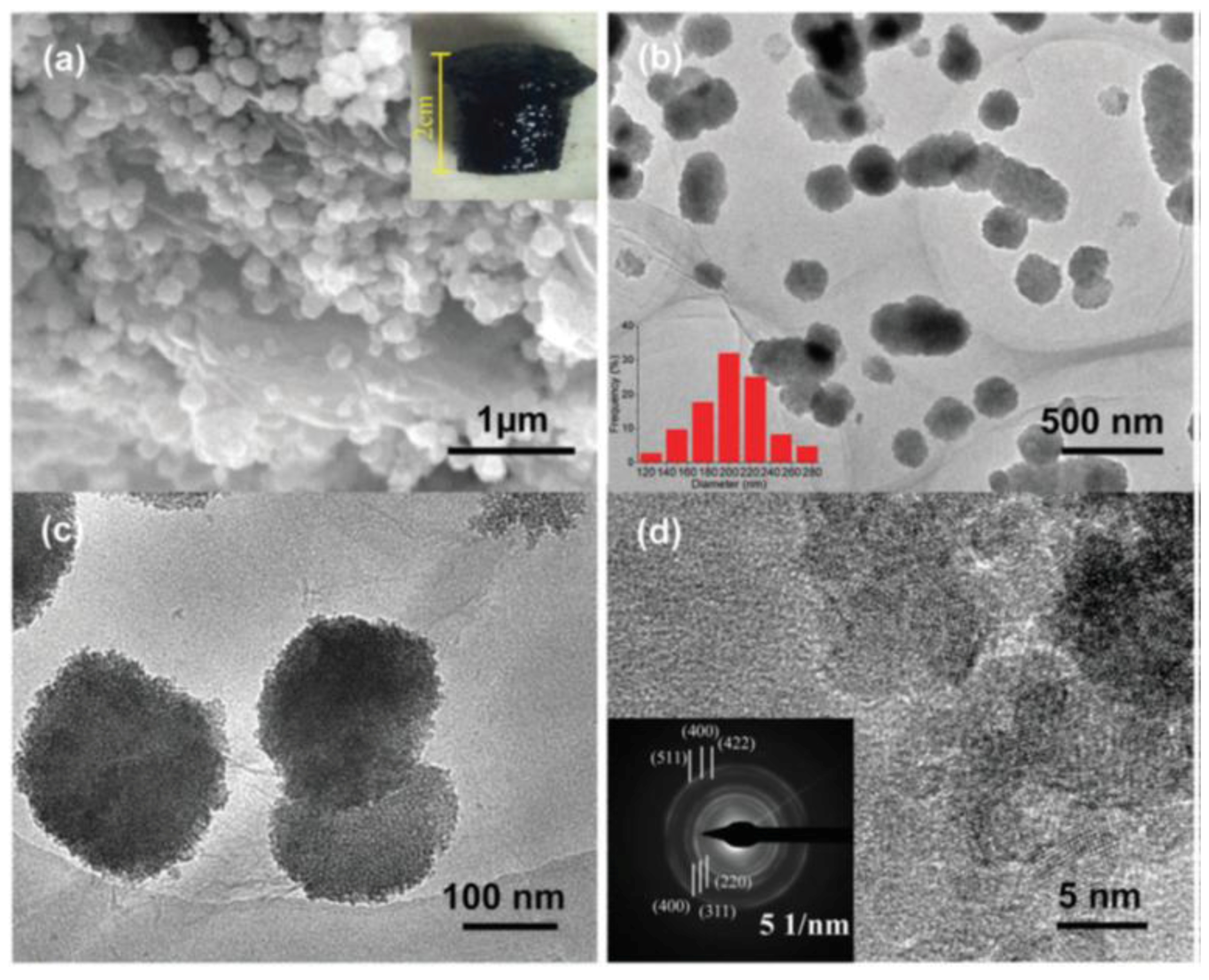
:1. Introduction
2. Application for Supercapacitors
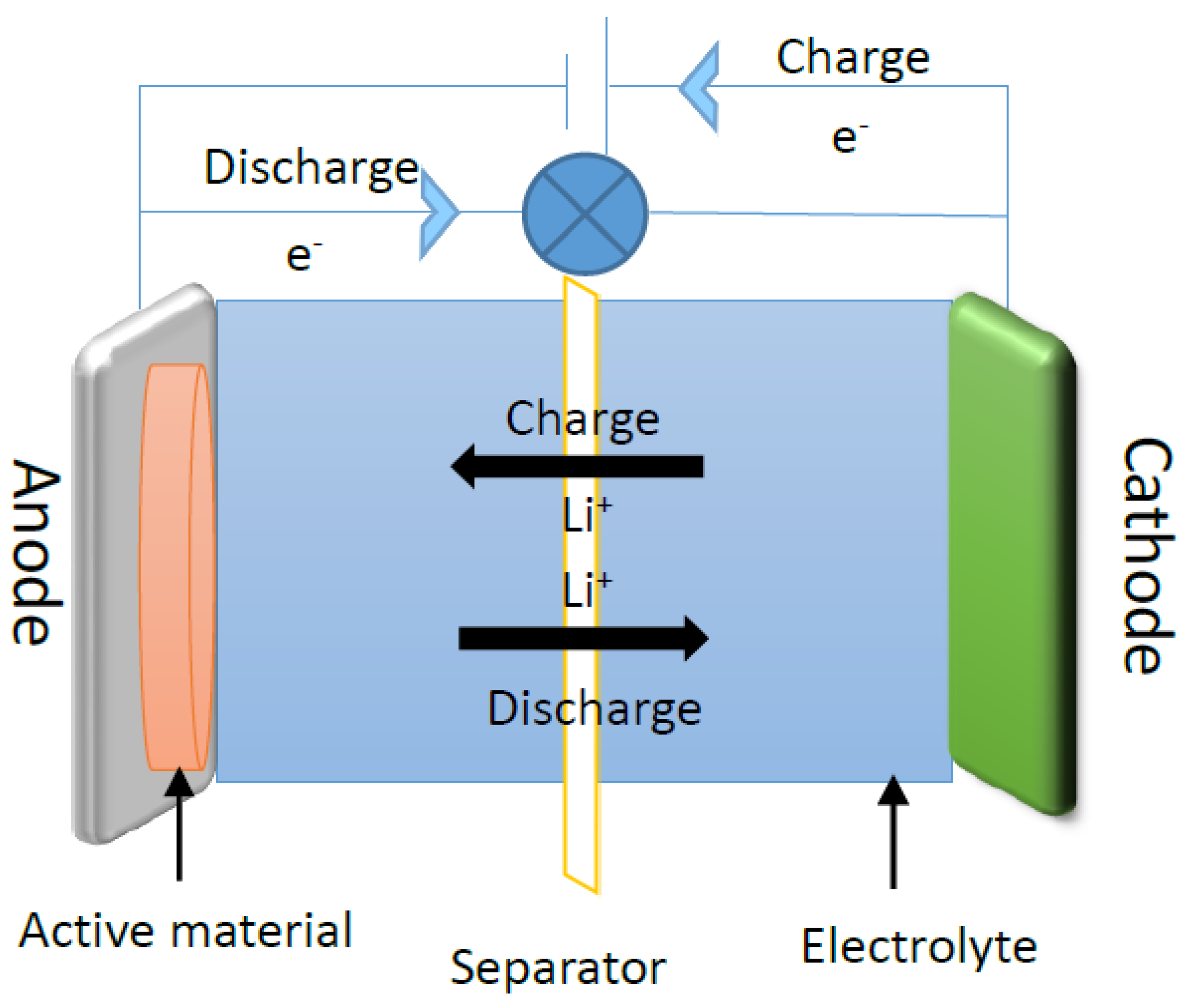
3. Application for Lithium-Ion Batteries
4. Application for Fuel Cells
5. Summary and Outlook
- Previous studies identified the critical roles of morphologies and structures of electrode materials in enhancement of electrochemical performance. Novel structures were desired to be synthesized for further improving their electrochemical activities. It is noteworthy to mention that nanostructured materials should focused considering their high specific areas and more active sites, 3D micron-structured or submicron-structured materials should also be addressed because of their excellent electrochemical performance.
- More work focused on electrode materials, contrasting few works emphasized on electrolytes, separators and counter electrodes. The future works should oversee all involved components for the establishment of optimal systems to realize better performance.
- More works emphasized on fabricating novel morphologies and structures, while few clearly addressed the essential mechanisms on charging-discharging process in energy storage and conversion. This missing correlation between morphologies and structures of CoFe2O4 and FeCo2O4 based materials has hampered their further optimization. The mechanical studies using DFT method was also highly recommended.
- Current applied approaches to fabricate CoFe2O4 and FeCo2O4 based materials were limited to some classical methods such as hydrothermal, solvothermal and sol-gel. Those methods likely introduced impurities into electrode materials which later affected their performance to some extent. There was actually some space to refine the current methods but the development of new synthesis strategies of CoFe2O4 and FeCo2O4 based materials were highly demanding.
- The scale-up preparation of CoFe2O4 and FeCo2O4 based materials was still challenging for their successful commercial applications. This was also true for other transition metal oxide electrode materials.
