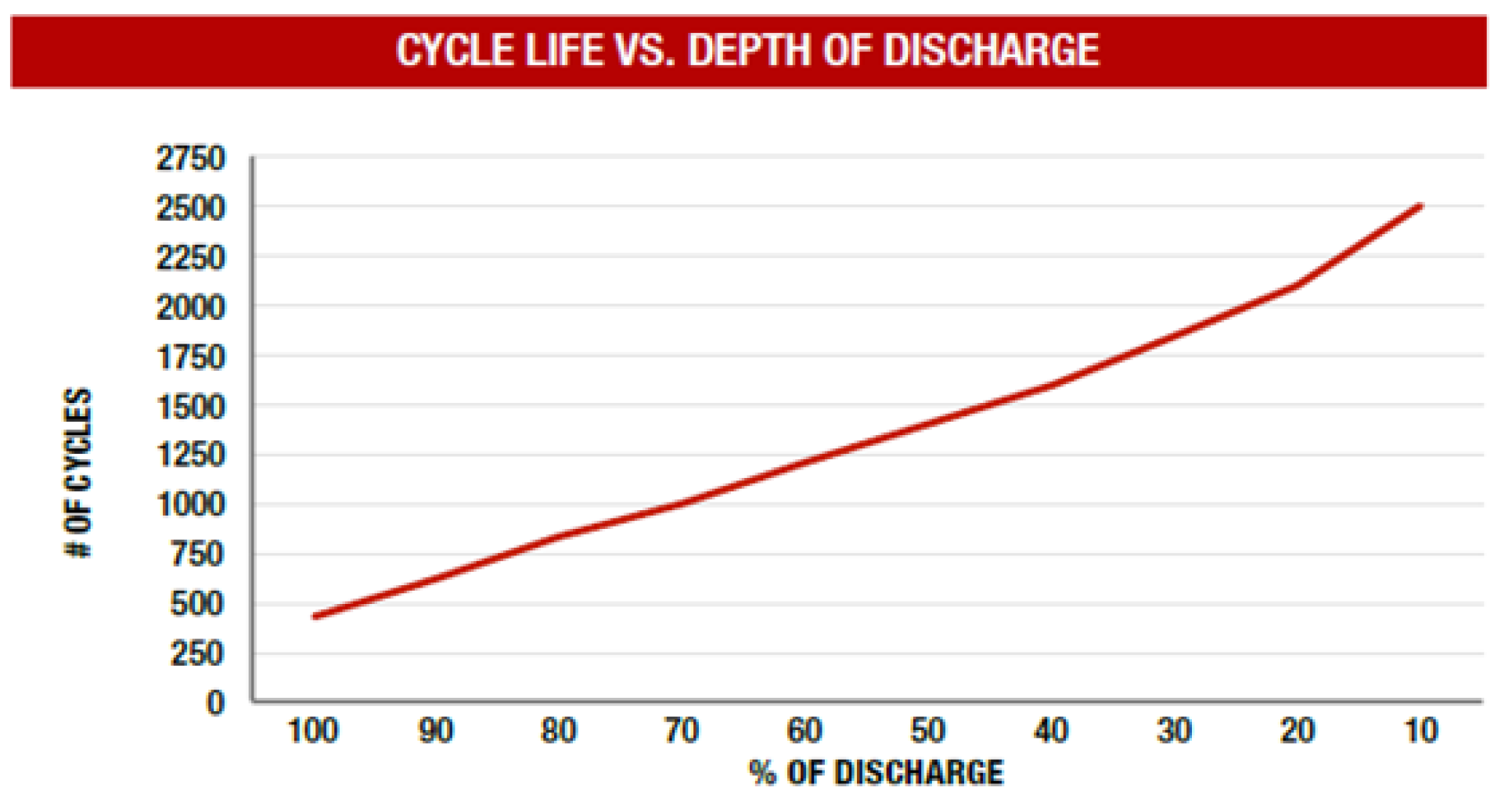
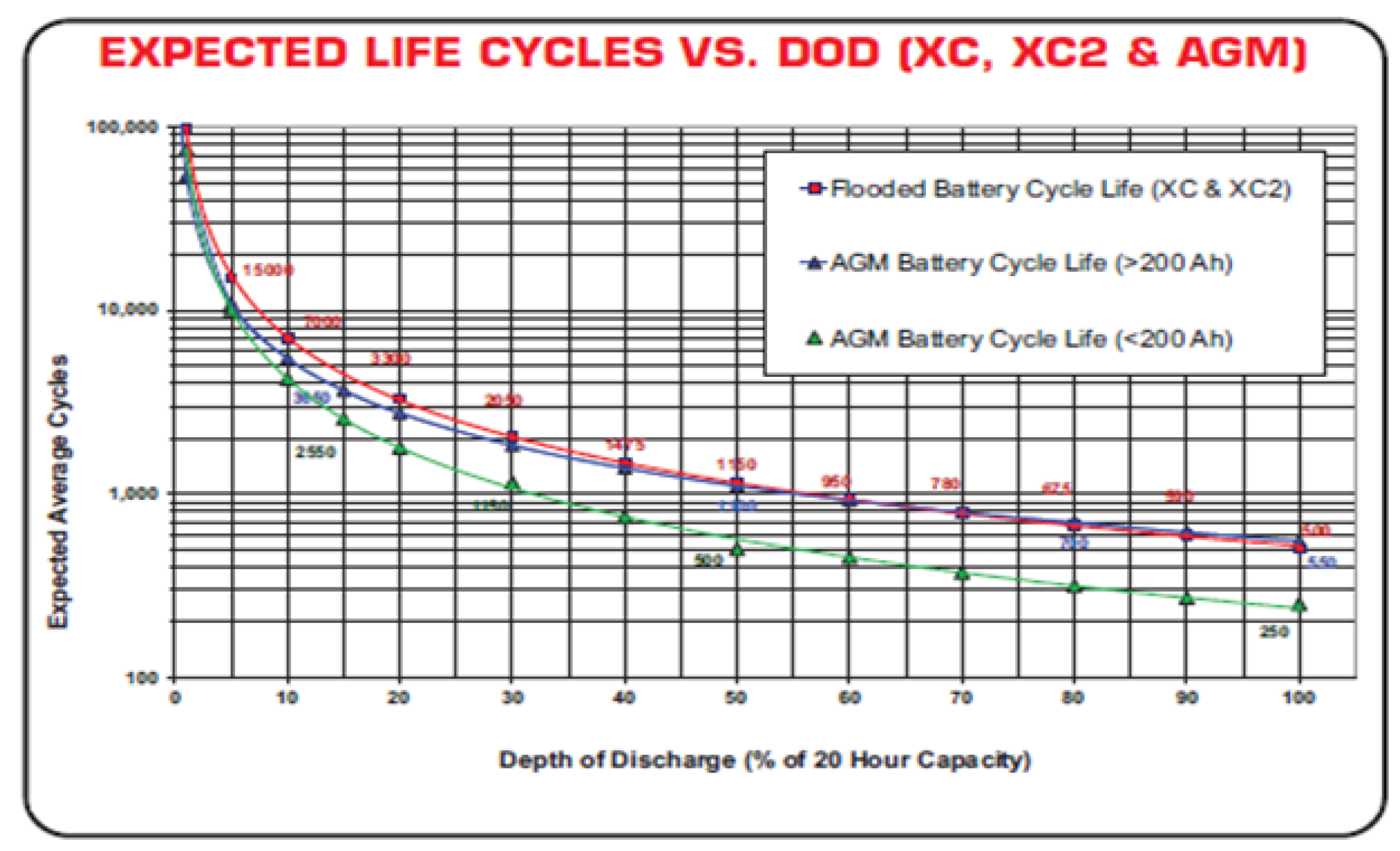
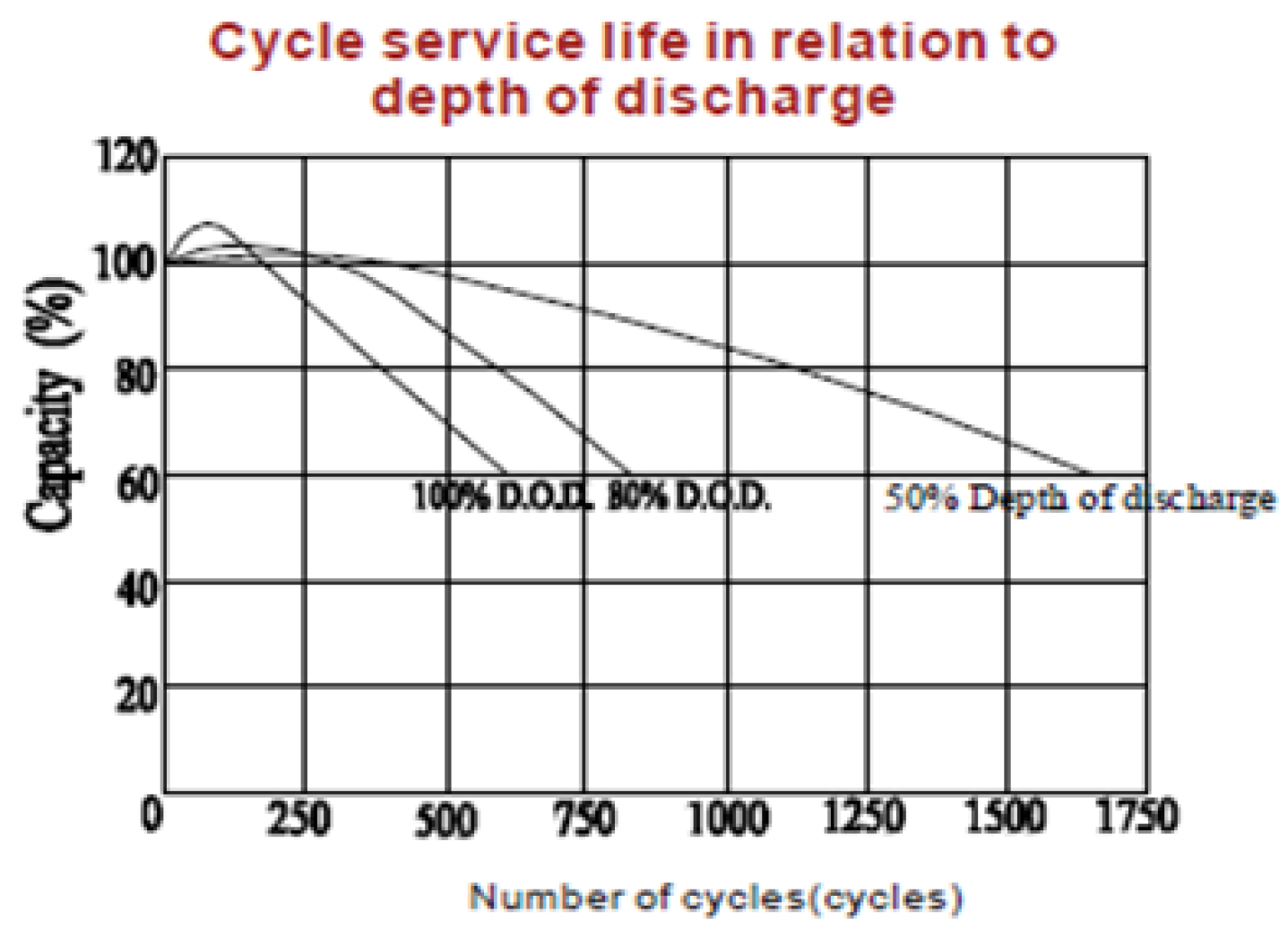
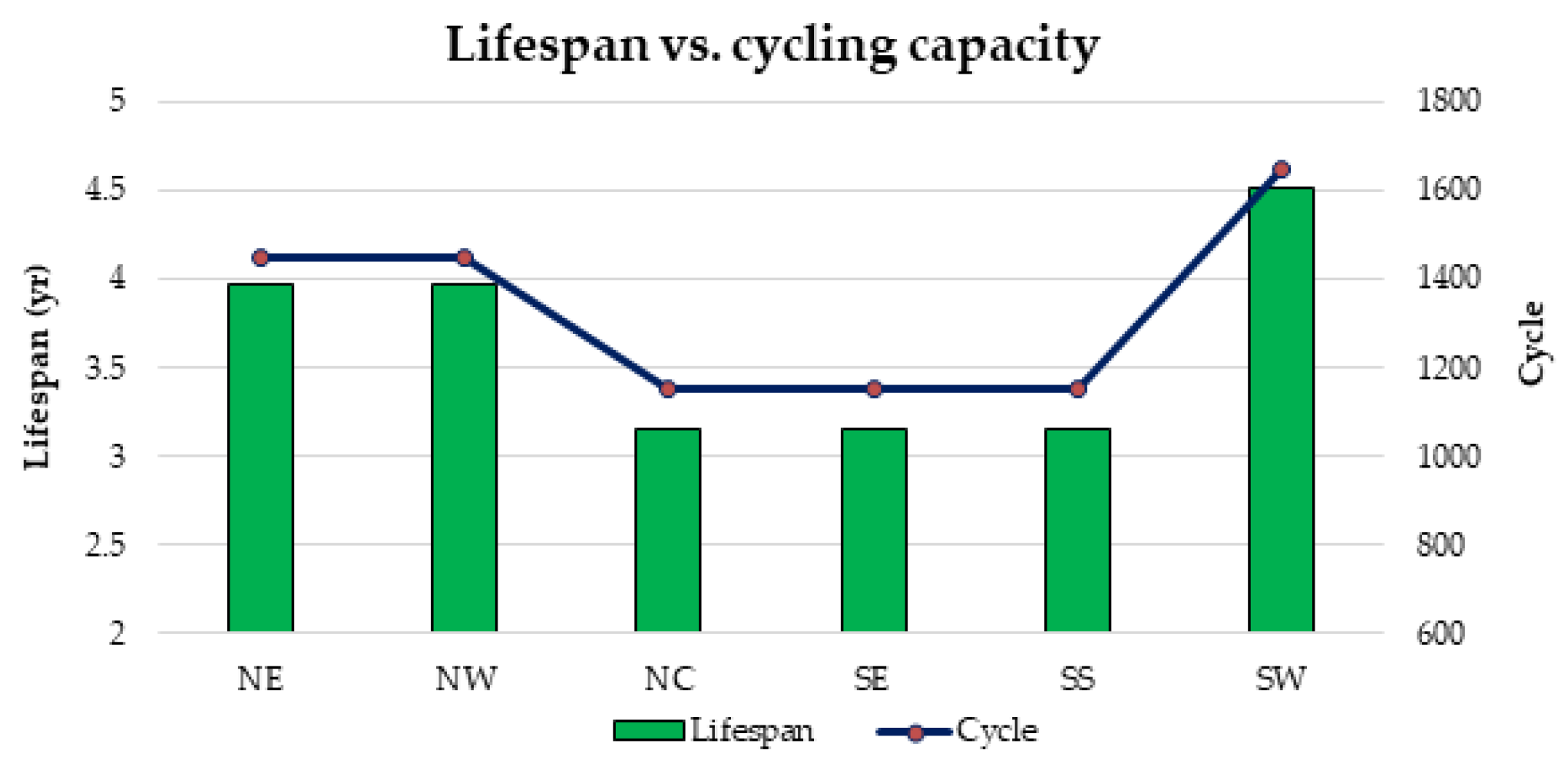
1. Introduction
Energy storage technologies are a key element of modern electrical power system, both for the conventional and the renewable energy systems applications [
1]. They have a wide range of applications in electrical systems both in the on-grid and off-grid electrical power generation systems, and are amongst the distributed energy resources (DERs) [
1,
2]. Some of their major applications include balancing the variable characteristics of the renewable energies, providing ancillary services such as frequency and voltage stability, ensuring a reliable energy supply and increased penetration of renewable energy technologies [
1,
2,
3].
The storage systems currently engaged for stationary applications include the pumped hydro, compressed-air, superconducting magnetic, capacitors and super-capacitors, flywheel, pumped heat and battery technologies [
3]. Battery technologies are one among the promising storage systems for stationary applications, because of their maturity and the ease with which they could be designed and installed compared to other storage technologies [
4,
5].
There are several challenges that exist in modern electrical power systems, which attract the attention and concerted efforts of energy experts, developers and researchers in the field of practice. Some of these problems include frequency and voltage stability, power quality issues such as voltage distortion and sag, intermittent renewable energy output, energy control, peak demand, reliability of energy supply, bi-directional power flow, integration of renewable energy-based microgrids and large-scale wind power etc. [
1,
5]. Energy storage systems have been used in different ways to address some of the mentioned challenges.
It is of interest in this current paper to form an appreciable background by detailing the different kinds of battery technologies and applications, and reviewing some of the existing studies in the literature. This will help to identify the milestones reached and the possible research gap. A critical review of the electrical energy storage has been presented [
1], which focus on the common energy storage systems that are engaged for stationary applications such as the pumped hydro, compressed-air, supercapacitor, superconducting magnetic, batteries, flywheel and thermal systems. Similarly, contributions of the studies in [
2,
3,
4] are on the comprehensive review of energy storage systems. The studies compared the characteristics and costs of the energy storage systems.
The energy storage sizing has been discussed for effective primary and secondary control of low-inertia microgrid systems, focusing on the frequency and voltage stability of the energy generation system [
6]. The optimization of a battery energy storage system has also been presented, focusing on primary frequency control [
7]. The dynamic frequency control support by energy storage has been discussed for reducing the impact of wind and solar generation on the isolated power system’s inertia [
8]. A review on the inertia response and frequency control techniques for renewable energy sources has been discussed, comparing the renewable energy generation systems without storage with those having a storage system [
9].
A study focusing on the battery energy storage has been presented for enabling the integration of distributed solar power generation [
10]. A review on the battery energy storage applications has been discussed with emphasis on wind integrated systems [
11]. The efficiency improvement of mini hydro pump storage power plant using Archimedes turbine has been discussed [
12]. A study on energy storage as the core of renewable energy technologies has been presented [
13], focusing on the key storage technologies and their characteristics. The electricity energy storage technology options have also been presented [
14].
The future-oriented analysis of battery technologies has also been published [
15], focusing on the available storage technologies, and selecting and synthetically characterizing the possible emerging technologies with a time horizon. The overview of current development in electrical energy storage technologies and the application potential in power system operation has been discussed [
16]. A review paper has also been published on the current and future electrical energy storage devices [
17], presenting the storage technologies used for renewable energy applications. The seasonal energy storage in a renewable energy system has been discussed emphasizing the energy storage capacity required to balance the intermittent renewable energy resources [
18]. A research on the technologies for mitigating fluctuation caused by renewable energy sources has been published [
19].
A review on redox flow batteries for the storage of renewable energy has been discussed. The authors first highlighted different storage technologies but placed emphasis on the characteristics, discharge cycles, round-trip efficiency and the applications of flow batteries in electrical power system [
20]. A comparative overview of large-scale battery systems for electricity storage has also been presented, focusing on the types, operational characteristics and the applications of battery systems in large scale solar and wind energies [
21]. A study on the application of battery-based storage systems in household-demand smoothening in electricity-distribution grids has been published [
22]. A battery energy storage system has been discussed, with emphasis on the residential electricity peak demand shaving application [
23].
The electrical energy storage systems in electricity generation have been discussed, introducing the energy policies, innovative technologies, and regulatory regimes for widespread application [
24]. A review of available methods and development on energy storage has been published. The authors provided the technology update [
25]. A comparative life cycle cost analysis of different electrical energy storage systems has been discussed [
