Complex Metal Hydrides for Hydrogen, Thermal and Electrochemical Energy Storage
Abstract
:1. Introduction
2. Complex Metal Hydrides for High-Density Hydrogen Storage
2.1. Complex Aluminum Hydrides
2.1.1. Aluminum Hydride
2.1.2. Metal Alanates
2.2. Metal Borohydrides
2.2.1. Monometallic Borohydrides
2.2.2. Bimetallic Borohydrides
2.2.3. Trimetallic Borohydrides
2.2.4. Metal Borohydrides Modified by Neutral Molecules
3. Complex Metal Hydrides for Electrochemical Applications
3.1. Metal Hydrides as Electrode Materials
3.2. Complex Metal Hydrides as Electrolytes
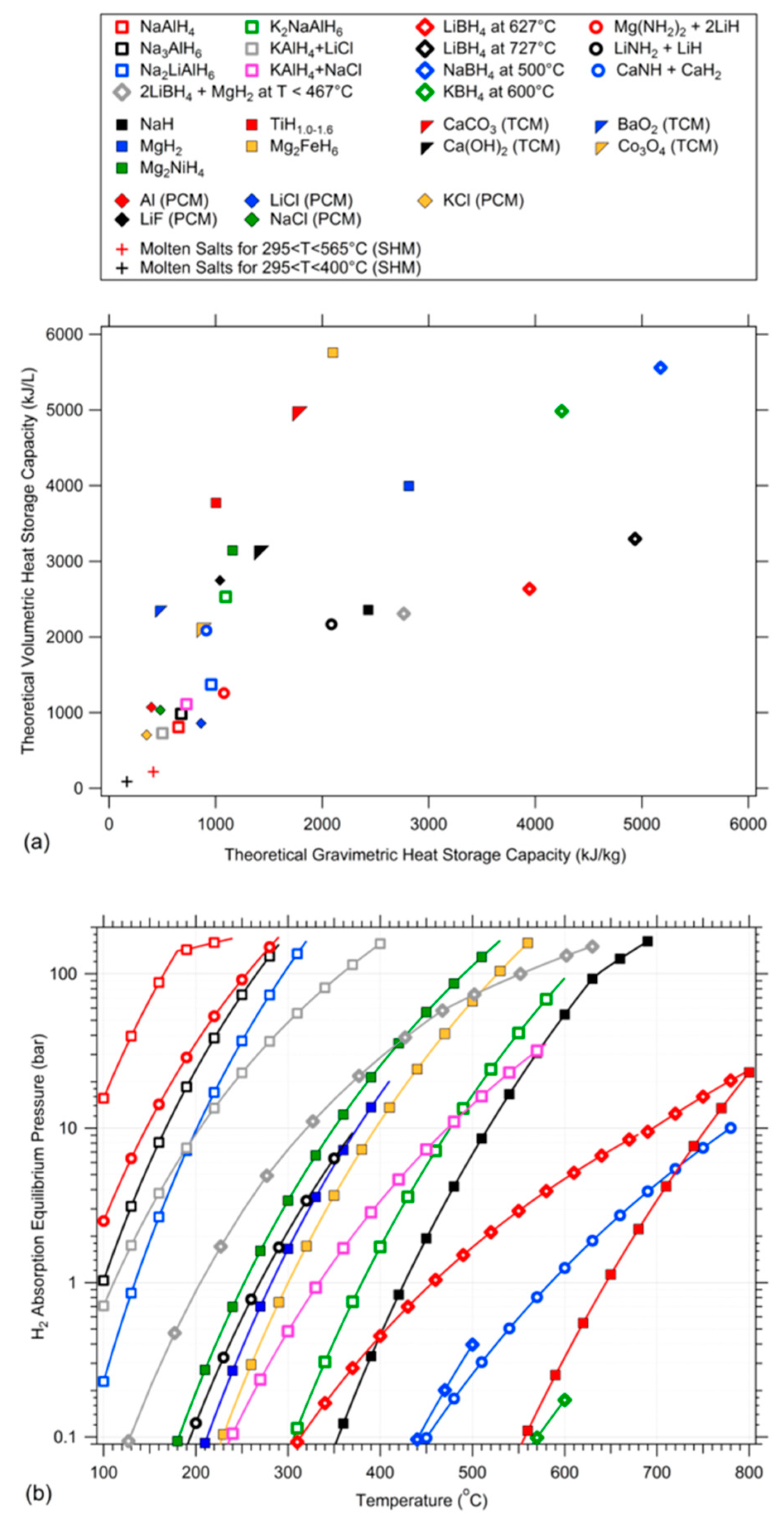
4. Complex Metal Hydrides for Thermal Energy Storage
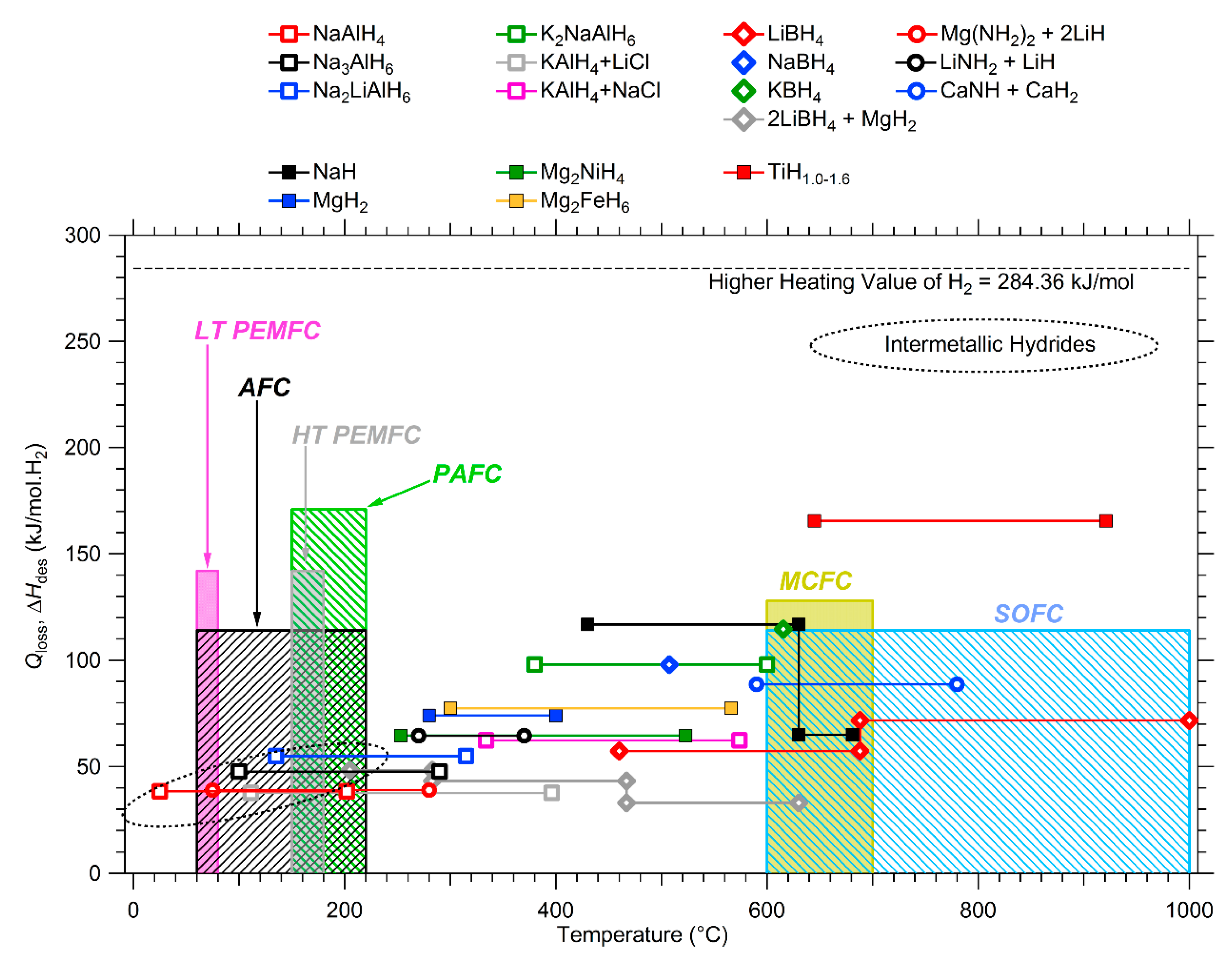
Complex Metal Hydrides and Fuel Cell Applications
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ley, M.B.; Jepsen, L.H.; Lee, Y.-S.; Cho, Y.W.; Bellosta von Colbe, J.M.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage—New perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Rude, L.H.; Nielsen, T.K.; Ravnsbæk, D.B.; Bösenberg, U.; Ley, M.B.; Richter, B.; Arnbjerg, L.M.; Dornheim, M.; Filinchuk, Y.; Besenbacher, F. Tailoring properties of borohydrides for hydrogen storage: A review. Phys. Status Solidi A 2011, 208, 1754–1773. [Google Scholar] [CrossRef]
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Černý, R.; Ravnsbæk, D.B.; Filinchuk, Y.; Dornheim, M.; Besenbacher, F.; Jensen, T.R. Metal borohydrides and derivatives—Synthesis, structure and properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, L.H.; Ley, M.B.; Lee, Y.-S.; Cho, Y.W.; Dornheim, M.; Jensen, J.O.; Filinchuk, Y.; Jørgensen, J.E.; Besenbacher, F.; Jensen, T.R. Boron–nitrogen based hydrides and reactive composites for hydrogen storage. Mater. Today 2014, 17, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Bogdanović, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloys Compd. 1997, 253–254, 1–9. [Google Scholar] [CrossRef]