- Many works only focused on the improvement of the basic electrochemical performance but missed the relevance toward their practical applications, such as adaptability to the load change and durability under severe working conditions. The current gap implied a long way ahead toward the commercialization.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharma, C.S.; Awasthi, R.; Singh, R.N.; Sinha, A.S.K. Graphene–cobaltite–Pd hybrid materials for use as efficient bifunctional electrocatalysts in alkaline direct methanol fuel cells. Phys. Chem. Chem. Phys. 2013, 15, 20333–20344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Johnston, C.; Grant, P.S. A novel hybrid supercapacitor with a carbon nanotube cathode and an iron oxide/carbon nanotube composite anode. J. Mater. Chem. 2009, 19, 8755–8760. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Electrochemical capacitors for energy management. Sci. Mag. 2008, 321, 651–652. [Google Scholar]
- Spinner, N.; Mustain, W.E. Effect of nickel oxide synthesis conditions on its physical properties and electrocatalytic oxidation of methanol. Electrochim. Acta 2011, 56, 5656–5666. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2003, 104, 4245–4269. [Google Scholar] [CrossRef]
- Liang, K.; Tang, X.; Hu, W. High-performance three-dimensional nanoporous NiO film as a supercapacitor electrode. J. Mater. Chem. 2012, 22, 11062–11067. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, S.; Cao, D.; Wang, G.; Yin, J. Electrochemical capacitance of Co3O4 nanowire arrays supported on nickel foam. J. Power Sources 2010, 195, 1757–1760. [Google Scholar] [CrossRef]
- Cheng, K.; Cao, D.; Yang, F.; Xu, Y.; Sun, G.; Ye, K.; Yin, J.; Wang, G. Facile synthesis of morphology-controlled Co3O4 nanostructures through solvothermal method with enhanced catalytic activity for H2O2 electroreduction. J. Power Sources 2014, 253, 214–223. [Google Scholar] [CrossRef]
- Yan, N.; Hu, L.; Li, Y.; Wang, Y.; Zhong, H.; Hu, X.; Kong, X.; Chen, Q. Co3O4 nanocages for high-performance anode material in lithium-ion batteries. J. Phys. Chem. C 2012, 116, 7227–7235. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, J.; Wu, X.; Han, Q.; Wang, X. Graphene oxide-MnO2 nanocomposites for supercapacitors. ACS NANO 2010, 4, 2822–2830. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS NANO 2012, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chen, J.; Liu, J.; Li, S.; Ma, Y.; Zhang, J.; An, J. Mesoporous NiCo2O4 nanoneedles grown on 3D graphene-nickel foam for supercapacitor and methanol electro-oxidation. Electrochim. Acta 2015, 151, 99–108. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, J.; Qu, B.; Lu, B.; Xu, Z. Graphene improving lithium-ion battery performance by construction of NiCo2O4/graphene hybrid nanosheet arrays. Nano Energy 2014, 3, 88–94. [Google Scholar] [CrossRef]
- Kang, W.; Tang, Y.; Li, W.; Li, Z.; Yang, X.; Xu, J.; Lee, C.-S. Porous CuCo2O4 nanocubes wrapped by reduced graphene oxide as high-performance lithium-ion battery anodes. Nanoscale 2014, 6, 6551–6556. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Pendashteh, A.; Palma, J.; Anderson, M.; Marcilla, R. Nanostructured porous wires of iron cobaltite: Novel positive electrode for high-performance hybrid energy storage devices. J. Mater. Chem. A 2015, 3, 16849–16859. [Google Scholar] [CrossRef]
- Lv, L.; Xu, Q.; Ding, R.; Qi, L.; Wang, H. Chemical synthesis of mesoporous CoFe2O4 nanoparticles as promising bifunctional electrode materials for supercapacitors. Mater. Lett. 2013, 111, 35–38. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, W.; Jiang, M.; Chen, C.; Li, Y. One-step accurate synthesis of shell controllable CoFe2O4 hollow microspheres as high-performance electrode materials in supercapacitor. Nano Res. 2016, 9, 2026–2033. [Google Scholar] [CrossRef]
- Kumbhar, V.S.; Jagadale, A.D.; Shinde, N.M.; Lokhande, C.D. Chemical synthesis of spinel cobalt ferrite (CoFe2O4) nano-flakes for supercapacitor application. Appl. Surf. Sci. 2012, 259, 39–43. [Google Scholar] [CrossRef]
- Mohamed, S.G.; Chen, C.-J.; Chen, C.K.; Hu, S.-F.; Liu, R.-S. High-performance lithium-ion battery and symmetric supercapacitors based on FeCo2O4 nanoflakes electrodes. ACS Appl. Mater. Interfaces 2014, 6, 22701–22708. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xiang, J.; Cao, Y. Hierarchically porous CoFe2O4 nanosheets supported on Ni foam with excellent electrochemical properties for asymmetric supercapacitors. Appl. Surf. Sci. 2017, 413, 351–359. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.; Mu, Y.; Bai, Y.; Wang, Y. Binary cobalt ferrite nanomesh arrays as the advanced binder-free electrode for applications in oxygen evolution reaction and supercapacitors. J. Power Sources 2016, 327, 599–609. [Google Scholar] [CrossRef]