26]. The authors considered the pumped hydropower storage, compressed air energy storage (CAES), flywheel, electrochemical batteries (e.g., lead-acid, NaS, Li-ion, and Ni–Cd), flow batteries (e.g., vanadium-redox), superconducting magnetic energy storage, supercapacitors, and the hydrogen energy storage. An optimized home energy management system has been presented with integrated renewable energy and storage resources [
27]. The authors focus on how the renewable energy resources and storage systems could be optimized for sustainable energy utilization.
The economic assessment of energy storage systems providing primary reserve and peak shaving in small isolated power systems has been discussed [
28]. A study on the energy storage and its use with intermittent renewable energy has been published [
29]. The research on the power systems’ optimal peak-shaving has been published with focus on the application to secondary storage [
30]. The authors consider the optimal peak shaving strategy, which enables the minimization of the power peak and the provision of an analytic design technique for achieving the optimal peak shaving. A study considering the state-of-the-art review of energy storage and real life applications has been presented [
31].
A study that considers improving the reliability of photovoltaic-based hybrid power system with battery storage in low wind locations has been discussed [
32]. The authors focus on how reliable energy could be supplied to the users by incorporating a battery storage system the energy system. The real-time testing of energy storage systems in renewable energy applications has been presented [
33]. A study has been published on lead-acid battery response to various formation levels, focusing on the off-grid solar and conventional applications [
34], while the research in [
35] by the same authors concentrate on internal resistance aspect of lead-acid battery response to various formation levels.
The development of phase change materials (PCMs) for low temperature energy storage applications has been presented, for buildings, solar water heating/drying/foot warmers applications [
36]. The research on the evaluation of grid-level adaptability for stationary battery energy storage system applications in Europe has been published [
37]. A review of recent developments of photovoltaics integrated with battery storage systems and related feed-in tariff policies has been presented [
38]. The evaluation of electrical energy storage (EES) technologies for renewable energy has also been presented, using the US Pacific Northwest as case study [
39].
Batteries pose threats to the environment and of course, to human health [
1]. Though several existing studies in the literature have presented quality research on the various battery technologies and their applications, some of which have been mentioned [
1,
2,
3,
4,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39], evaluating the environmental impact of batteries in electrical systems remains a gap that requires concerted research efforts. Every component in the electrical system has its environmental impact [
40], but the focus of this study is not only to present an overview of the battery systems, but also to examine their GHG impact in the energy system.
The existing studies provide relevant and useful background for understanding the different types of energy storage technologies, their features and characteristics, developments and applications in electric power systems. Furthermore, some of the authors have presented how the storage systems can be employed as possible solutions to the challenges of variable characteristics of renewable energies, frequency and voltage instability, peak demand, reliability, power quality issues, grid-integrated microgrids and large-scale wind etc. Some of the studies also consider the life-cycle cost of the storage options, while a few others discussed future-oriented evaluation of battery technologies. However, none of these studies [
1,
2,
3,
4,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39] has considered the evaluation of the environmental impact of batteries in the electrical system. Such an evaluation will be useful for better understanding of the battery lifecycle impact and the possible ways to achieve environmentally friendly energy solutions and operation in the future, in the wake of increased renewable energy-based electricity systems around the world.
Therefore, while this current paper focuses on the review of the state-of-the art of battery storage technologies, it analyses the environmental impact of battery storage technologies in a renewable energy system application. To achieve a sustainable energy requires the consideration of some enabling planning, development and management perspectives, which include the social, technical, economic and the environmental aspects [
40]. Though some of the studies have considered the techno-economic aspects, which is an acceptable analysis technique for ascertaining the technical and economic feasibility of the system [
1,
4,
15,
26,
37], it is also important to examine the environmental impact of the storage system over the energy system’s lifetime. Therefore, this research attempts to consider the critical gap by first introducing an optimal battery technology sizing approach, which is crucial for determining the battery capacity and selecting the suitable battery cells for energy generation applications. It also analyses the life cycle environmental impact of batteries in a renewable energy system application.
In order to determine an optimal battery size, this paper proposes a novel approach that interfaces two critical energy generation design factors such as the reliability and cost. The study considers the system reliability in terms of loss of power probability (LOPP), which is relates the unmet energy demand with the total energy demand over the year [
4]. Obtaining a minimum cost at a given LOPP is an important objective and criterion that informs the technology selection. The paper considers the cost in terms of the cost of energy (COE) in
$/kWh, using the life cycle cost analysis [
31].