- Züttel, A.; Wenger, P.; Rentsch, S.; Sudan, P.; Mauron, P.; Emmenegger, C. LiBH4 a new hydrogen storage material. J. Power Sources 2003, 118, 1–7. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.; Luo, J.; Lin, J.; Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Soulié, J.-P.; Renaudin, G.; Černý, R.; Yvon, K. Lithium boro-hydride LiBH4: I. Crystal structure. J. Alloys Compd. 2002, 346, 200–205. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.-W.; Chen, P. Amides and borohydrides for high-capacity solid-state hydrogen storage—Materials design and kinetic improvements. MRS Bull. 2013, 38, 480–487. [Google Scholar] [CrossRef]
- Nakamori, Y.; Kitahara, G.; Orimo, S. Synthesis and dehydriding studies of Mg–N–H systems. J. Power Sources 2004, 138, 309–312. [Google Scholar] [CrossRef]
- Leng, H.Y.; Ichikawa, T.; Hino, S.; Hanada, N.; Isobe, S.; Fujii, H. New Metal–N–H System Composed of Mg(NH2)2 and LiH for Hydrogen Storage. J. Phys. Chem. B 2004, 108, 8763–8765. [Google Scholar] [CrossRef]
- Luo, W. (LiNH2–MgH2): A viable hydrogen storage system. J. Alloys Compd. 2004, 381, 284–287. [Google Scholar] [CrossRef]
- Paik, B.; Li, H.-W.; Wang, J.; Akiba, E. A Li–Mg–N–H composite as H2 storage material: A case study with Mg(NH2)2–4LiH–LiNH2. Chem. Commun. 2015, 51, 10018–10021. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Hu, J.; Wu, G.; Chen, P.; Luo, W.; Gross, K.; Wang, J. Thermodynamic and kinetic investigations of the hydrogen storage in the Li–Mg–N–H system. J. Alloys Compd. 2005, 398, 235–239. [Google Scholar] [CrossRef]
- Torre, F.; Valentoni, A.; Milanese, C.; Pistidda, C.; Marini, A.; Dornheim, M.; Enzo, S.; Mulas, G.; Garroni, S. Kinetic improvement on the CaH2-catalyzed Mg(NH2)2 + 2LiH system. J. Alloys Compd. 2015, 645, S284–S287. [Google Scholar] [CrossRef]