- Zhu, B.; Tang, S.; Vongehr, S.; Xie, H.; Zhu, J.; Meng, X. FeCo2O4 submicron-tube arrays grown on Ni foam as high rate-capability and cycling-stability electrodes allowing superior energy and power densities with symmetric supercapacitors. Chem. Commun. 2016, 52, 2624–2627. [Google Scholar] [CrossRef] [PubMed]
- Sankar, K.V.; Selvan, R.K.; Meyrick, D. Electrochemical performances of CoFe2O4 nanoparticles and a rGO based asymmetric supercapacitor. RSC Adv. 2015, 5, 99959–99967. [Google Scholar] [CrossRef]
- Xiong, P.; Huang, H.; Wang, X. Design and synthesis of ternary cobalt ferrite/graphene/polyaniline hierarchical nanocomposites for high-performance supercapacitors. J. Power Sources 2014, 245, 937–946. [Google Scholar] [CrossRef]
- Zhu, B.; Tang, S.; Vongehr, S.; Xie, H.; Meng, X. Hierarchically MnO2–nanosheet covered submicrometer-FeCo2O4-tube forest as binder-free electrodes for high energy density all-solid-state supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 4762–4770. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Cao, S.; Cao, Y. Hierarchical Core-Shell Nanosheet Arrays with MnO2 Grown on Mesoporous CoFe2O4 Support for High-Performance Asymmetric Supercapacitors. Electrochim. Acta 2017, 240, 31–42. [Google Scholar] [CrossRef]
- Lin, L.; Tang, S.; Zhao, S.; Peng, X.; Hu, N. Hierarchical three-dimensional FeCo2O4@MnO2 core-shell nanosheet arrays on nickel foam for high-performance supercapacitor. Electrochim. Acta 2017, 228, 175–182. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Hao, X.D.; Diao, Z.P.; Li, J.; Guan, Y.M. One-pot controllable synthesis of flower-like CoFe2O4/FeOOH nanocomposites for high-performance supercapacitors. Mater. Lett. 2014, 123, 229–234. [Google Scholar] [CrossRef]
- Sankar, K.V.; Selvan, R.K. Fabrication of flexible fiber supercapacitor using covalently grafted CoFe2O4/reduced graphene oxide/polyaniline and its electrochemical performances. Electrochim. Acta 2016, 213, 469–481. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Liu, B.; Yu, G.; Hou, X.; Chen, D.; Shen, G. NiCo2O4 nanowire arrays supported on Ni foam for high-performance flexible all-solid-state supercapacitors. J. Mater. Chem. A 2013, 1, 2468–2473. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Hou, L.; Zhang, X.; Shen, L.; Lou, X.W.D. Ultrathin mesoporous NiCo2O4 nanosheets supported on Ni foam as advanced electrodes for supercapacitors. Adv. Funct. Mater. 2012, 22, 4592–4597. [Google Scholar] [CrossRef]
- Liu, S.; Hui, K.S.; Hui, K.N. Flower-like copper cobaltite nanosheets on graphite paper as high-performance supercapacitor electrodes and enzymeless glucose sensors. ACS Appl. Mater. Interfaces 2016, 8, 3258–3267. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Wang, X.; Zhao, X.; Wang, Y.; Ji, Y.; Zhang, H.; Liu, X. Controlled growth of mesoporous ZnCo2O4 nanosheet arrays on Ni foam as high-rate electrodes for supercapacitors. RSC Adv. 2014, 4, 2393–2397. [Google Scholar] [CrossRef]
- Chien, H.C.; Cheng, W.Y.; Wang, Y.H.; Lu, S.Y. Ultrahigh specific capacitances for supercapacitors achieved by nickel cobaltite/carbon aerogel composites. Adv. Funct. Mater. 2012, 22, 5038–5043. [Google Scholar] [CrossRef]
- Xu, K.; Huang, X.; Liu, Q.; Zou, R.; Li, W.; Liu, X.; Li, S.; Yang, J.; Hu, J. Understanding the effect of polypyrrole and poly (3,4-ethylenedioxythiophene) on enhancing the supercapacitor performance of NiCo2O4 electrodes. J. Mater. Chem. A 2014, 2, 16731–16739. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Z.; Ma, J.; Zhang, X.; Wang, J.; Liu, J. Hierarchical NiCo2O4@ NiO core–shell hetero-structured nanowire arrays on carbon cloth for a high-performance flexible all-solid-state electrochemical capacitor. J. Mater. Chem. A 2014, 2, 1448–1457. [Google Scholar] [CrossRef]
- Ji, L.; Rao, M.; Aloni, S.; Wang, L.; Cairns, E.J.; Zhang, Y. Porous carbon nanofiber–sulfur composite electrodes for lithium/sulfur cells. Energy Environ. Sci. 2011, 4, 5053–5059. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Zhao, T.P.; Zhan, X.Y.; Gao, D.S.; Xiao, Q.Z.; Lei, G.T. High capacity three-dimensional ordered macroporous CoFe2O4 as anode material for lithium ion batteries. Electrochim. Acta 2010, 55, 4594–4598. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, D.-W.; Li, F.; Zhang, L.; Li, N.; Wu, Z.-S.; Wen, L.; Lu, G.Q.; Cheng, H.-M. Graphene-wrapped Fe3O4 anode material with improved reversible capacity and cyclic stability for lithium ion batteries. Chem. Mater. 2010, 22, 5306–5313. [Google Scholar] [CrossRef]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacin, M.R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting Through Conversion Reactions. Adv. Mater. 2010, 22. [Google Scholar] [CrossRef]
- Li, S.; Wang, B.; Liu, J.; Yu, M. In situ one-step synthesis of CoFe2O4/graphene nanocomposites as high-performance anode for lithium-ion batteries. Electrochim. Acta 2014, 129, 33–39. [Google Scholar] [CrossRef]
- Lavela, P.; Tirado, J.L. CoFe2O4 and NiFe2O4 synthesized by sol–gel procedures for their use as anode materials for Li ion batteries. J. Power Sources 2007, 172, 379–387. [Google Scholar] [CrossRef]