To assess the impact of batteries, this study uses a stand-alone or grid-independent solar PV energy generation system as a case study. It then examines how the lifespan, cycling capacity and the number of replacements of the lead-acid battery cells contribute to the environmental impact of the system over the project lifetime of 25 years. Lead-acid battery has been selected for the analysis because of its commercial maturity and cost-effectiveness compared to other systems [
5,
26]; more so, it is the most widely used battery storage technology. The proposed contributions of this paper are expected to provide insights not only into the global progress in battery technologies, but also stand as a reference point for ascertaining the impact of batteries in electrical power systems, especially the off-grid systems that are associated with frequent replacements of battery banks over the project life [
5].
The study uses the lifecycle emission rate (LCER) of the lead-acid battery and the number of times the battery cells are replaced over the PV energy system’s lifespan of 25 years, to assess the GHG impact of different sizes of batteries. In addition to the impact assessment, the paper highlights the possible strategies to minimize the environmental impact of battery cells, and discussed the important criteria for selecting battery technologies for energy systems application. These can serve as a basis for environmental sustainability planning. This paper maintains a position that while it is important for the research community to continually work towards improving the cycling capacity, round-trip efficiency and the energy density of batteries, it is also necessary to intensify research in the aspect of environmental impact of batteries in the modern-day energy system.
The remaining part of the paper is arranged as follows:
Section 2 discusses the existing battery technologies and their comparison;
Section 3 presents the case study and methodology;
Section 4 focuses on the results of the impact analysis;
Section 5 presents the new and future battery technologies;
Section 6 presents the criteria for battery selection while
Section 7 concludes the paper.
2. Battery Storage Technologies
Rechargeable (secondary) battery storage systems comprise of a wide range of technologies and are classified based on the type of electrodes and electrolytes used in their storage system arrangements [
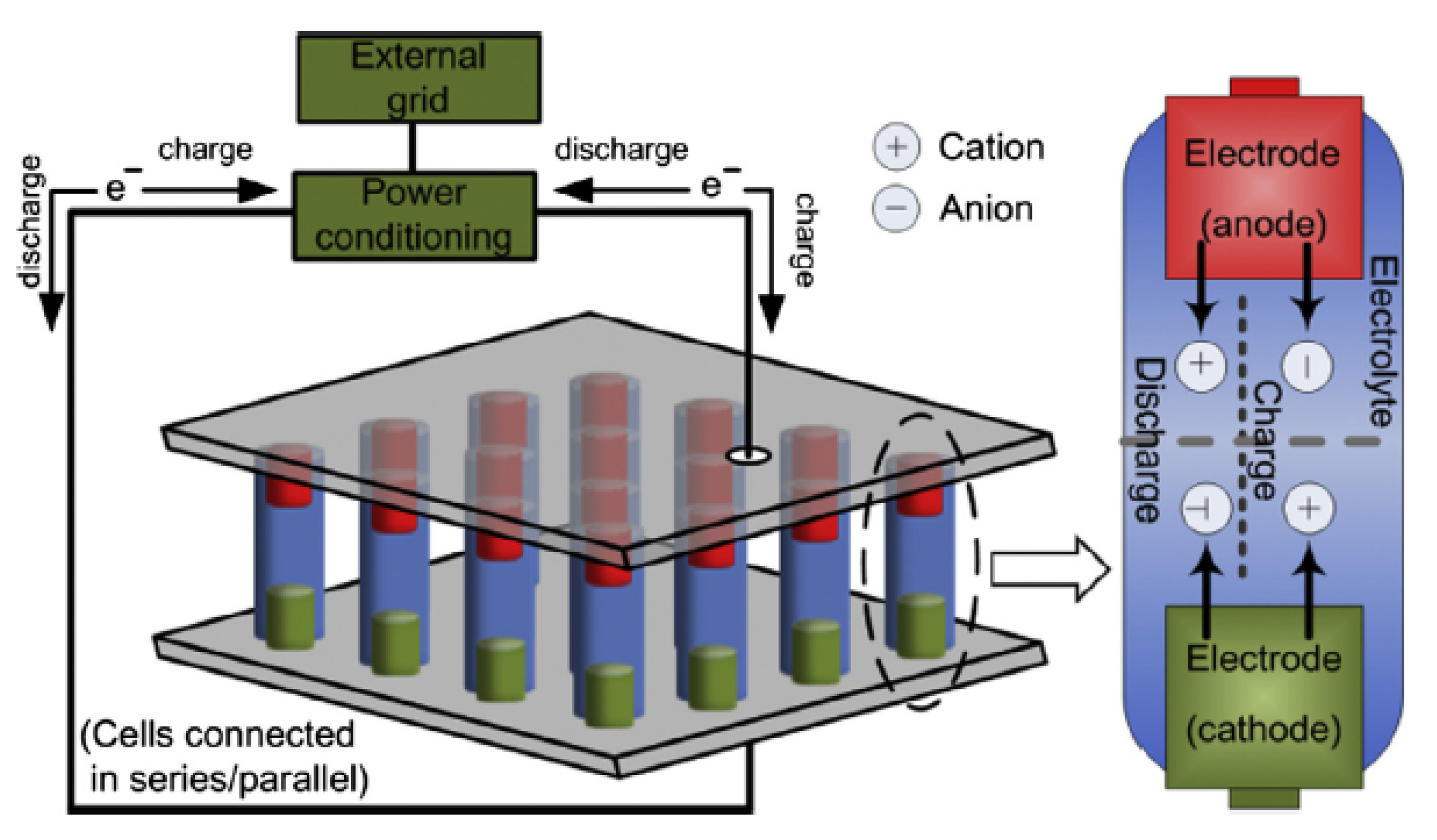
26]. The operation of a typical battery energy system is presented in
Figure 1. The battery system is made up of electrochemical cells that are wired in series, and which generate electrical energy at a specified voltage through an electrochemical reaction. Each electrochemical cell has two electrodes (i.e., anode and cathode) and an electrolyte [
16]. An electrochemical cell can convert energy from electrical to chemical energy and vice-versa. At discharge, the electrochemical reactions occur at the two electrodes at the same time. Therefore, electrons are provided from the anodes and are collected at the cathodes at the external circuit. During the charge state, the reverse reactions occur and the battery is recharged through an external voltage that is applied to the electrodes.
The battery technologies are practically the most widely used storage technologies because of the ease with which they are designed and manufactured [
41], and are employed for bridging power applications in electrical systems [
3]. This is because of their relatively lower cycling capacity compared to super-capacitors, flywheel and superconducting magnetic storage systems that are popularly used for power quality purposes.
The popular battery technologies systems currently engaged for stationary applications include the lead-acid, sodium sulfur (NaS), sodium-nickle chloride (NaNiCl
), also known as ZEBRA, nickel cadmium (Ni–Cd), lithium-ion (Li-ion), zinc-bromide (Zn–Br), polysulfide bromine (PSB) and vanadium-redox (VRFB) [
26], including the new systems such as advanced valve regulated (VRLA) lead-acid, lead-carbon, metal-air technologies, UltraBattery, battery with current collector improvement, advanced sodium-metal chloride, high performance sodium-copper chloride and nanostructured energy materials in lithium batteries.
The history of battery advancements is presented in
Table 1. The information details the various inventions, the Scientists who developed the ideas/technologies between the year 1600 and 2002. The voltaic cell technology was invented in 1800 by Alessandro Volta, which sets the pace for the battery storage system, even though the idea of electrochemistry dates to 1600.
Some notable current practical renewable energy-based and power applications of batteries are summarized as follows [
43]:
Bulk wind to distributed energy storage. Coupling the wind resource with distributed battery storage will help address the issue of intermittent energy generation. As such, large scale wind may be integrated with the existing grid in the future.
Community-based energy storage (CES). A community battery storage system may be designed to island, meaning that when a localized section of the distribution power system is isolated from the grid, it can support the users’ demand, e.g., a back-up power. Battery systems can also be used as part of a completely autonomous power generation system with a solar PV, wind, small hydro, biomass and/or distributed diesel power plant serving an off-grid homes or community.
Distributed grid-integrated PV system. A grid-connected or on-site storage located/connected near the PV system helps to manage the challenges of rapid output variations, daily variations, power quality issues-harmonics and the mismatch between the PV output and the users’ demand that are posed by grid-connected PV systems without storage.
Energy storage and plug-in vehicles. A distributed battery storage system for electric vehicle charging can be a part of a localized energy system strategy to integrate a distributed solar PV power to improve the reliability of energy supply at a specified portion of the electric grid.
End-user bill management. Storage system could be employed to reduce the cost of electricity service. Such an application could also help the end-users to “time-shift” their energy from PV or other generation options or it could be employed to integrate a nearby solar PV system. In this case, a time-of-use (TOU) energy pricing may use storage to reduce electricity cost when the demand and the price of energy are low, and then used later when the demand and price are high, instead of purchasing high-priced electricity.
Flexible peaking resource. “Peakers” or “Peaking resources” are designed to serve peak electricity demand. One of the alternatives to peakers is the energy storage option, e.g., battery, which can provide a responsive and high flexible peaking resource compared to the demand-side management approaches. Using modular battery storage for serving a peaking demand could also be cost-effective.
Frequency regulation. Traditionally, frequency regulation is essentially the “ramping” of electric generation assets in a timeframe of minutes. However, electricity storage has the potential of serving this purpose in milliseconds, which also provides an economic prospect. For instance, the Pacific Northwest National Laboratory (PNNL) suggested that storage systems on a millisecond timeframe could have a value of at least that of 20 mins timeframe electric power assets. Battery storage system is one the popular storage options that can contribute to the grid stability.
Spinning reserve. Utilities are usually charged with the responsibility of being able to accommodate the loss of the largest power generator in the network with minimum load flow and frequency deviation. In this situation, all generators have a proportion of their reserve capacity associated with their primary energy source and inertia. For a steam-power option, additional fuel is wasted as the generator will be operated below its rated capacity. Nowadays, storage systems are used for this capability through dedicated power converters that interact the with the power grid.
Transmission and distribution (T & D) upgrade deferral. Storage systems are a key option for deferring or avoiding the need for upgrading the transmission and distribution apparatus. Since a modular configuration of a storage system, e.g., battery could be employed to serve a small proportion of peak demand, there will not be the need to increase the capacity of the T and D in the short-term. This also helps to extend the life span of the T and D equipment.
Uninterruptible power systems (UPS). Battery systems are a crucial component of the UPS used to provide a stable and reliable power for critical loads, e.g., medical, emergency lighting and communication equipment, to minimize or forestall lost productivity, facilities or equipment damage.
2.1. Lead-Acid
The lead acid technologies are the oldest form of battery energy storage system developed by a French physicist Gaston Planté in 1859 [
1,
3,
26,
41]. They have been a common storage option for mini-or micro-grids or the grid-independent electrical power systems, uninterrupted power supply and spinning reserve applications [
1].
The electrochemistry of lead-acid technologies in the charge state consists of a lead dioxide (PbO
) and lead (Pb) in a concentrated tetraoxosulphate (VI) acid electrolyte [
41]. The PbO
and the Pb are the positive and negative electrodes, respectively. However, in the discharge state, the electrodes—lead dioxide and the lead are converted to lead sulphate (PbSO
); thus, they consumed the sulphate ions. This development reduces the specific gravity of the electrolyte to a level similar that of water, meaning that the electrolyte loses its dissolved tetraoxosulphate (VI) acid and turns to water [
1,
41].
The PbO
(positive electrode) is the key factor that influences the performance and cycle life of a lead-acid technologies [
41], while Pb (negative electrode) determines the cold-temperature performance of the systems. The electrochemical reactions of lead-acid batteries are as follows. At the anode (i.e., positive electrode)
At the cathode (i.e., negative electrode)
There are different types of lead acid technologies including the flooded type that requires regular topping up with distilled water and the sealed
maintenance free type that has a gelled/absorbed electrolyte, and the valve regulated type [
1,
3].