- Sharma, Y.; Sharma, N.; Rao, G.V.S.; Chowdari, B.V.R. Studies on spinel cobaltites, FeCo2O4 and MgCo2O4 as anodes for Li-ion batteries. Solid State Ion. 2008, 179, 587–597. [Google Scholar] [CrossRef]
- Nam, K.T.; Kim, D.-W.; Yoo, P.J.; Chiang, C.-Y.; Meethong, N.; Hammond, P.T.; Chiang, Y.-M.; Belcher, A.M. Virus-enabled synthesis and assembly of nanowires for lithium ion battery electrodes. Science 2006, 312, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.W.; Deng, D.; Lee, J.Y.; Feng, J.; Archer, L.A. Self-supported formation of needlelike Co3O4 nanotubes and their application as lithium-ion battery electrodes. Adv. Mater. 2008, 20, 258–262. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, B.; Corr, S.A.; Shi, Q.; Hu, Y.-S.; Heier, K.R.; Chen, L.; Seshadri, R.; Stucky, G.D. Ordered mesoporous metallic MoO2 materials with highly reversible lithium storage capacity. Nano Lett. 2009, 9, 4215–4220. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, T.; Chen, W.; Liu, L.; Yang, X.; Wang, Y.; Guo, Y. General design of hollow porous CoFe2O4 nanocubes from metal–organic frameworks with extraordinary lithium storage. Nanoscale 2014, 6, 15168–15174. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, H.; Mu, Y.; Yang, J.; Wang, Y. Porous iron cobaltate nanoneedles array on nickel foam as anode materials for lithium-ion batteries with enhanced electrochemical performance. ACS Appl. Mater. Interfaces 2016, 8, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, Z.; Sun, L.; Gao, G.; Liu, X. Controlled synthesis and enhanced electrochemical performance of Prussian blue analogue-derived hollow FeCo2O4 nanospheres as lithium-ion battery anodes. RSC Adv. 2015, 5, 36575–36581. [Google Scholar] [CrossRef]
- Zhu, H.; Sun, Y.; Zhang, X.; Tang, L.; Guo, J. Evaporation-induced self-assembly synthesis of mesoporous FeCo2O4 octahedra with large and fast lithium storage properties. Mater. Lett. 2016, 166, 1–4. [Google Scholar] [CrossRef]
- Yao, X.; Kong, J.; Tang, X.; Zhou, D.; Zhao, C.; Zhou, R.; Lu, X. Facile synthesis of porous CoFe2O4 nanosheets for lithium-ion battery anodes with enhanced rate capability and cycling stability. RSC Adv. 2014, 4, 27488–27492. [Google Scholar] [CrossRef]
- Wang, B.; Li, S.; Wu, X.; Li, B.; Liu, J.; Yu, M. Nanocrystal-constructed mesoporous CoFe2O4 nanowire arrays aligned on flexible carbon fabric as integrated anodes with enhanced lithium storage properties. Phys. Chem. Chem. Phys. 2015, 17, 21476–21484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, Y.; Sun, Y.; Zhang, Q.; Zhu, Q.; Hou, D.; Guo, J. Self-template synthesis of CoFe2O4 nanotubes for high-performance lithium storage. RSC Adv. 2015, 5, 29837–29841. [Google Scholar] [CrossRef]
- Mao, J.; Hou, X.; Wang, X.; Hu, S.; Xiang, L. The cubic aggregated CoFe2O4 nanoparticle anode material for lithium ion battery with good performance. Mater. Lett. 2015, 161, 652–655. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Zou, R.; Kang, W.; San Chui, Y.; Yuen, M.F.; Lee, C.-S.; Zhang, W. Layer-stacked cobalt ferrite (CoFe2O4) mesoporous platelets for high-performance lithium ion battery anodes. J. Mater. Chem. A 2015, 3, 6990–6997. [Google Scholar] [CrossRef]
- Wang, N.; Xu, H.; Chen, L.; Gu, X.; Yang, J.; Qian, Y. A general approach for MFe2O4 (M = Zn, Co, Ni) nanorods and their high performance as anode materials for lithium ion batteries. J. Power Sources 2014, 247, 163–169. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.; Sun, Y.; Zhang, X. Mesoporous CoFe2O4 octahedra with high-capacity and long-life lithium storage properties. RSC Adv. 2016, 6, 18–22. [Google Scholar] [CrossRef]
- Wang, Y.; Su, D.; Ung, A.; Ahn, J.-H.; Wang, G. Hollow CoFe2O4 nanospheres as a high capacity anode material for lithium ion batteries. Nanotechnology 2012, 23, 055402. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.Q.; Tu, J.P.; Shi, S.J.; Liu, X.Y.; Wang, X.L.; Gu, C.D. Ascorbic acid-assisted synthesis of cobalt ferrite (CoFe2O4) hierarchical flower-like microspheres with enhanced lithium storage properties. J. Power Sources 2014, 256, 153–159. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Selvan, R.K.; Lee, Y.S. An overview of AB2O4-and A2BO4-structured negative electrodes for advanced Li-ion batteries. RSC Adv. 2016, 6, 21448–21474. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Xing, M.; Zhang, J. Mesoporous TiO2 nanocrystals grown in situ on graphene aerogels for high photocatalysis and lithium-ion batteries. J. Am. Chem. Soc. 2014, 136, 5852–5855. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Ren, W.; Wen, L.; Gao, L.; Zhao, J.; Chen, Z.; Zhou, G.; Li, F.; Cheng, H.-M. Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. ACS NANO 2010, 4, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, G.; Lv, Z.; Wang, H. In situ synthesis of hierarchical CoFe2O4 nanoclusters/graphene aerogels and their high performance for lithium-ion batteries. Phys. Chem. Chem. Phys. 2015, 17, 27109–27117. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhuo, L.; Zhang, C.; Zhao, F. Carbon dioxide-induced homogeneous deposition of nanometer-sized cobalt ferrite (CoFe2O4) on graphene as high-rate and cycle-stable anode materials for lithium-ion batteries. J. Power Sources 2015, 275, 650–659. [Google Scholar] [CrossRef]