Lead-acid batteries have a low cycle life ranging from ~2000–2500, a round trip-efficiency (RTE) of ~70–90%, and a lifespan of ~5–15 years and a low energy density of ~40–50 Wh/kg [
26,
41]. They are identified as low-cost secondary battery technologies, which is one of the reasons for their widespread application electrical and renewable energy applications.
They possess a moderately good operating temperature ranging from
–60
[
41]. However, they have a potential for generating a negative environmental influence because of the toxic remnants they produce [
1]. Their grids also contain antimony and arsenic, which constitute health hazards [
41].
2.2. Sodium-Sulfur
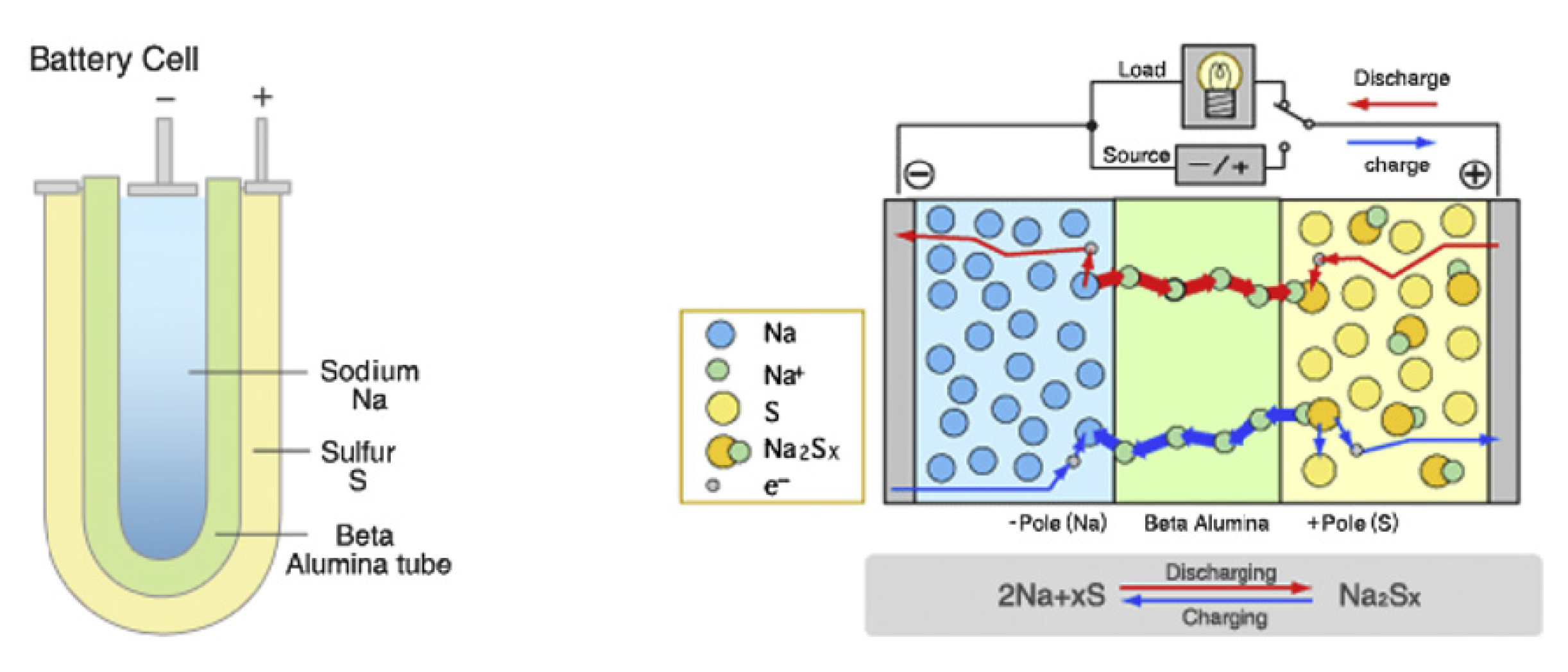
The NaS technologies were developed by NGK Insulators and Tokyo Electric Power in 1987 [
26]. They are one of the most proven battery storage technologies for the mega-watt scale electrical applications [
14]. They NaS batteries have been applied for power quality and power time shift purposes, because of their relatively higher RTE ranging from 75–~90% [
1,
26]. The NaS battery consists of molten sulphur at the anode and molten sodium at the cathode, which are separated by a solid beta alumina ceramic electrolyte [
1]. The electrolyte allows the positive sodium (Na
) ions to flow through it and then combines with sulphur to form sodium polysulphides as
In the discharge state, the Na
ions flow through the electrolyte and this causes electrons to flow in the battery’s external circuit, thus, delivering 2 V. The electrochemistry is a reversible process, as charging causes the Na
S
to release the Na
back through the electrolyte, to recombine as elemental sodium (Na). The schematic diagram of the structure of NaS is presented in
Figure 2.
They have a cycle life, lifespan and discharge time of 2500–4500, ~10–15 yr and up to 7 h, respectively [
44]. The power rating of the technology is scalable, and this promises widespread utility-scale applications in the future. The typical energy and power densities of the NaS technology range from 150–240 Wh/kg and 150–230 W/kg, respectively [
1]. However, the main drawback of this technology is that a heat source is usually required that uses a part of the battery’s energy; thus, reducing the battery performance, as it needs to operate at a high temperature of about 300–350
, see [
1].
2.3. Nickel-Cadmium
This technology is one of the oldest battery storage technologies [
1,
26,
45], for instance, the nickel-cadmium (Ni–Cd) battery was invented by Waldmar Jungner in 1899. However, the application of the Ni–Cd was limited because of material cost and the difficulty to manufacture. There exist five different battery technologies that use the nickel-electrode in their design, manufacture and operation [
3,
45]. These include the nickel-iron (NiFe), Ni–Cd, nickel hydrogen (Ni–H
), nickel metal hydride (Ni–MH), and nickel-zinc (Ni–Zn). Ni–Cd and Ni–MH are the widely used technologies compared to others; however, Ni–Cd is currently the most utilized nickel-electrode in modern utilities around the world [
45].
They have energy and power density of 50–75 Wh/kg and 150–300 W/kg, respectively, cycle life of 2000–2500, a lifespan of ~10–20 years and RTE of ~70–72%, see [
3,
11,
44]. The Ni–Cd battery is employed for different applications such as power quality and for forestalling unscheduled outage, i.e., emergency reserve for communication services, for power tools, portable devices and emergency lighting, UPS and generator starting [
1,
26].
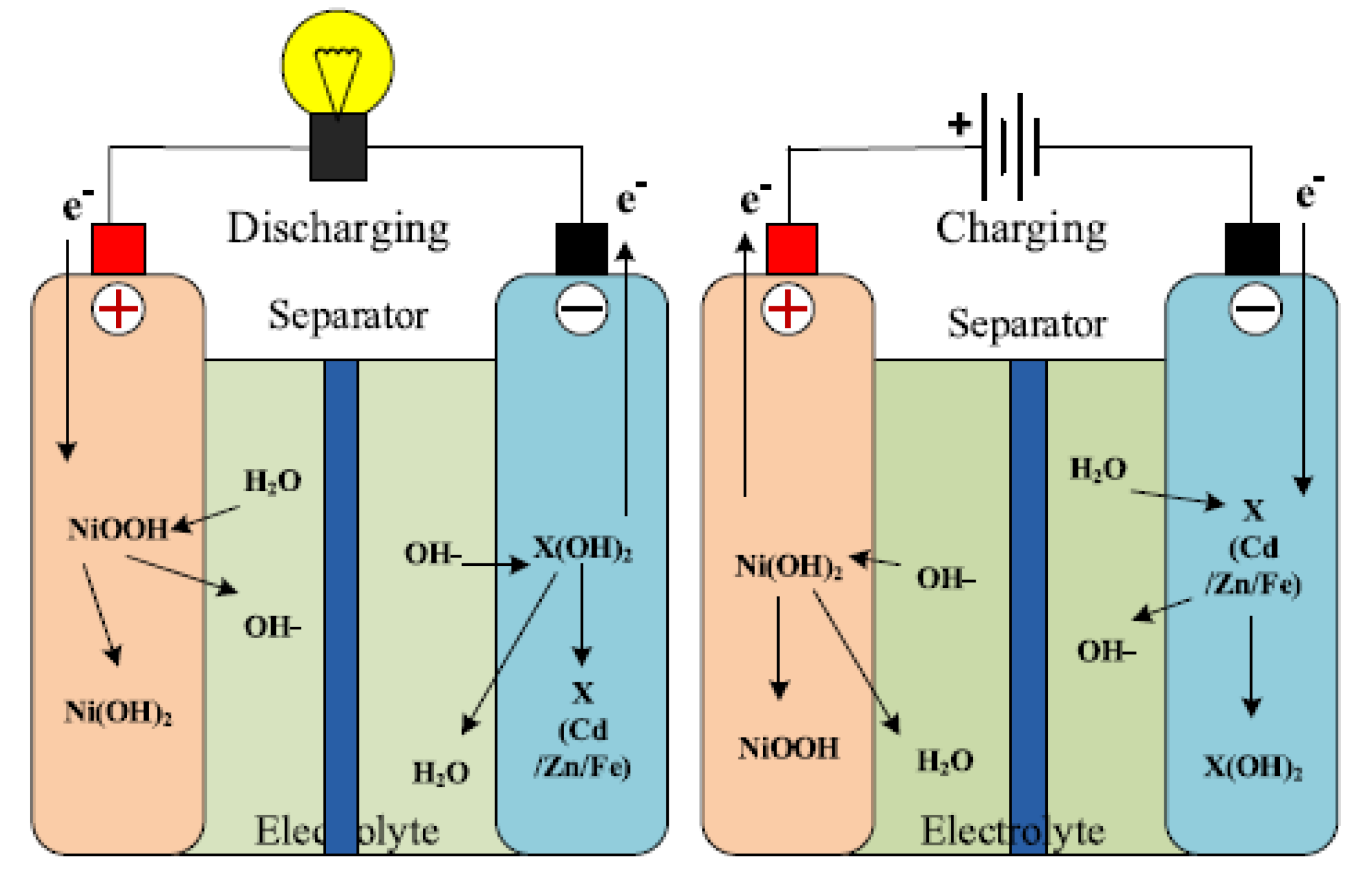
The anode of the Ni–Cd battery is nickel hydroxide (Ni(OH)
) while the cathode is a cadmium hydroxide plate. The battery also has a separator, and an alkaline electrolyte [
1]. The battery usually designed with a metal case and a sealing plate, equipped with a safety valve. During the charging process, the Ni(OH)
is converted to nickel oxyhydroxide–NiOOH, while the anode is converted to cadmium hydroxide Cd(OH)
[
3,
46,
47]. The conversion process of the anode is possible through oxidation when it is charged in the presence of aqueous potassium hydroxide KOH. In the discharge state, NiOOH reacts with H
O to produce the Ni(OH)
and hydroxide ion at the anode [
45,
47]. The electrochemistry of the battery system is described by
and illustrated in
Figure 3.