- Wang, Y.; Park, J.; Sun, B.; Ahn, H.; Wang, G. Wintersweet-Flower-Like CoFe2O4/MWCNTs Hybrid Material for High-Capacity Reversible Lithium Storage. Chem. Asian J. 2012, 7, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Zhao, X.; Chen, R.; Fichtner, M. A facile synthesis of encapsulated CoFe2O4 into carbon nanofibres and its application as conversion anodes for lithium ion batteries. J. Power Sources 2014, 260, 205–210. [Google Scholar] [CrossRef]
- Sener, T.; Kayhan, E.; Sevim, M.; Metin, O. Monodisperse CoFe2O4 nanoparticles supported on Vulcan XC-72: High performance electrode materials for lithium-air and lithium-ion batteries. J. Power Sources 2015, 288, 36–41. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Wang, L.; Yan, W.; Wei, W. C@CoFe2O4 fiber-in-tube mesoporous nanostructure: Formation mechanism and high electrochemical performance as an anode for lithium-ion batteries. J. Alloy. Compd. 2017, 693, 110–117. [Google Scholar] [CrossRef]
- Liu, X.; Wu, N.; Cui, C.; Zhou, P.; Sun, Y. Enhanced rate capability and cycling stability of core/shell structured CoFe2O4/onion-like C nanocapsules for lithium-ion battery anodes. J. Alloy. Compd. 2015, 644, 59–65. [Google Scholar] [CrossRef]
- Kumar, P.R.; Kollu, P.; Santhosh, C.; Rao, K.E.V.; Kim, D.K.; Grace, A.N. Enhanced properties of porous CoFe2O4–reduced graphene oxide composites with alginate binders for Li-ion battery applications. New J. Chem. 2014, 38, 3654–3661. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, X.; Zhang, L.; Guo, X.; Liu, D.; Chen, J.; Ji, J. Liquid-Solid-Solution Assembly of CoFe2O4/Graphene Nanocomposite as a High-Performance Lithium-Ion Battery Anode. Electrochim. Acta 2016, 215, 247–252. [Google Scholar] [CrossRef]
- Xia, H.; Zhu, D.; Fu, Y.; Wang, X. CoFe2O4-graphene nanocomposite as a high-capacity anode material for lithium-ion batteries. Electrochim. Acta 2012, 83, 166–174. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, H.; Zhang, K.H.L.; Coto, M.; Zhao, T.; Chen, S.; Dong, B.; Lu, S.; Abdelkader, A.; Guo, Y. Quick one-pot synthesis of amorphous carbon-coated cobalt–ferrite twin elliptical frustums for enhanced lithium storage capability. J. Mater. Chem. A 2017, 5, 8062–8069. [Google Scholar] [CrossRef]
- Dong, B.; Li, M.; Xiao, C.; Ding, D.; Gao, G.; Ding, S. Tunable growth of perpendicular cobalt ferrite nanosheets on reduced graphene oxide for energy storage. Nanotechnology 2016, 28, 055401. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, J.; Fang, C.; Cao, G.; Zhu, T.; Zhao, X. Self-assembly of a CoFe2O4/graphene sandwich by a controllable and general route: Towards a high-performance anode for Li-ion batteries. J. Mater. Chem. 2012, 22, 19738–19743. [Google Scholar] [CrossRef]
- Rai, A.K.; Gim, J.; Thi, T.V.; Ahn, D.; Cho, S.J.; Kim, J. High rate capability and long cycle stability of Co3O4/CoFe2O4 nanocomposite as an anode material for high-performance secondary lithium ion batteries. J. Phys. Chem. C 2014, 118, 11234–11243. [Google Scholar] [CrossRef]
- Li, M.; Yin, Y.-X.; Li, C.; Zhang, F.; Wan, L.-J.; Xu, S.; Evans, D.G. Well-dispersed bi-component-active CoO/CoFe2O4 nanocomposites with tunable performances as anode materials for lithium-ion batteries. Chem. Commun. 2012, 48, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yu, C.; Liu, S.; Yang, J.; Fan, X.; Qiu, J. Facile Fabrication of Bicomponent CoO/CoFe2O4-N-Doped Graphene Hybrids with Ultrahigh Lithium Storage Capacity. Part. Part. Syst. Charact. 2015, 32, 91–97. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, T.; Mao, M.; Ren, W.; Li, Q. Enhanced electrochemical performance of hierarchical CoFe2O4/MnO2/C nanotubes as anode materials for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 12328–12333. [Google Scholar] [CrossRef]
- Guan, H.; Wang, X.; Li, H.; Zhi, C.; Zhai, T.; Bando, Y.; Golberg, D. CoO octahedral nanocages for high-performance lithium ion batteries. Chem. Commun. 2012, 48, 4878–4880. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, B.; Wu, Y. Mesoporous Co3O4 nanowire arrays for lithium ion batteries with high capacity and rate capability. Nano Lett. 2008, 8, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fu, H.; Peng, A.; Zhai, T.; Ma, Y.; Yuan, F.; Yao, J. One-Pot Solution Synthesis of Cubic Cobalt Nanoskeletons. Adv. Mater. 2009, 21, 1636–1640. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, J.; Cheng, C.; Li, H.; Zhang, J.; Gong, H.; Fan, H.J. Co3O4 Nanowire@ MnO2 Ultrathin Nanosheet Core/Shell Arrays: A New Class of High-Performance Pseudocapacitive Materials. Adv. Mater. 2011, 23, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, G.; Liu, J.; Qiao, S.; Ahn, H. Highly ordered mesoporous NiO anode material for lithium ion batteries with an excellent electrochemical performance. J. Mater. Chem. 2011, 21, 3046–3052. [Google Scholar] [CrossRef]