2.4. Lithium-Ion
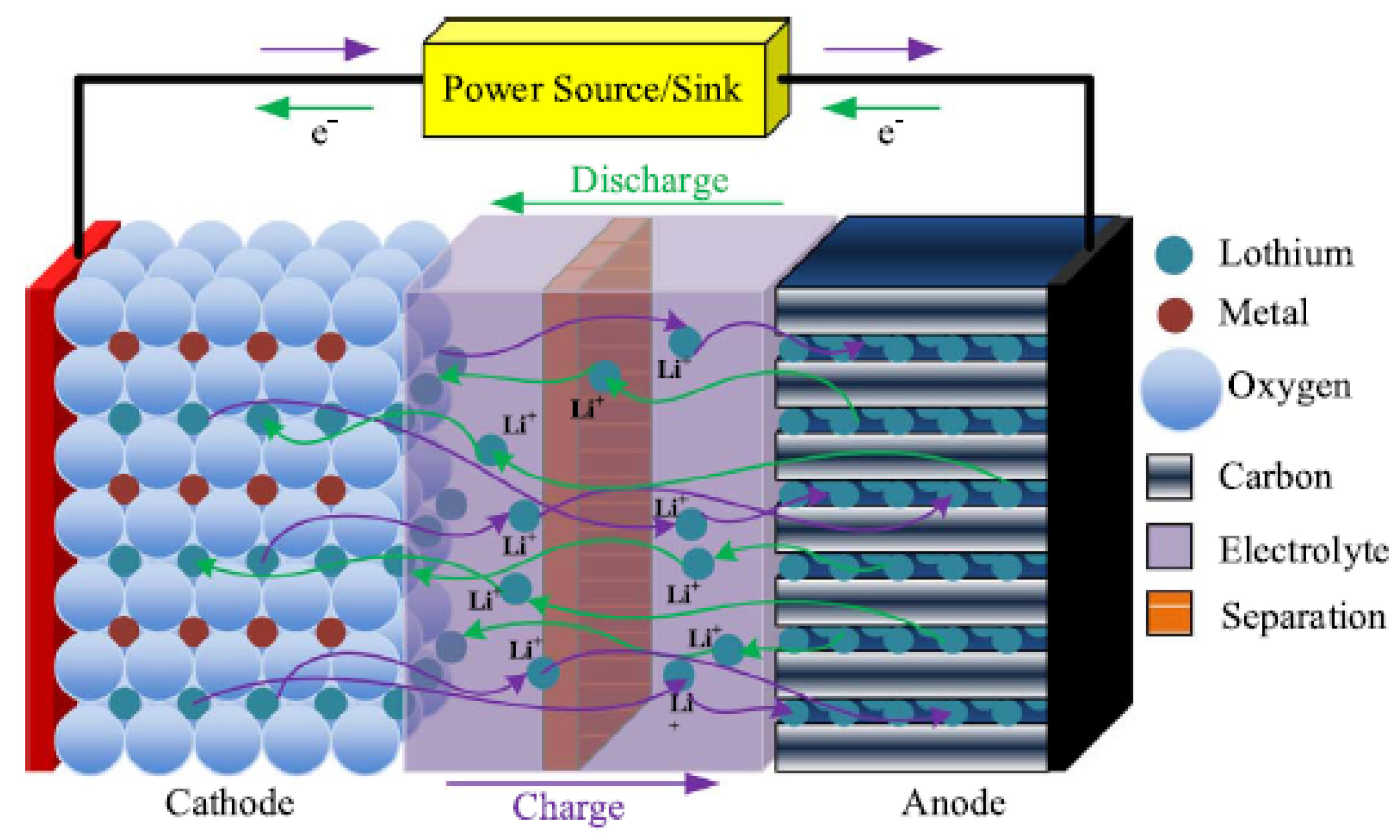
Traditionally, lithium-based batteries are widely used in smaller appliances, such as mobiles and laptops but not employed for electric power supply purposes [
11]. Lithium batteries are fast developing since their invention in the 1960s and are categorized into lithium polymer cells and lithium-ion technologies [
1,
11,
42,
49]. In lithium-ion battery technology, the organic carbonates of lithium (LiPF
) are used as electrolyte. The negative electrode is usually a lithium metal oxide such as LiMO
, LiCoO
or LiNiO
, etc., while the positive electrode is made of a
graphitic carbon [
1].
During charging, the lithium atoms in the negative electrode become ions and migrate to the positive electrode (i.e., carbon) where they recombine with external electrons to form the lithium atoms [
1,
11], as
and
During discharging, the process is reversed. The battery electrochemistry is illustrated in
Figure 4.
As research and development in the technology advances, the energy density has increased from 75–200 Wh/kg, with an increased cycle life of 10,000 cycles [
1]. The RTE of the Li-ion batteries is about 100%. This is an important of this technology over other battery technologies. The lithium polymer batteries, on the other hand, have relatively lower efficiency and lifespan [
11].
2.5. Flow Batteries
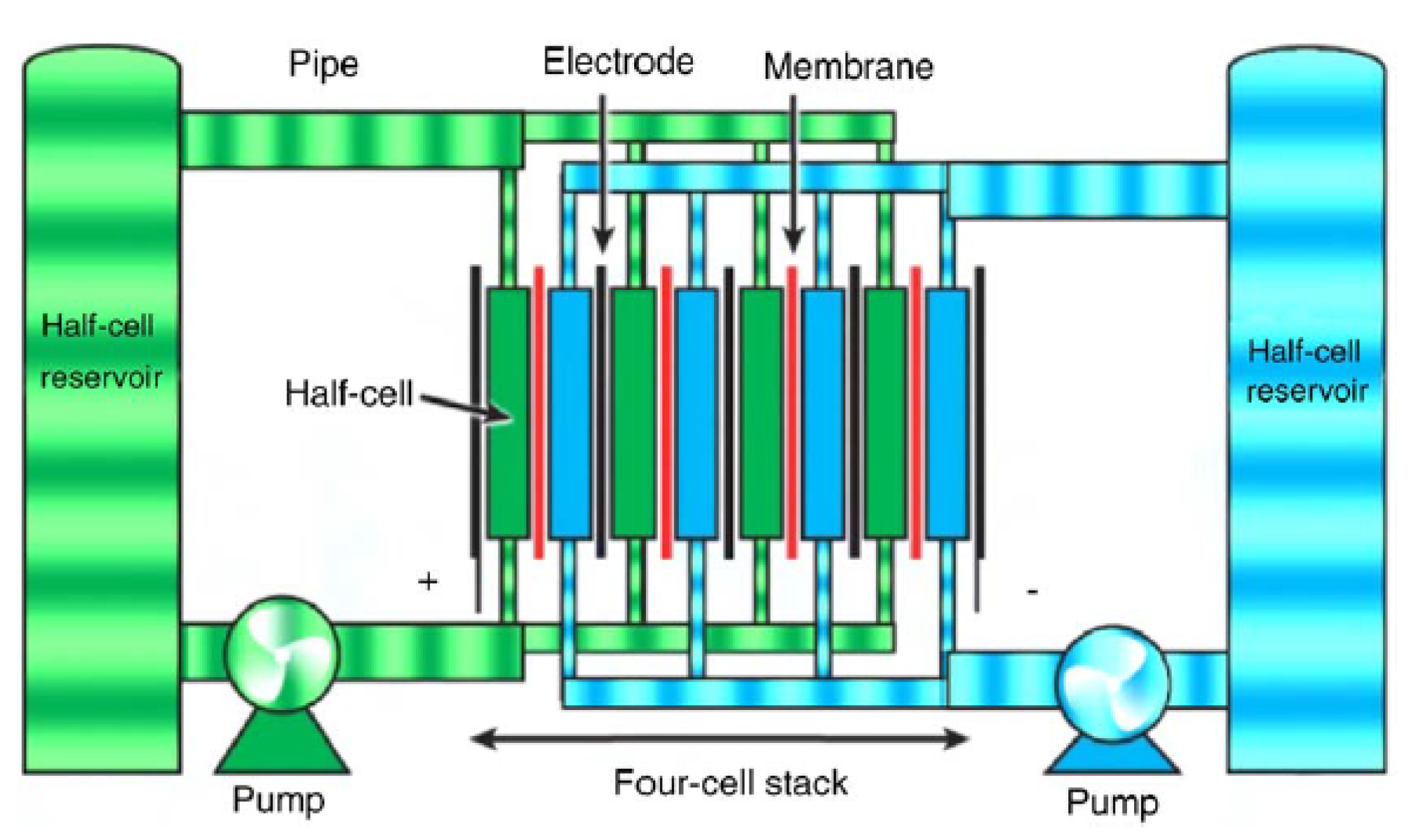
These battery technologies are designed to store energy in the electrolyte solutions—a feature that is opposite to the conventional battery technologies in which the electrodes are used for such a task [
1,
26,
50]. The battery arrangement also includes additional electrolyte that is stored externally in tanks, and is pumped through the cell (or cells) of the reactor [
1].
The mode of operation of flow battery technologies is based on reduction-oxidation (redox) reactions of the electrolytes. In the charging state, one electrolyte is oxidized at the positive electrode while the other is reduced at the negative electrode [
16]. This way, the electrical energy is converted to chemical energy. The chemical reaction is reversible and it allows the battery to be charged, discharged and recharged as desired.
The power and energy capacities of these battery technologies could be designed independently, i.e., the energy capacity is determined by the amount of electrolyte that is stored in external tanks but the power rating is ascertained through the active area of the cell compartment [
26,
50,
51,
52,
53]. These technologies have the potential for continuous release of energy at a high rate, with a discharge of up to 10 h [
1].
Flow batteries are categorized into two technologies such as redox flow batteries and hybrid flow batteries, depending on if all electro-active materials could be dissolved in the electrolyte [
16]. Examples of the redox type are all vanadium (e.g., vanadium redox flow (VRB)), polysulphide bromide (PSB), iron-chromium etc., while the hybrid types are zinc-bromine and zinc-cerium [
50]. The schematic diagram of the structure of a flow battery is shown in
Figure 5.
2.5.1. Zinc-Bromine
In these battery technologies, two aqueous electrolyte solutions contain the reactive materials made of zinc (Zn) and bromine (Br) elements, which are stored in two external tanks [
16]. During the discharge process, the reactive materials combine to form zinc-bromide, and then generate 1.8 V across each of the battery cells [
1]. This then increase the Zn
and Br-ion densities in the two electrolyte tanks. However, in the charge state, zinc is deposited as a thin layer on a side of the composite electrode, while bromine is developed as a dilute solution on the other side of the membrane. It then reacts with other agents, i.e.,
organic amines to develop thick bromine oil, which sinks to the bottom of the electrolytic tank [
1]. This is mixed with the remaining electrolyte during discharge. The chemical reactions at the anode and the cathode are described by
and
respectively [
1], while battery structure is illustrated in
Figure 6.
The zinc-bromine batteries have a lower RTE and lifespan of ~65–75% and over 2000, respectively, compared to the conventional lead-acid batteries [
1,
16]. They have an energy density ranging from 30–50 Wh/kg [
1]. However, the major problems with ZnBr battery are material corrosion and dendrite formation [
16].
2.5.2. Sodium-Nickel Chloride
This technology has been commercially available since about two decades ago [
26]. It is otherwise known as Zero Emission Battery Research (ZEBRA) and is also recognized as a high-temperature battery with temperature ranging from 270–350
[
1,
26]. In the case of NaNiCl
, the nickel chloride is used as the anode instead of sulphur as is the case with NaS. The electrochemistry for ZEBRA is described by
ZEBRA battery technologies can withstand limited
overcharge and
discharge; they possess better safety characteristics, a higher cell voltage of 2.58 V, compared to the NaS technologies [
1]. They have a cycle life of ~2500 and a lifespan ranging from 10–14 years, with a discharge time of seconds to hours and RTE of ~85–90% [
55]. However, they possess energy and power densities of ~100–120 Wh/kg and ~150–200 W/kg, respectively, which are better than the values reported for lead-acid technologies.