- Teh, P.F.; Sharma, Y.; Pramana, S.S.; Srinivasan, M. Nanoweb anodes composed of one-dimensional, high aspect ratio, size tunable electrospun ZnFe2O4 nanofibers for lithium ion batteries. J. Mater. Chem. 2011, 21, 14999–15008. [Google Scholar] [CrossRef]
- Guo, X.; Han, J.; Zhang, L.; Liu, P.; Hirata, A.; Chen, L.; Fujita, T.; Chen, M. A nanoporous metal recuperated MnO2 anode for lithium ion batteries. Nanoscale 2015, 7, 15111–15116. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Si, W.; Xi, L.; Oswald, S.; Yan, C.; Schmidt, O.G. High-rate amorphous SnO2 nanomembrane anodes for Li-ion batteries with a long cycling life. Nanoscale 2015, 7, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Kirubakaran, A.; Jain, S.; Nema, R.K. A review on fuel cell technologies and power electronic interface. Renew. Sustain. Energy Rev. 2009, 13, 2430–2440. [Google Scholar] [CrossRef]
- Li, Y.S.; Zhao, T.S.; Liang, Z.X. Effect of polymer binders in anode catalyst layer on performance of alkaline direct ethanol fuel cells. J. Power Sources 2009, 190, 223–229. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Jin, Y.; Qiao, S.Z. Nanostructured Metal-Free Electrochemical Catalysts for Highly Efficient Oxygen Reduction. Small 2012, 8, 3550–3566. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Luo, N.; Miley, G.H. Cathode electrocatalyst selection and deposition for a direct borohydride/hydrogen peroxide fuel cell. J. Power Sources 2007, 173, 77–85. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Z.; Yang, Y.; Su, D. The growth and enhanced catalytic performance of Au@Pd core–shell nanodendrites. Nanoscale 2013, 5, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Kua, J.; Goddard, W.A. Oxidation of methanol on 2nd and 3rd row group VIII transition metals (Pt, Ir, Os, Pd, Rh, and Ru): Application to direct methanol fuel cells. J. Am. Chem. Soc. 1999, 121, 10928–10941. [Google Scholar] [CrossRef]
- Xia, B.Y.; Wu, H.B.; Wang, X.; Lou, X.W. One-pot synthesis of cubic PtCu3 nanocages with enhanced electrocatalytic activity for the methanol oxidation reaction. J. Am. Chem. Soc. 2012, 134, 13934–13937. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bian, W.; Wu, J.; Tian, J.-H.; Yang, R. Preparation and electrocatalytic activity of 3D hierarchical porous spinel CoFe2O4 hollow nanospheres as efficient catalyst for oxygen reduction reaction and oxygen evolution reaction. Electrochim. Acta 2015, 151, 276–283. [Google Scholar] [CrossRef]
- Yan, W.; Cao, X.; Ke, K.; Tian, J.; Jin, C.; Yang, R. One-pot synthesis of monodispersed porous CoFe2O4 nanospheres on graphene as an efficient electrocatalyst for oxygen reduction and evolution reactions. RSC Adv. 2016, 6, 307–313. [Google Scholar] [CrossRef]
- Yan, W.; Yang, Z.; Bian, W.; Yang, R. FeCo2O4/hollow graphene spheres hybrid with enhanced electrocatalytic activities for oxygen reduction and oxygen evolution reaction. Carbon 2015, 92, 74–83. [Google Scholar] [CrossRef]
- Zhao, X.; Fu, Y.; Wang, J.; Xu, Y.; Tian, J.-H.; Yang, R. Ni-doped CoFe2O4 Hollow Nanospheres as Efficient Bi-functional Catalysts. Electrochim. Acta 2016, 201, 172–178. [Google Scholar] [CrossRef]
- Liu, S.; Bian, W.; Yang, Z.; Tian, J.; Jin, C.; Shen, M.; Zhou, Z.; Yang, R. A facile synthesis of CoFe2O4/biocarbon nanocomposites as efficient bi-functional electrocatalysts for the oxygen reduction and oxygen evolution reaction. J. Mater. Chem. A 2014, 2, 18012–18017. [Google Scholar] [CrossRef]
- Li, P.; Ma, R.; Zhou, Y.; Chen, Y.; Zhou, Z.; Liu, G.; Liu, Q.; Peng, G.; Liang, Z.; Wang, J. In situ growth of spinel CoFe2O4 nanoparticles on rod-like ordered mesoporous carbon for bifunctional electrocatalysis of both oxygen reduction and oxygen evolution. J. Mater. Chem. A 2015, 3, 15598–15606. [Google Scholar] [CrossRef]
- Yan, W.; Bian, W.; Jin, C.; Tian, J.-H.; Yang, R. An Efficient Bi-functional Electrocatalyst Based on Strongly Coupled CoFe2O4/Carbon Nanotubes Hybrid for Oxygen Reduction and Oxygen Evolution. Electrochim. Acta 2015, 177, 65–72. [Google Scholar] [CrossRef]
- Bian, W.; Yang, Z.; Strasser, P.; Yang, R. A CoFe2O4/graphene nanohybrid as an efficient bi-functional electrocatalyst for oxygen reduction and oxygen evolution. J. Power Sources 2014, 250, 196–203. [Google Scholar] [CrossRef]
- Yan, W.; Cao, X.; Tian, J.; Jin, C.; Ke, K.; Yang, R. Nitrogen/sulfur dual-doped 3D reduced graphene oxide networks-supported CoFe2O4 with enhanced electrocatalytic activities for oxygen reduction and evolution reactions. Carbon 2016, 99, 195–202. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Zhang, L.; Hu, T.; Liu, W.; Liu, N.; Du, F.; Li, Q.; Wang, Y. One-pot synthesis of Ag–CoFe2O4/C as efficient catalyst for oxygen reduction in alkaline media. Int. J. Hydrog. Energy 2016, 41, 22547–22553. [Google Scholar] [CrossRef]
- Huo, R.; Jiang, W.-J.; Xu, S.; Zhang, F.; Hu, J.-S. Co/CoO/CoFe2O4/G nanocomposites derived from layered double hydroxides towards mass production of efficient Pt-free electrocatalysts for oxygen reduction reaction. Nanoscale 2014, 6, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Q.; Hu, T.; Zhang, L.; Deng, Y. Carbon supported MnO2-CoFe2O4 with enhanced electrocatalytic activity for oxygen reduction and oxygen evolution. Appl. Surf. Sci. 2017, 403, 51–56. [Google Scholar] [CrossRef]
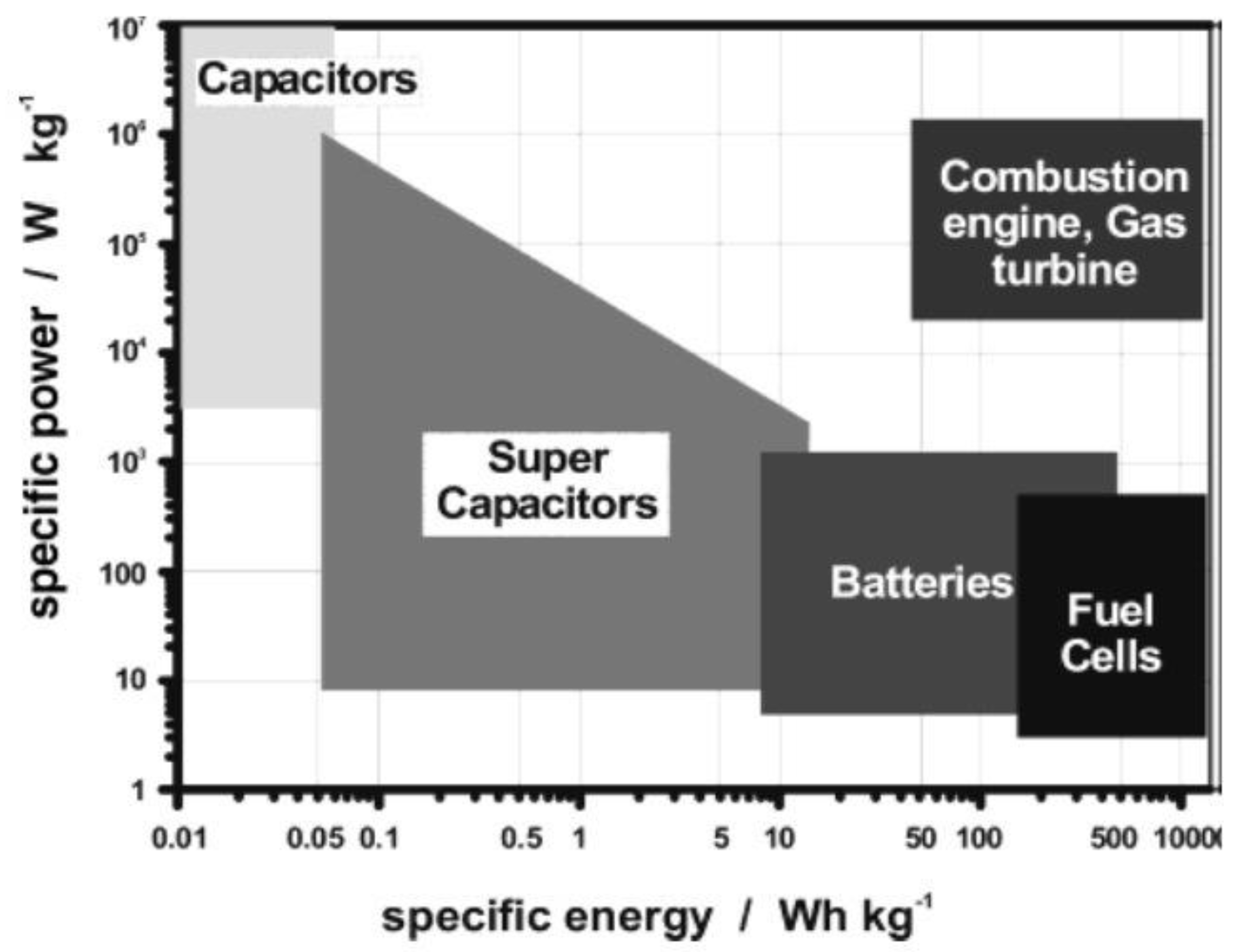
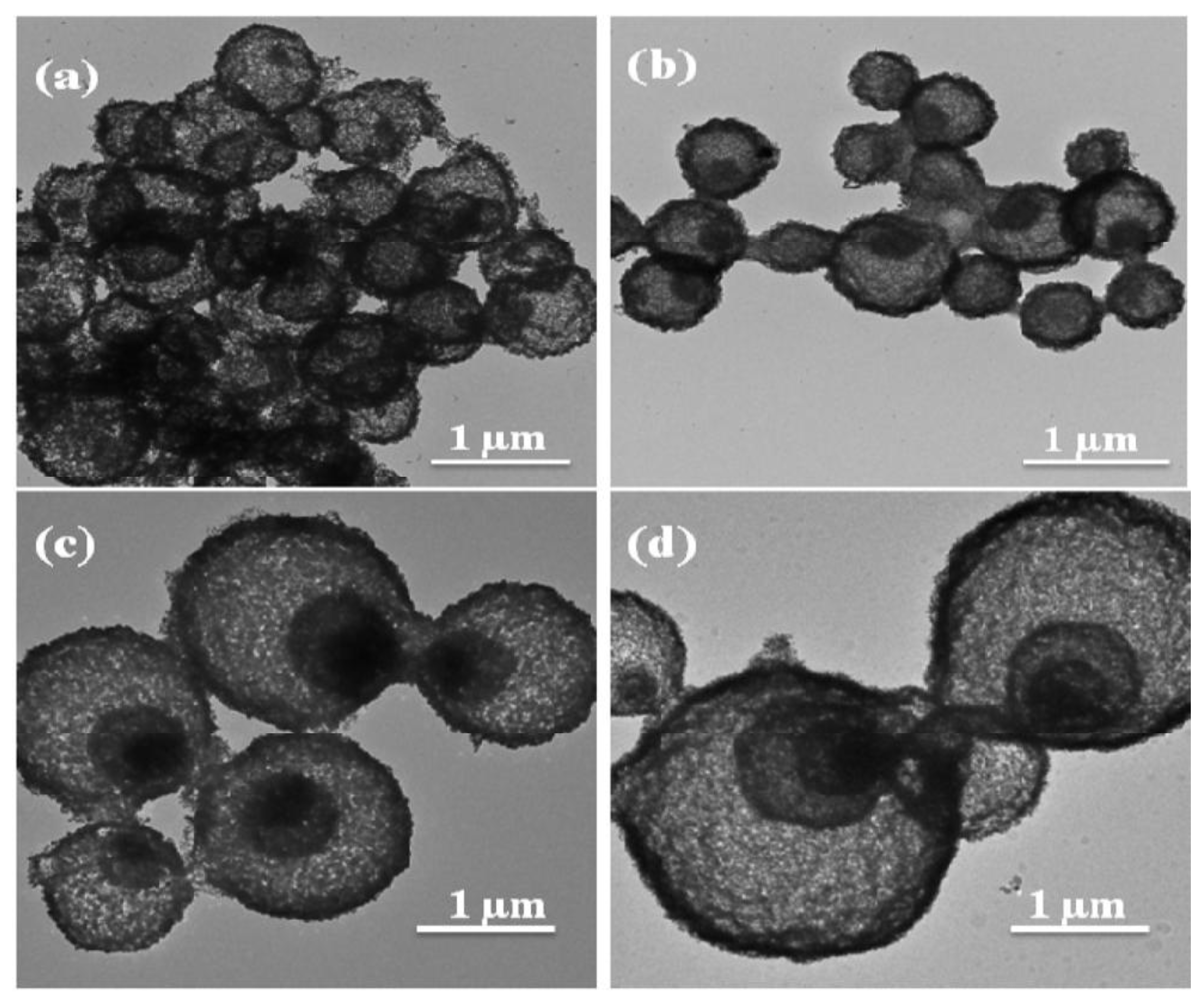
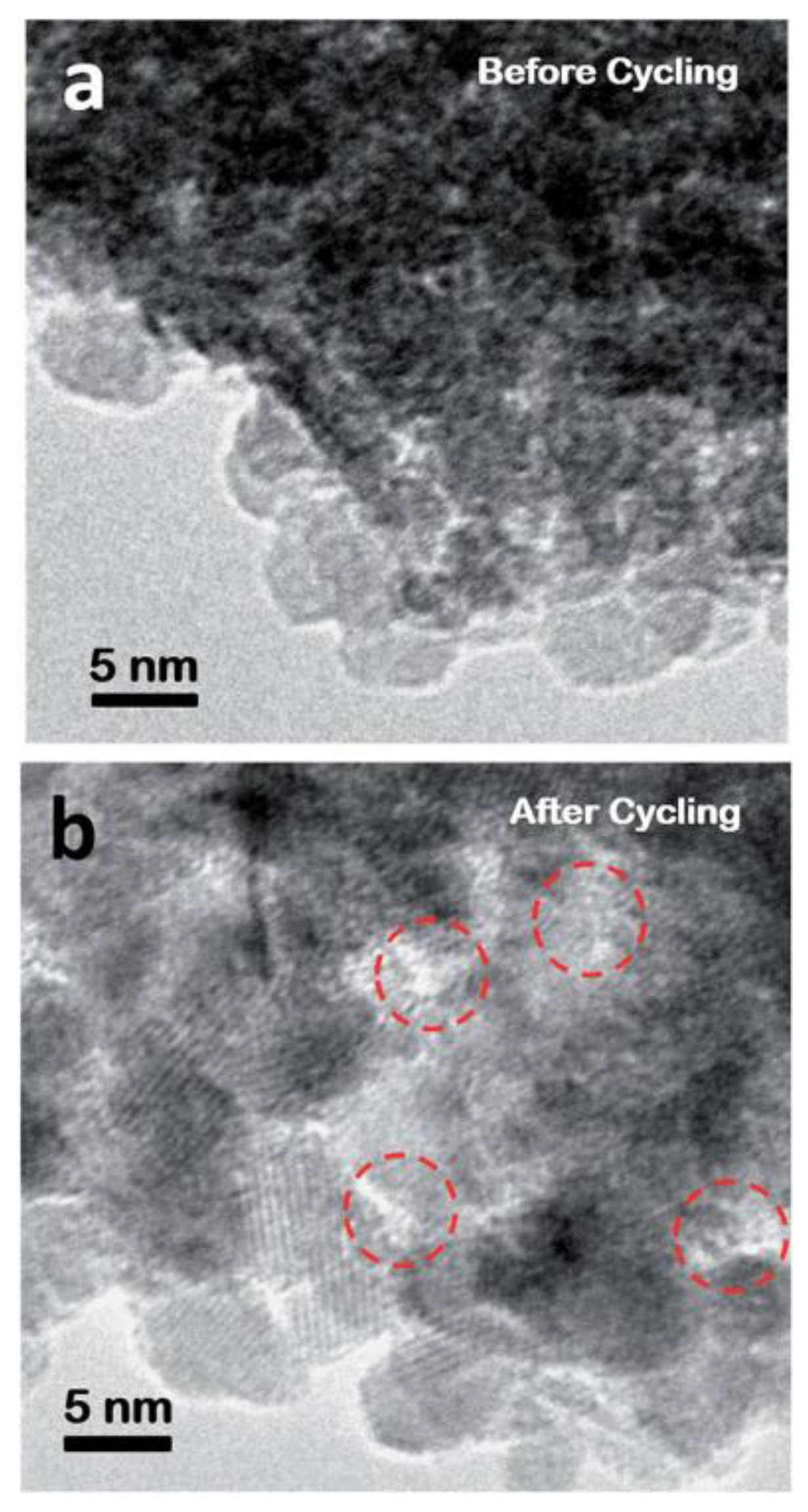

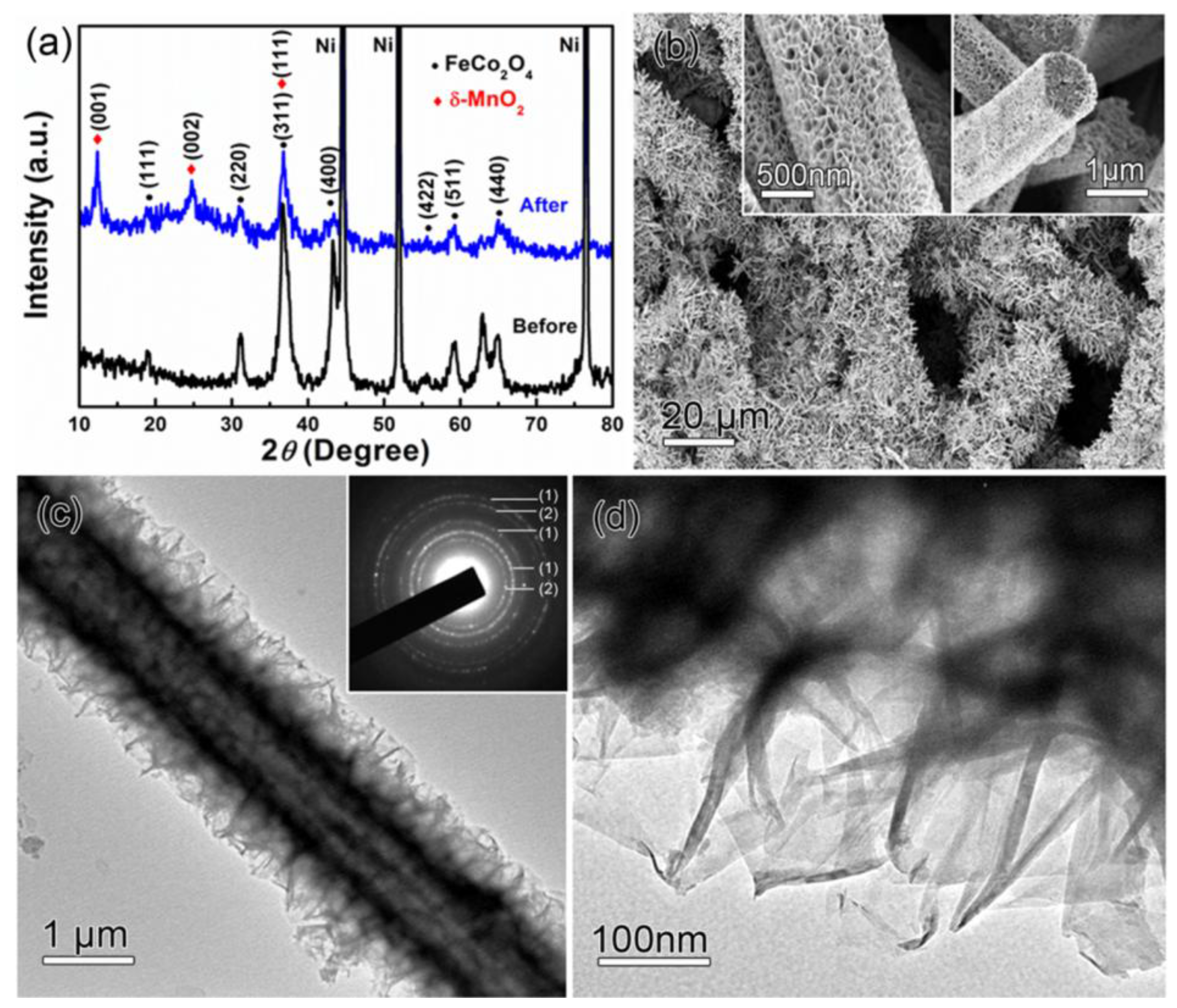
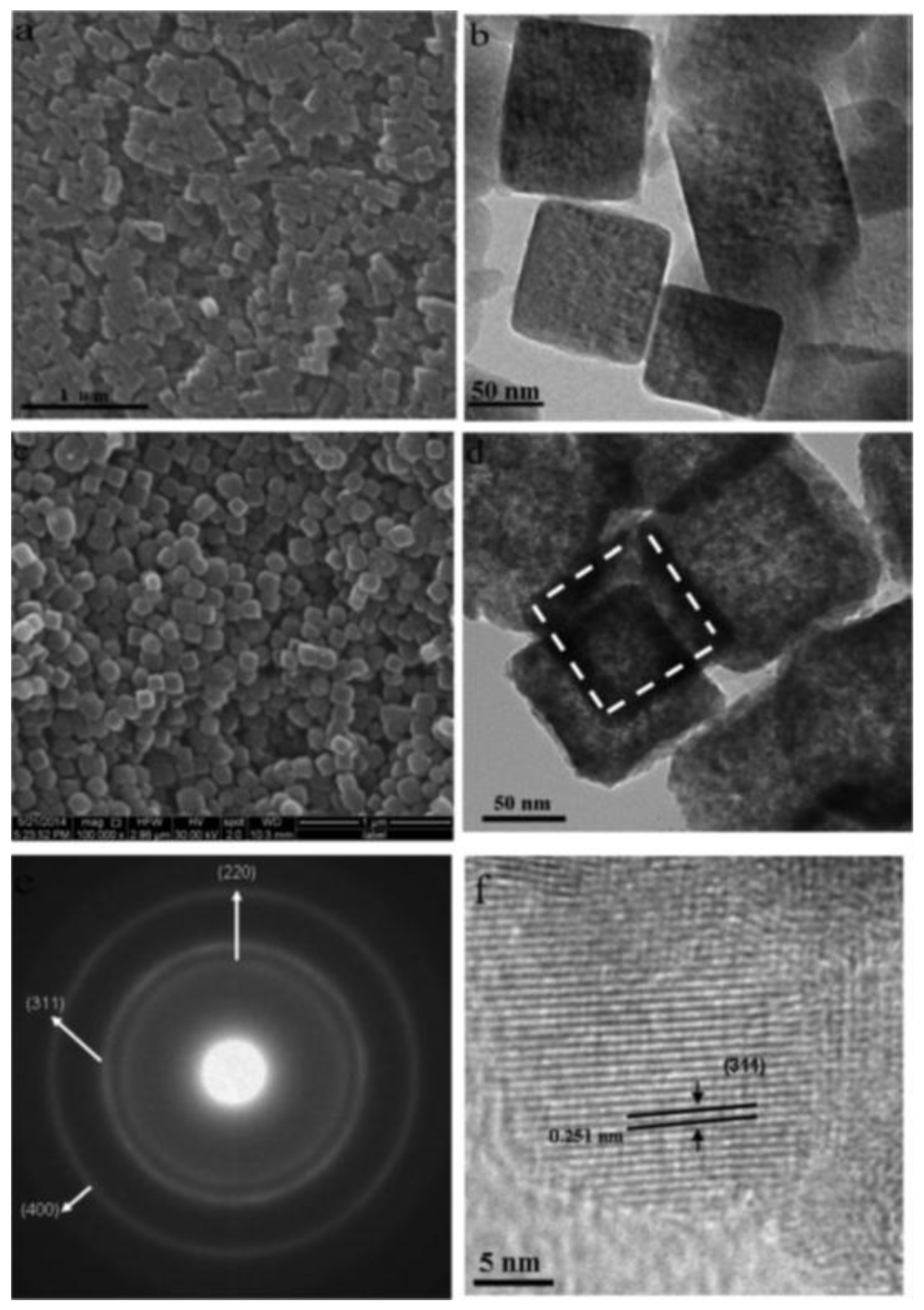
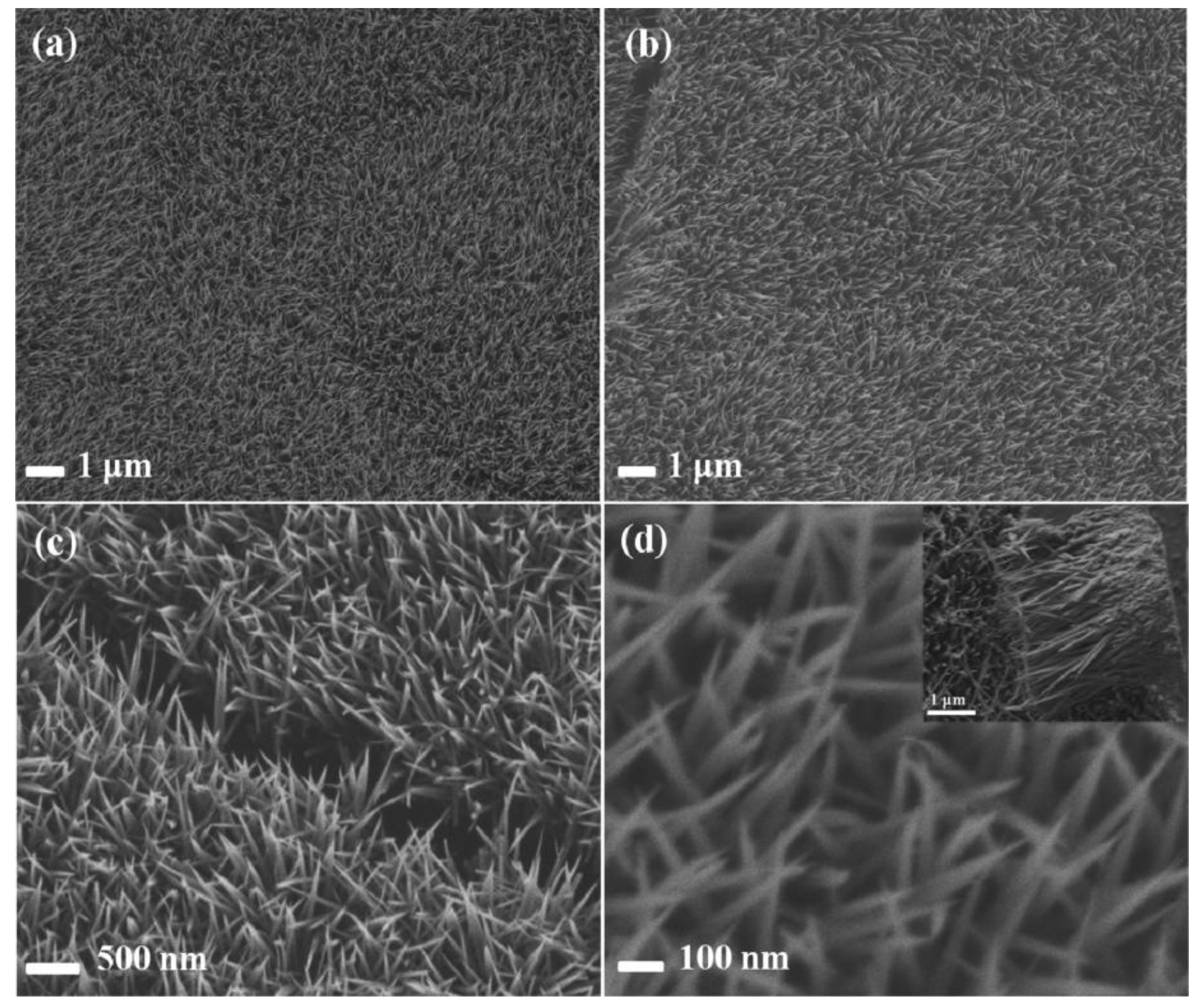
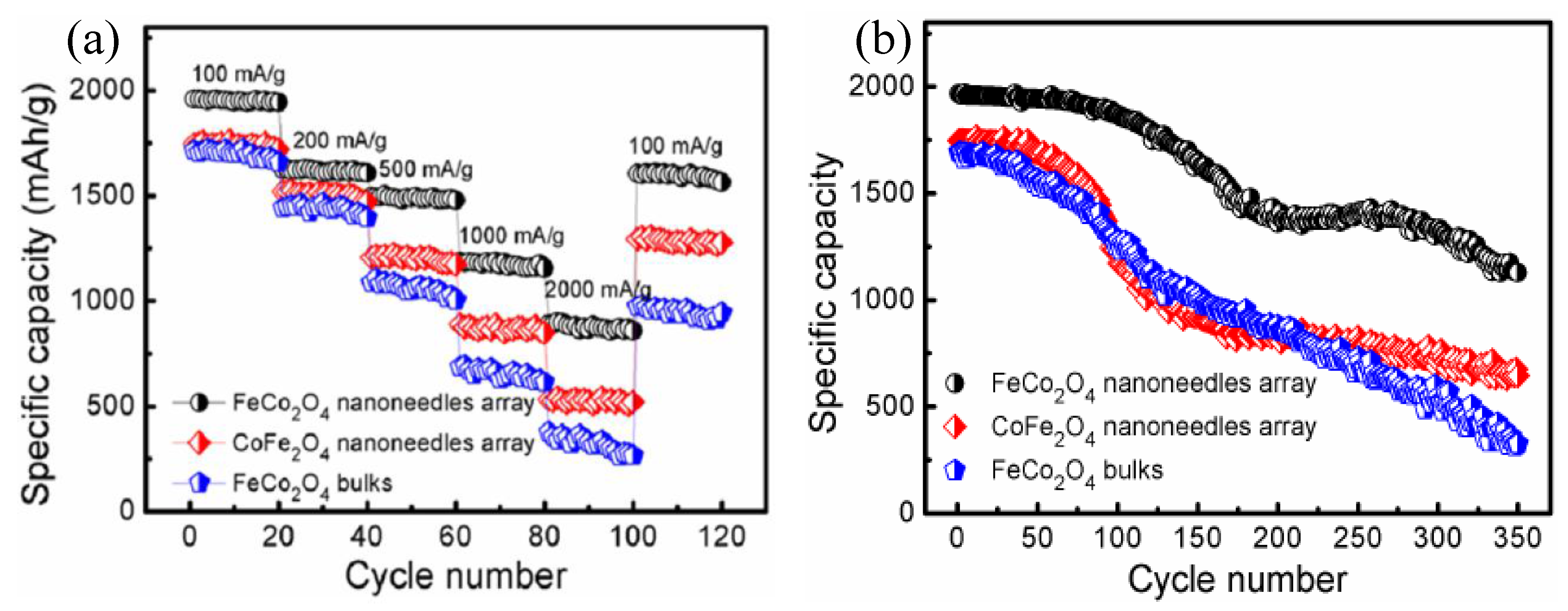

| Materials | Synthetic Method | Potential Window | Specific Capacitance | Cycling Stability | Ref. |
|---|---|---|---|---|---|
| FeCo2O4 nanowires/NF | Hydrothermal | 0 to 0.55 V (vs. Hg/HgO) | 428 F g−1 (5 mV s−1) | 142% retention after 2000 cycles | [18] |
| CoFe2O4 nanoparticles | Hard-templating | −1.0 to 0.5 V (vs. Hg/HgO) | 142 F g−1 (2 mV s−1) | 71.8% retention after 1000 cycles | [19] |
| CoFe2O4 hollow microspheres | Hydrothermal | 0 to 0.4 V (vs. Ag/AgCl) | 1790 F g−1 (2 A g−1) | 98% retention after 500 cycles | [20] |
| CoFe2O4 nanoflakes/SS | Chemical bath deposition | −1.0 to −0.2 V (vs. SCE) | 366 F g−1 (5 mV s−1) | 90.6% retention after 1000 cycles | [21] |
| FeCo2O4 nanoflakes/NF | Hydrothermal | 0 to 2.5 V (two-electrode systems) | 433 F g−1 (0.1 A g−1) | 62.5% retention after 2500 cycles | [22] |
| CoFe2O4 nanosheets/NF | Hydrothermal | 0–0.4 V (vs. Ag/AgCl) | 503 F g−1 (2 A g−1) | 98% retention after 5000 cycles | [23] |
| CoFe2O4 nanomesh/NF | Hydrothermal | 0 to 0.6 V (vs. SCE) | 1426 F g−1 (1 A g−1) | 92.6% retention after 3000 cycles | [24] |
| FeCo2O4 submicron-tube/NF | Chemical bath deposition | −0.2 to 0.6 V (vs. Ag/AgCl) | 1254 F g−1 (2 mA cm−2) | 91% retention after 5000 cycles | [25] |
| CoFe2O4 nanoparticles | Solution combustion | −1.1 to 0 V (vs. Hg/HgO) | 195 F g−1 (1 mV s−1) | 67% retention after 3000 cycles | [26] |
| CoFe2O4/graphene/PANI | Hydrothermal + polymerization | −0.8 to 0.2 V (vs. Hg/HgO) | 1133.3 F g−1 (1 mV s−1) | 96% retention after 5000 cycles | [27] |
| FeCo2O4-Tube/MnO2 nanosheets | Chemical bath deposition + Hydrothermal | 0 to 0.6 V (vs. Ag/AgCl) | 3.3 F cm−2 (1 mA cm−2) | 94% retention after 2000 cycles | [28] |
| CoFe2O4/MnO2 nanosheet arrays | Hydrothermal | −0.2 to 0.4 V (vs. Ag/AgCl) | 3.59 F cm−2 (2 mA cm−2) | 91.5% retention after 2250 cycles | [29] |
| FeCo2O4/MnO2 nanosheet arrays | Hydrothermal | 0 to 0.45 V (vs. SCE) | 2491.8 F g−1 (4 mA cm−2) | 87.2 retention after 5000 cycles | [30] |
| CoFe2O4/FeOOH | Hydrothermal | −0.2 to 0.3 V (vs. Hg/HgO) | 332.4 F g−1 (0.5 A g−1) | 91.3% retention after 1000 cycles | [31] |
| CoFe2O4/RGO/PANI | Solution combustion + polymerization | −0.174 to 0.926 V (vs. SHE) | 239 F g−1 (1.5 A g−1) | 100% retention after 1000 cycles | [32] |
| Materials | Synthetic Method | Reversible Capacity (mA h g−1) | Rate Capability | Ref. |
|---|---|---|---|---|
| FeCo2O4 nanoflakes/NF | Hydrothermal | 905 at 200 mA g−1 after 170 cycles | 1222 mA h g−1 at 800 mA g−1 | [22] |
| 3D ordered macroporous CoFe2O4 | Templating | 702 at 0.2 mA cm−2 after 30 cycles | 816 mA h g−1 at 5 mA cm-2 | [42] |
| Hollow CoFe2O4 nanocubes | Metal-organic frameworks | 1115 at 1 C after 200 cycles | 815 mA h g−1 at 20 C | [51] |
| FeCo2O4 nanoneedles/NF | Hydrothermal | 1129 at 100 mA g−1 after 350 cycles | 875 mA h g−1 at 2 A g−1 | [52] |
| Hollow FeCo2O4 nanospheres | Soft-templating | 1060 at 100 mA g−1 after 50 cycles | 823 mA h g−1 at 1 A g−1 | [53] |
| FeCo2O4 octahedra | Evaporation-induced self-assembly | 1101 at 1000 mA g−1 after 200 cycles | 518 mA h g−1 at 10 A g−1 | [54] |
| CoFe2O4 nanosheets | Thermal decomposition | 806 at 1 A g−1 after 200 cycles | 303 mA h g−1 at 10 A g−1 | [55] |
| CoFe2O4 nanowires/FCF | Hydrothermal | 954.3 at 200 mA g−1 after 150 cycles | 595.3 mA h g−1 at 3.2 A g−1 | [56] |
| CoFe2O4 nanotubes | Self-templating | 988 at 100 mA g−1 after 100 cycles | 654 mA h g−1 at 5 A g−1 | [57] |
| Cubic aggregated CoFe2O4 nanoparticles | Hydrothermal | 1133.5 at 100 mA g−1 after 120 cycles | 679 mA h g−1 at 3.2 A g−1 | [58] |
| Layer-stacked CoFe2O4 platelets | Co-precipitation | 580 at 5 A g−1 after 2000 cycles | 654 mA h g−1 at 10 A g−1 | [59] |
| CoFe2O4 nanorods | Templating | 800 at 1 A g−1 after 300 cycles | 840 mA h g−1 at 1 A g−1 | [60] |
| CoFe2O4 octahedra | Sol-gel | 992 at 100 mA g−1 after 200 cycles | 366 mA h g−1 at 5 A g−1 | [61] |
| Hollow CoFe2O4 nanospheres | Hydrothermal | 1185 at 90 mA g−1 after 50 cycles | 1000 mA h g−1 at 900 mA g−1 | [62] |