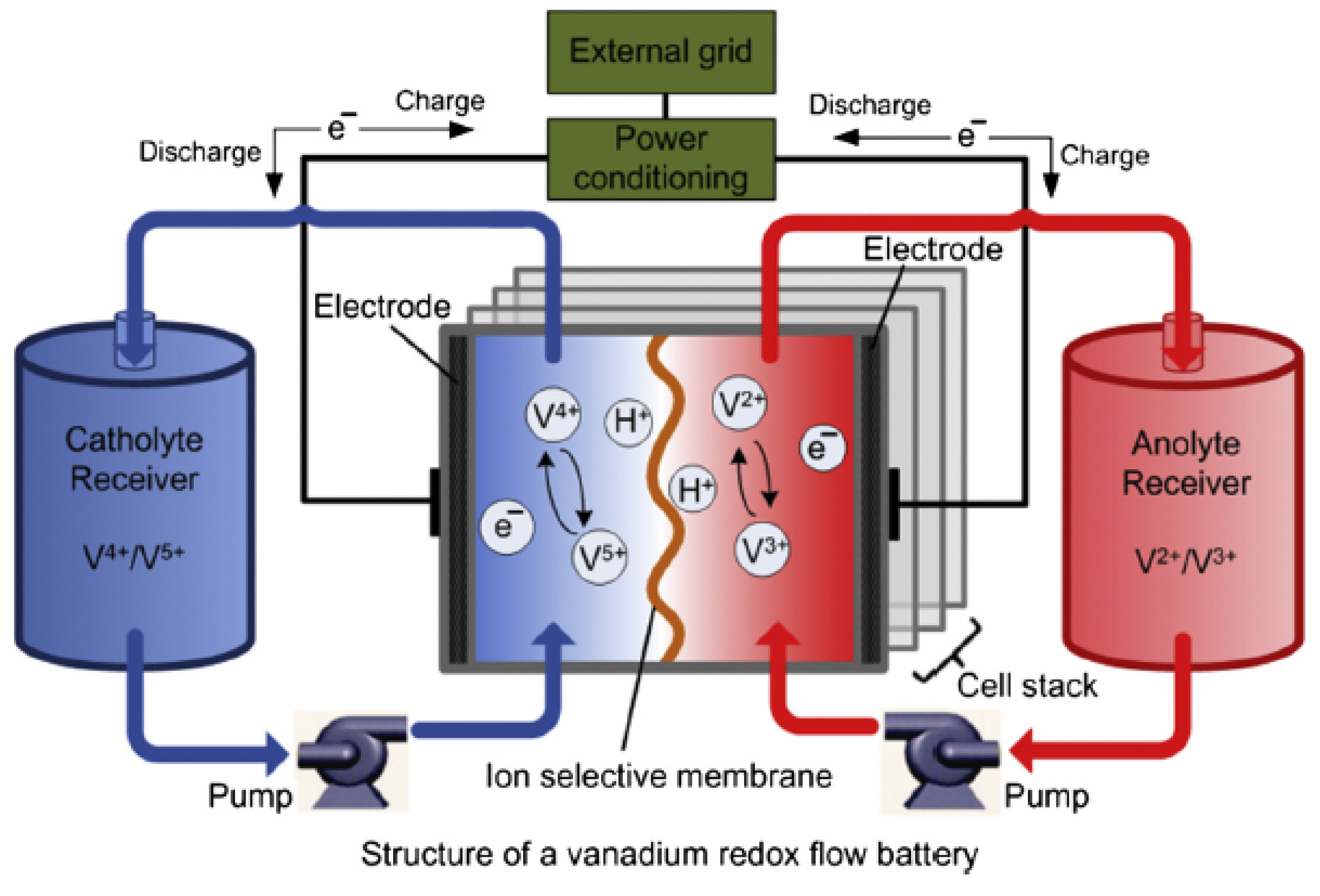
2.5.3. Vanadium Redox
The vanadium redox flow (VRB) is one among the mature flow battery technologies, and it stores energy by using the vanadium redox ions V
/V
and V
/V
in two different electrolytic tanks [
1,
16,
56,
57,
58]. It uses the vanadium in the four oxidation states, thus, making it to have only one active element in the
anolyte and
catholyte [
16,
50]. In the charge/discharge states, the process involves the exchange of H
ions through the selective membrane of the ion, and a cell voltage of 1.4 V is developed in the process [
16]. The chemical reactions are described by
and
while the structure of VRB is illustrated in
Figure 7.
Vanadium redox batteries possess quick responses, even faster than 0.001 s and they have cycle life in the range of 10,000 to over 16,000 cycles [
16,
29]. They have a RTE of ~85% and can guarantee continuous power with a discharge duration of over 24 h [
16]. They have an energy density of 10–30 Wh/kg and lifespan of 5–10 years [
1]. They are suitable for enhancing power quality, uninterruptible power supply UPS, forestalling unscheduled power outages, and balancing the intermittent characteristics of renewable energies [
16]. However, the major challenges with VRBs are low electrolyte stability and solubility, which results in low energy density, and relatively higher operating cost [
1,
16].
2.5.4. Polysulphide Bromine
In these batteries, a reversible electrochemical reaction is allowed between two electrolytes (salt solution) viz. sodium bromide and sodium polysulfide, as described in [
1]
The electrolytes are separated in the cells by a polymer membrane that only allows the migration of positive sodium ions, making the battery to generate ~1.5 V across the membrane [
1].
The main advantages of PSB batteries are the abundance and cost-effectiveness of the materials for producing the two electrolytes and their solubility in aqueous electrolyte [
16]. They also have a fast response time of around 0.02 s [
1,
58], and this makes them a suitable technology for power system frequency control and voltage control. They have a lifespan ranging from 10–15 years, and a RTE efficiency of ~75% and they also operate at room temperature [
1,
16]. However, during the electrochemical reactions, bromine and sodium sulphate crystals are produced and this may result in negative environmental impact [
16].
2.6. Comparison of the Battery Technologies
The comparison of the battery energy storage systems is presented in
Table 2 and
Table 3. This includes the development status of the different technologies, the energy and power capital cost and their environmental influence, advantages and shortcomings.
It is clearly presented that the lead-acid is a mature technology; NaS, NiCd and ZEBRA have a commercial status, while that of VRB is early commercialization. Li-ion, ZnBr and the VRB are also developed, while PSB is currently still in its development stage. Apart from these technologies, there are some new technologies that are currently being researched and developed by some laboratories/Scientists around the world. These will be discussed later in this study.
The information presented in
Table 2 further demonstrates that the lead-acid is not only a mature technology, but also one of the cheapest battery storage option of the all the technologies presented. Its energy and power capital costs range from 200–400 (
$/kWh) and 300–600 (
$/kW), compared to the values presented for the other technologies. It also obvious that the ZEBRA presents cost effective power and energy solutions.
Though the lead-acid battery is mature and presents a cheap energy storage option, it produces toxic remains, which has a negative environmental influence. Furthermore, the NiCd, VRB and PSB technologies are also toxic and they have a negative environmental influence. One of the possible measures to address issue of the negative effect is by using an effective recycling system [
1,
16]. Such a measure will minimize the cumulative environmental impact.
6. Criteria for Battery Selection
It is necessary to consider certain decision variables in the selection of battery systems. These include the initial cost, lifetime, installation cost, maintenance cost, shipping cost, replacement cost, disposal cost, safety, cycling capacity and round-trip efficiency, reliability and environmental impact [
108]. This is because they basically affect the type of battery technology that is employed for a specific power application. This study has demonstrated how the reliability and cost factors form a major criterion for selecting battery technologies for off-grid solar energy generation applications.
Generally, the cost of solar PV modules has drastically reduced over the past decade [
91], which is one of the factors that have contributed to the widespread application of photovoltaic electricity both for on-grid and off-grid applications around the world. However, this is not the same with the battery systems. Batteries are more expensive than the PV modules in a typical PV system application, for instance, one of the recent studies on the techno-economic evaluation of PV microgrid reveals that the cost of the battery bank ranges from 40–46% of the total microgrid cost [
109]. This demonstrates that the total cost of battery—initial, installation, maintenance, shipping, replacement and disposal cost is one of the key factors that affect the selection of batteries.
Moreover, the initial cost can, to a large extent, determine the expected lifetime, efficiency and cycle performance of batteries, since the battery cells with higher performances will be more expensive than those with relatively lower performances, e.g., Li-ion versus lead acid batteries in terms of efficiency and energy density. Some batteries have a higher maintenance cost, such as the vented or flooded batteries because of the routine top up of the electrolyte in the cells [
1,
5,
41], while some others have relatively low maintenance cost, e.g., the VRLA battery technologies that do not require topping up of the electrolyte, OPzS battery, UltraBattery etc.
In addition, battery cells are usually replaced a number of times over an energy system’s project life, which gives rise to replacement costs over the system’s lifecycle; the disposal cost is incurred at the end of the battery’s useful life. Batteries are usually transported from a manufacturer in one country or location to another and are finally delivered to the site where they are installed, which give rise to the shipping and the installation costs. The optimal battery solution is the one that has the lowest lifecycle cost, i.e.,
$/kWh, which is strongly connected to the mentioned decision variables [
5].
Apart from the economic consideration, it is crucial to consider the safety of operation and the environmental impact of the battery. The use of batteries with a high degree of operational safety and a low environmental impact is encouraged and advocated [
14]. Therefore, battery technologies are usually taking through extensive compliance testing before they are certified safe to be operated by the users [
93]. The minds of the intended users will be at rest when the battery system is safe to use.
Ascertaining the environmental risk or impact of using or operating a type of battery is another important consideration. The impact is a function of the battery materials. For instance, lead and cadmium are very toxic materials [
1], and the energy planners, developers and users-alike are being conscious of the risks of using these batteries. Therefore, the standard procedures of operation are recommended and the use of effective recycling process is employed after the battery system’s useful life. Apart from cost, the lifetime of a battery is dependent on its use. A properly operated and maintained battery system will have longer life, less replacements over time, thus, resulting in lower environmental impacts.
6.1. Future Work
The lead-acid batteries remain the commonly used storage system for off-grid energy generation applications both in the developed and the developing countries of the world [
91]. The developed countries have standards and policies guiding the use of these batteries, but these are not available in several developing countries, including Nigeria.
Over 60% of Nigeria’s citizens, for instance, do not have access to the national grid [
109]; it is against this backdrop that grid-independent solar PV systems are proposed for off-grid communities and households in the country. This is also the case with several other developing countries such as Bangladesh, Malawi, etc. [
110]. Based on this development, as the rate of deployment of PV/battery systems increases, it is also important to consider the possible standards and policies that guide the use and end-of-life management of the batteries. Therefore, our future work will be based on the recommendation of policies that can possibly guide the deployment and widespread application of PV/battery systems in developing countries, and the modality for recycling decommissioned plant components toward actualising the environmental sustainability.
7. Conclusions
This research has presented an overview of the battery energy storage technologies for electrical power application. It has also compared the technical properties and performance of the various systems such as the cycling capacity, power and energy densities, round trip-efficiencies and the costs. The existing battery technologies considered include lead-acid, sodium sulfur, sodium-nickle chloride, nickel cadmium, lithium-ion, zinc-bromide, polysulfide bromine and vanadium-redox.