| Flower-like CoFe2O4 microspheres | Hydrothermal | 733.5 at 200 mA g−1 after 50 cycles | 717 mA h g−1 at 1 A g−1 | [63] |
| Materials | Synthetic Method | Reversible Capacity (mA h g−1) | Rate Capability | Ref. |
|---|---|---|---|---|
| CoFe2O4 nanoclusters/graphene aerogels | Solvothermal | 966 at 500 mA g−1 after 300 cycles | 221 mA h g−1 at 8 A g−1 | [68] |
| CoFe2O4 nanoparticles/graphene | Carbon dioxide-induced deposition | 1114 at 100 mA g−1 after 100 cycles | 636 mA h g−1 at 3 A g−1 | [69] |
| Flower-like CoFe2O4/MWCNTs | Hydrothermal | 823 at 45 mA g−1 after 50 cycles | 359 mA h g−1 at 1.8 A g−1 | [70] |
| CoFe2O4/carbon nanofibers | Pyrolysis-oxidation | 705 at 100 mA g−1 after 250 cycles | - | [71] |
| CoFe2O4 nanoparticles/Vulcan XC-72 | Thermal decomposition | 766 at 100 mA g−1 after 25 cycles | 478 mA h g−1 at 1 C | [72] |
| C/CoFe2O4 fiber-in-tube nanostructure | Electro-spinning | 740 at 200 mA g−1 after 200 cycles | 488 mA h g−1 at 1.6 A g−1 | [73] |
| Core/shell structured CoFe2O4/onion-like C | Arc discharge | 914.2 at 91.6 mA g−1 after 500 cycles | 617.1 mA h g−1 at 916 mA g−1 | [74] |
| CoFe2O4 nanoclusters/RGO | Solvothermal | 1040 at 91.4 mA g−1 after 30 cycles | 380 mA h g−1 at 18.28 A g−1 | [75] |
| CoFe2O4/graphene | Liquid-solid-solution assembly | 1102 at 200 mA g−1 after 100 cycles | 410 mA h g−1 at 6.4 A g−1 | [76] |
| CoFe2O4/graphene nanocomposite | Hydrothermal | 910 at 100 mA g−1 after 50 cycles | 406 mA h g−1 at 2 A g−1 | [77] |
| CoFe2O4/C twin elliptical frustums | One-pot refluxing reaction | 875 at 500 mA g−1 after 600 cycles | 631 mA h g−1 at 4 A g−1 | [78] |
| CoFe2O4 nanosheets/RGO | Solvent method | 835.6 at 400 mA g−1 after 200 cycles | 848.6 mA h g−1 at 1 A g−1 | [79] |
| CoFe2O4/graphene sandwich | Solvothermal | 1047 at 200 mA g−1 after 160 cycles | 440 mA h g−1 at 1.6 A g−1 | [80] |
| Co3O4/ CoFe2O4 nanocomposites | Auto combustion | 896.4 at 64.1 mA g−1 after 60 cycles | 328.1 mA h g−1 at 6 A g−1 | [81] |
| CoO/CoFe2O4 nanocomposites | Separate nucleation and aging | 1040 at 100 mA g−1 after 30 cycles | 490 mA h g−1 at 6.4 A g−1 | [82] |
| CoO/CoFe2O4/N-doped graphene | Hydrothermal | 1172 at 500 mA g−1 after 100 cycles | 680 mA h g−1 at 2 A g−1 | [83] |
| CoFe2O4/MnO2/C nanotubes | Electrospinningand hydrothermal | 713.6 at 100 mA g−1 after 250 cycles | 310.6 mA h g−1 at 1 A g−1 | [84] |
| Materials | Synthetic Method | Onset Potential | Tafel Slope | Durability | Ref. |
|---|---|---|---|---|---|
| 3D CoFe2O4 hollow nanospheres | Hydrothermal | 0.78 V (vs. RHE) | - | 34% decay after 43,200 s | [101] |
| Ni-doped CoFe2O4 hollow nanospheres | Hydrothermal | −0.15 V (vs. Ag/AgCl) | - | - | [104] |
| FeCo2O4/hollow graphene spheres | Electrostatically induced assembly method | −0.09 V (vs. Ag/AgCl) | 56 mV/dec | 7.9% decay after 86,400 s | [103] |
| CoFe2O4 nanospheres/graphene | Solvothermal | −0.11 V (vs. Ag/AgCl) | 61 mV/dec | 6% decay after 72,000 s | [105] |
| CoFe2O4/biocarbon nanocomposites | Biotemplate and chemical precipitation | −0.14 V (vs. Ag/AgCl) | - | 15.1% decay after 43,000 s | [105] |
| CoFe2O4/rod-like carbon | Hydrothermal | −0.1 V (vs. Ag/AgCl) | 99 mV/dec | 9.5% decay after 20,000 s | [106] |
| CoFe2O4/Carbon nanotubes | Solvothermal | −0.124 V (vs. Ag/AgCl) | - | - | [107] |
| CoFe2O4/graphene | Solvothermal | −0.136 V (vs. Ag/AgCl) | 67 mV/dec | 5.5% decay after 43,200 s | [108] |
| N,S dual-doped 3D RGO/CoFe2O4 | Hydrothermal | −0.14 V (vs. Ag/AgCl) | 70 mV/dec | 6.7% decay after 43,200 s | [109] |
| Ag/CoFe2O4/C | Solvothermal | E1/2 at −0.13 V (vs. Hg/HgO) | - | 5.2% decay after 5000 s | [110] |
| Co/CoO/CoFe2O4/graphene nanocomposites | Separate nucleation and aging | E1/2 at −0.25 V (vs. Ag/AgCl) | - | 20% decay after 20,000 s | [111] |
| MnO2-CoFe2O4/C | Solvothermal | 0.85 V (vs. RHE) | - | 7.3% decay after 10,000 s | [112] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Liu, S.; Li, Y.; Conte, E.; Cao, Y. A Critical Review of Spinel Structured Iron Cobalt Oxides Based Materials for Electrochemical Energy Storage and Conversion. Energies 2017, 10, 1787. https://doi.org/10.3390/en10111787
Gao H, Liu S, Li Y, Conte E, Cao Y. A Critical Review of Spinel Structured Iron Cobalt Oxides Based Materials for Electrochemical Energy Storage and Conversion. Energies. 2017; 10(11):1787. https://doi.org/10.3390/en10111787
Chicago/Turabian StyleGao, Hongyan, Shuai Liu, Yafei Li, Eric Conte, and Yan Cao. 2017. "A Critical Review of Spinel Structured Iron Cobalt Oxides Based Materials for Electrochemical Energy Storage and Conversion" Energies 10, no. 11: 1787. https://doi.org/10.3390/en10111787
APA StyleGao, H., Liu, S., Li, Y., Conte, E., & Cao, Y. (2017). A Critical Review of Spinel Structured Iron Cobalt Oxides Based Materials for Electrochemical Energy Storage and Conversion. Energies, 10(11), 1787. https://doi.org/10.3390/en10111787