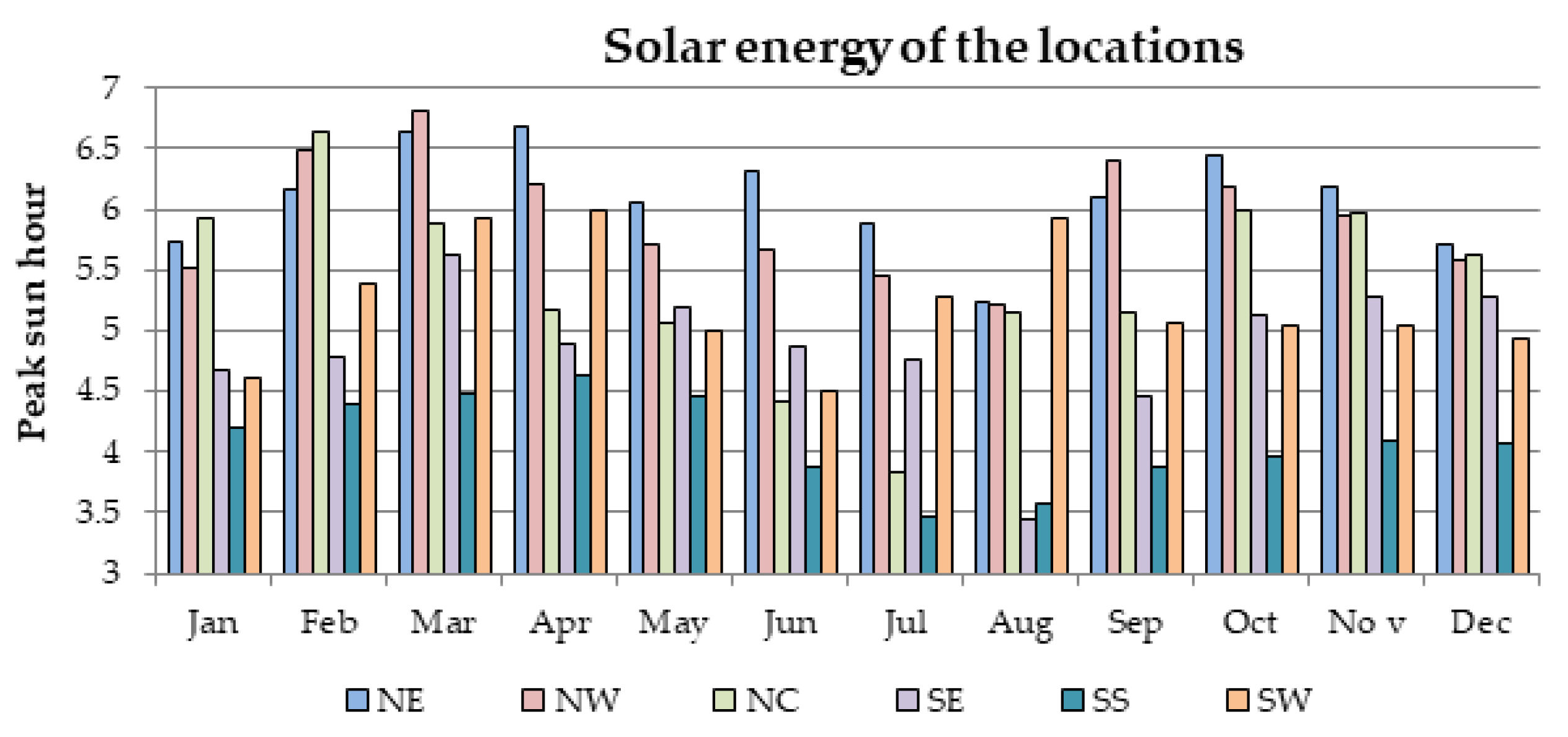
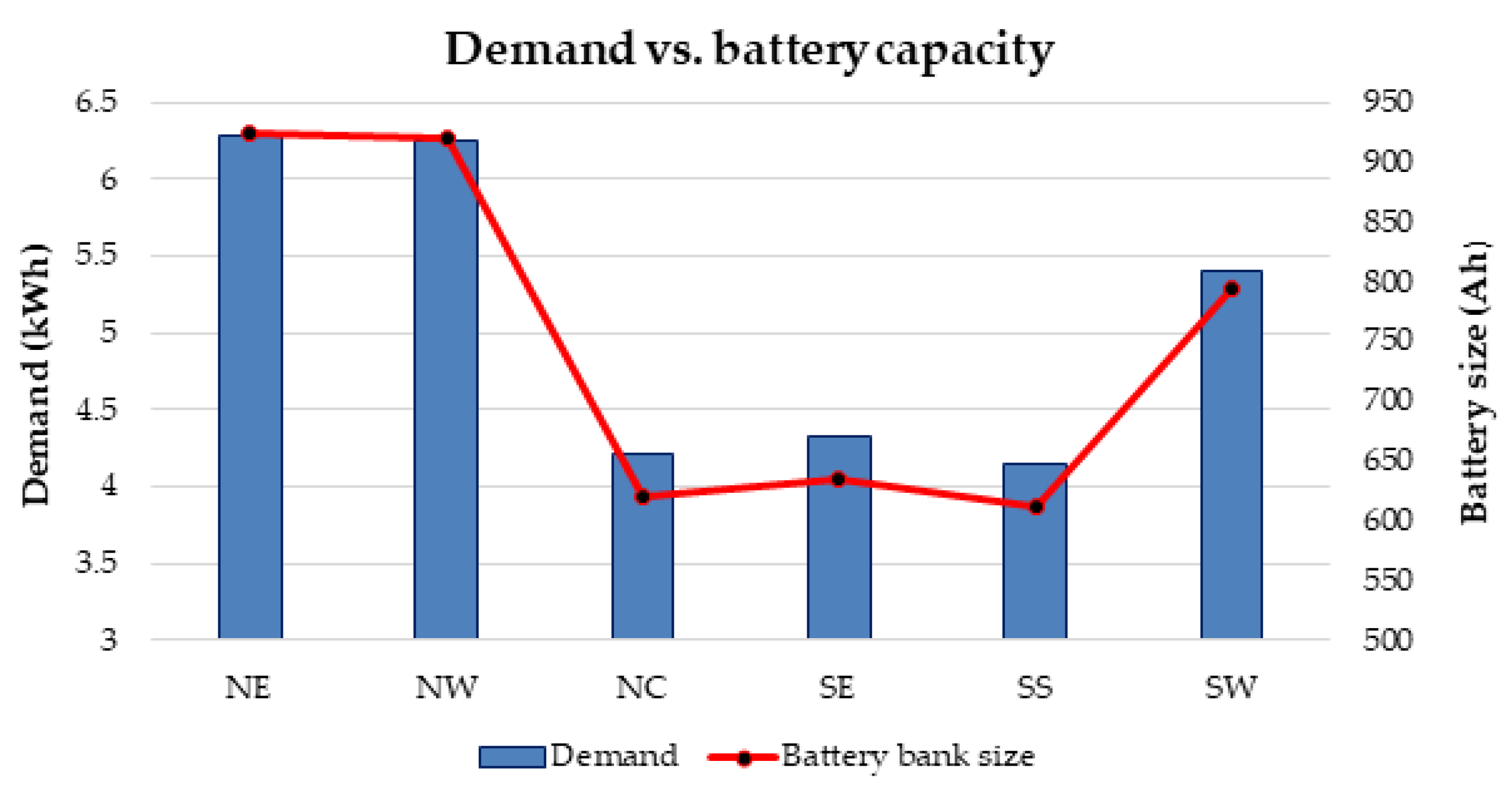
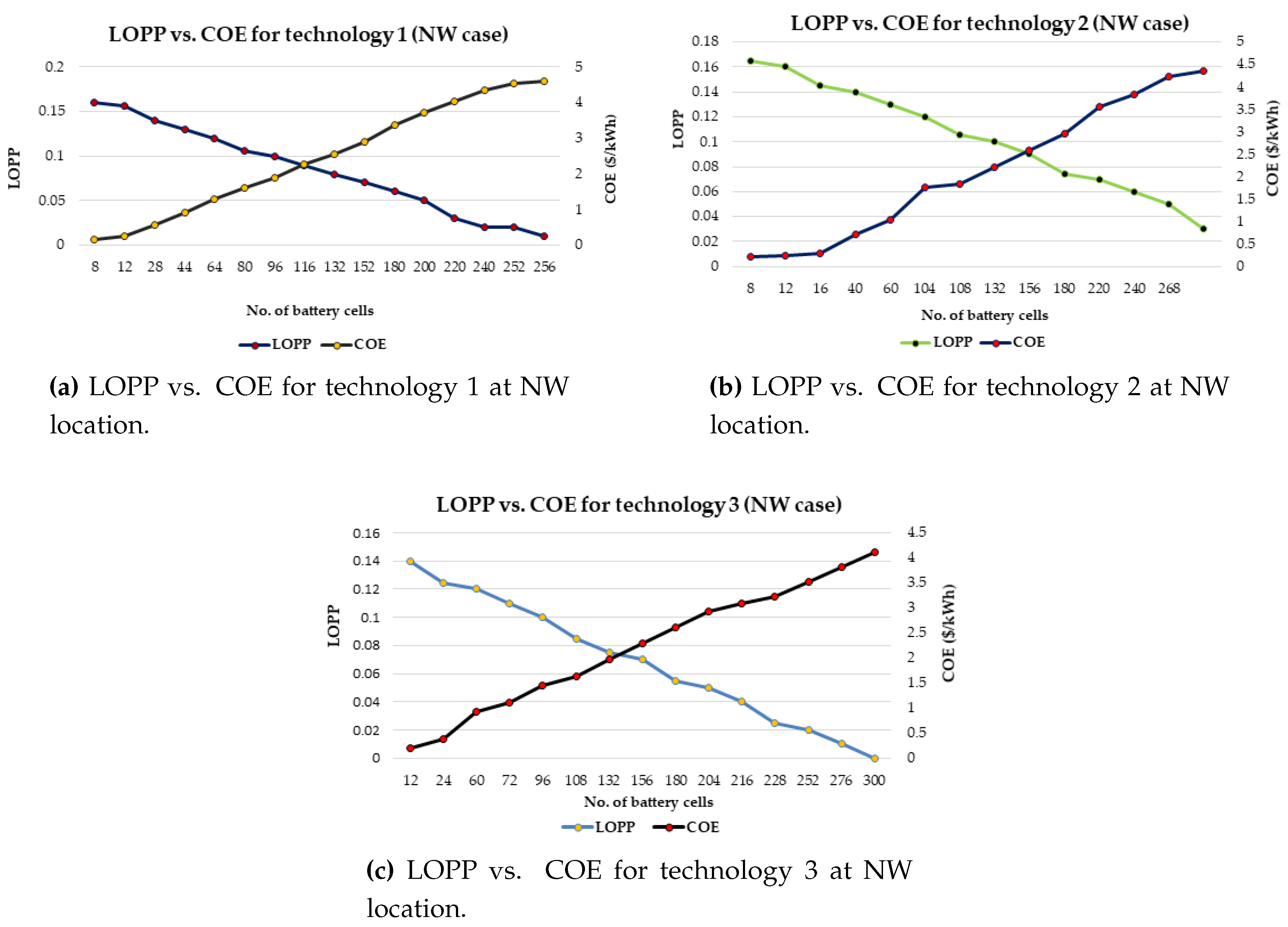
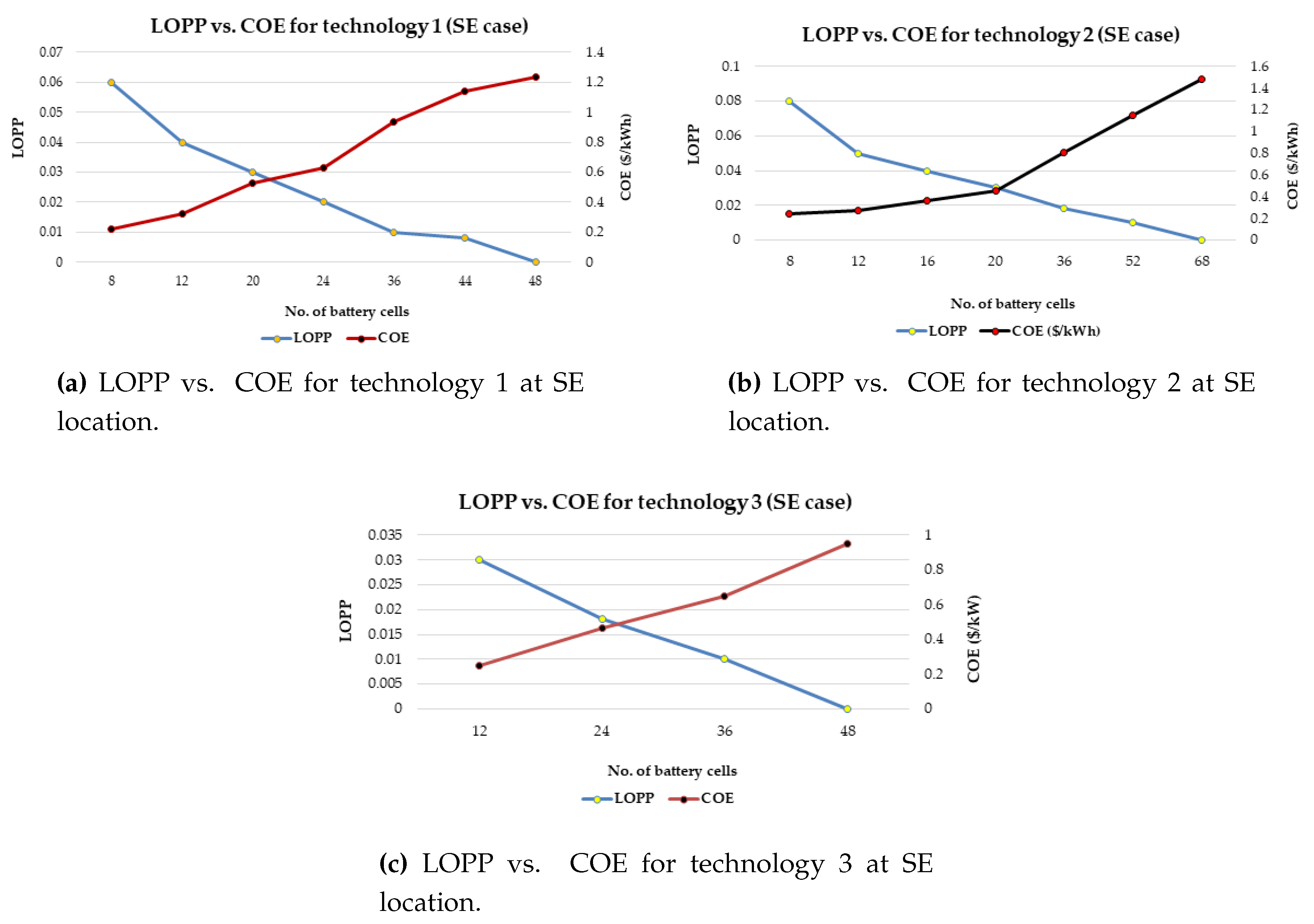
It has also proposed a general strategy for optimal battery sizing and technology selection in a typical stand-alone photovoltaic (PV) system. This approach considers two critical energy generation design factors such as the reliability and cost. The reliability has been examined in terms of the loss of power probability (LOPP), while the cost has been considered in terms of the cost of energy (COE), using the life cycle cost analysis. The battery banks were simulated in Hybrid Optimisation Model for Electric Renewables (HOMER) environment. The selection strategy results demonstrate that the LOPP decreases as the number of battery cells increases, while the COE increases as the number of battery cells increases for the six different energy configurations. Therefore, the configurations with the minimum LOPPs or COE have been selected as the optimum options. The approach and results can be repeated for any other location around the world.
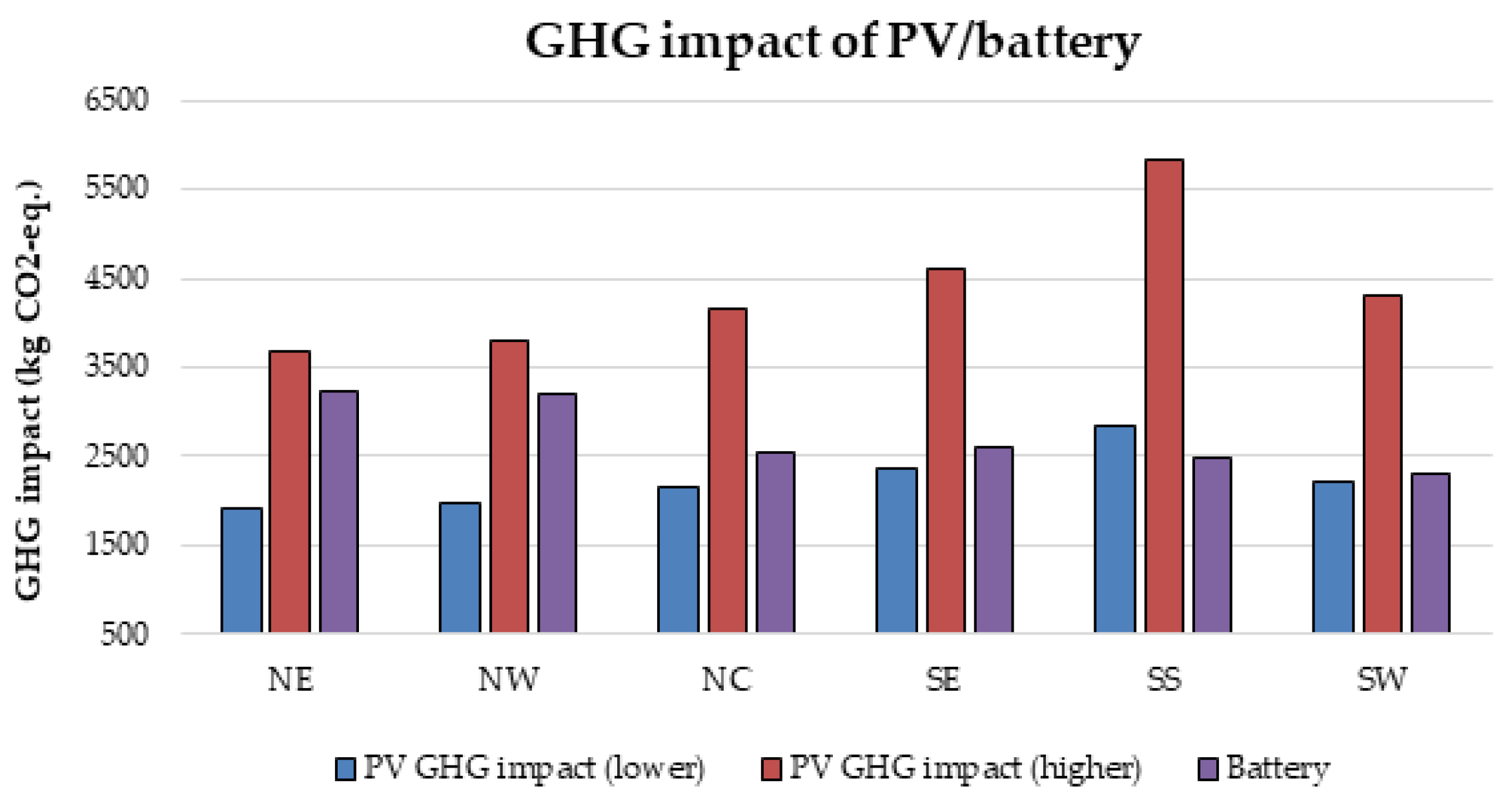
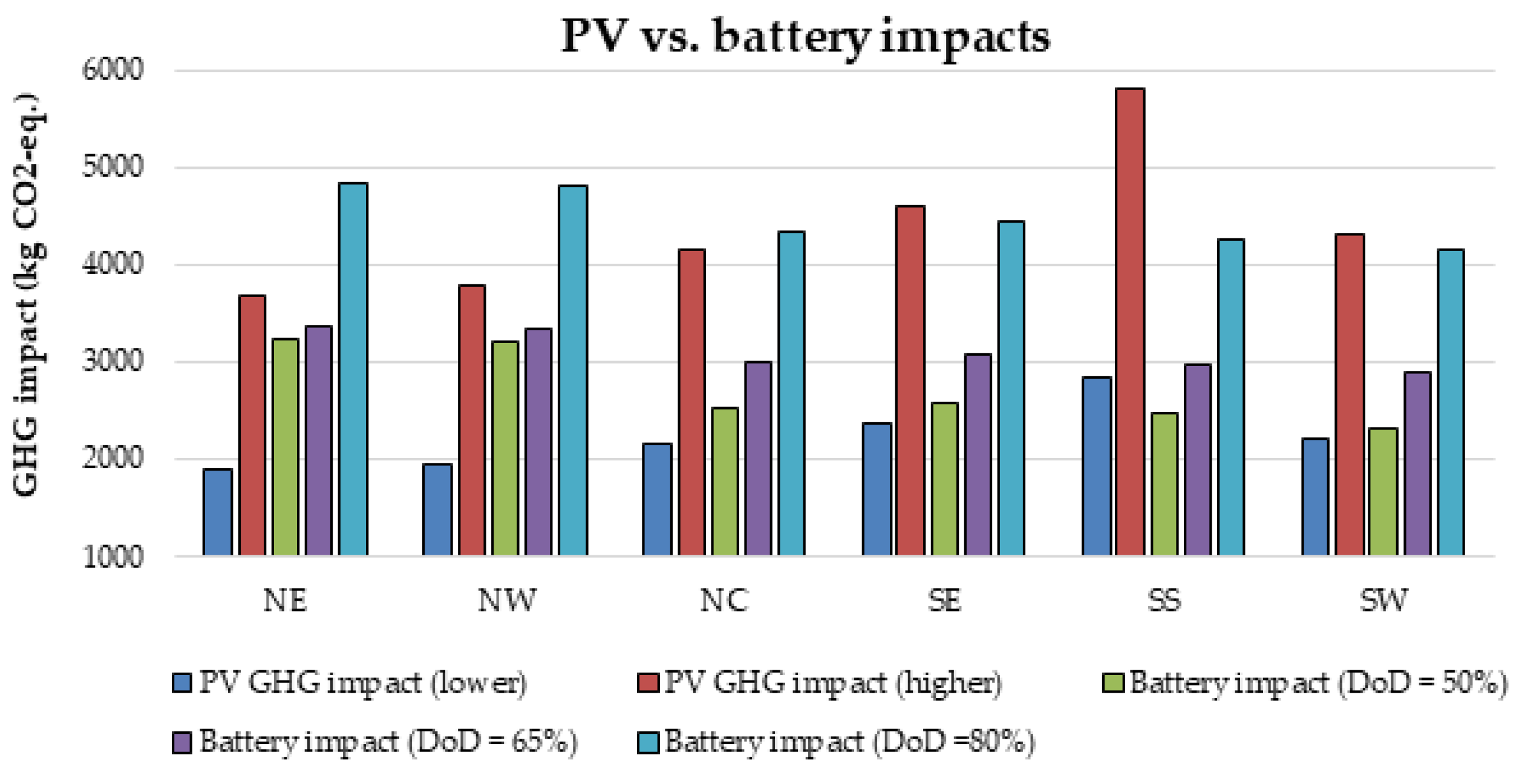
Furthermore, there is currently an increased use of batteries than ever due to the widespread application of renewable energy systems around the world. As a result, the issue of environmental risk and the potential hazard to human health by the use of batteries is very crucial in the energy system field of practice. This is because some of the battery materials are toxic, e.g., lead, cadmium etc. Therefore, efforts have been made in this paper to examine the environmental impact of batteries in a renewable energy system, using a 1.5 kW stand-alone solar PV system as a case study. The energy system was considered for 6 different locations in Nigeria and the GHG impact was estimated based on the life cycle emission rate reported in the literature. The results demonstrate that the battery has a significant impact in the off-grid PV system, achieving GHG impact of about 36–68% in the PV system for battery depth of discharge (DoD) values of 50–80%.
The study demonstrates that the battery impact is attributed to the shorter lifespan of the battery cells and their frequent replacements over a 25-year project life. Though the results are approximate, they can be useful for better understanding of the life cycle environmental impact of batteries in a typical stand-alone PV system. Therefore, making concerted efforts to lengthen the battery lifetime, for instance, by improving their charge/discharge control system and educating the users on battery maintenance and management, will be useful for achieving decreased environmental impacts.
The paper discussed new batteries such as advanced valve regulated lead-acid, lead-carbon, metal-air technologies, UltraBattery, battery with current collector improvement, advanced sodium-metal chloride, high performance sodium-copper chloride and nanostructured energy materials in lithium batteries, which are new and promising battery technologies for future applications with improved cycle life performance, higher lifespan and lower cost that can achieve lower environmental impacts. The study also highlighted the decision variables for selecting battery systems for electrical power applications, which can be useful for planning purposes.
The absence of effective policies and recycling systems for batteries in several developing countries means that there is the danger of serious environmental risks in the future, as there is an increased interest in the adoption and deployment of PV/battery system in these countries. Our future work will focus on the policy recommendation for widespread use and management of battery systems.