- Shaw, L.L.; Ren, R.; Markmaitree, T.; Osborn, W. Effects of mechanical activation on dehydrogenation of the lithium amide and lithium hydride system. J. Alloys Compd. 2008, 448, 263–271. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, K.; Luo, K.; Gao, M.; Pan, H.; Wang, Q. Size-Dependent Kinetic Enhancement in Hydrogen Absorption and Desorption of the Li–Mg–N–H System. J. Am. Chem. Soc. 2009, 131, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Santoru, A.; Pistidda, C.; M. Richter, T.M.; Chaudhary, A.-L.; Gizer, G.; Niewa, R.; Chen, P.; Klassen, T.; Dornheim, M. New synthesis route for ternary transition metal amides as well as ultrafast amide–hydride hydrogen storage materials. Chem. Commun. 2016, 52, 5100–5103. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Richter, T.M.M.; Pistidda, C.; Chaudhary, A.-L.; Santoru, A.; Gizer, G.; Niewa, R.; Chen, P.; Klassen, T.; Dornheim, M. Ternary Amides Containing Transition Metals for Hydrogen Storage: A Case Study with Alkali Metal Amidozincates. ChemSusChem 2015, 8, 3777–3782. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Pistidda, C.; Richter, T.M.M.; Santoru, A.; Milanese, C.; Garroni, S.; Bednarcik, J.; Chaudhary, A.-L.; Gizer, G.; Liermann, H.-P.; et al. In situ X-ray diffraction studies on the de/rehydrogenation processes of the K2[Zn(NH2)4]-8LiH system. J. Phys. Chem. C 2017, 121, 1546–1551. [Google Scholar] [CrossRef]
- Janot, R.; Eymery, J.-B.; Tarascon, J.-M. Decomposition of LiAl(NH2)4 and Reaction with LiH for a Possible Reversible Hydrogen Storage. J. Phys. Chem. C 2007, 111, 2335–2340. [Google Scholar] [CrossRef]
- Jepsen, L.H.; Wang, P.; Wu, G.; Xiong, Z.; Besenbacher, F.; Chen, P.; Jensen, T.R. Thermal decomposition of sodium amide, NaNH2, and sodium amide hydroxide composites, NaNH2-NaOH. Phys. Chem. Chem. Phys. 2016, 18, 25257–25264. [Google Scholar] [CrossRef] [PubMed]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 88th ed.; CRC Press: Boca Raton, FL, USA, 25 June 2007; ISBN 0-8493-0488-1. [Google Scholar]
- Orimo, S.I.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex Hydrides for Hydrogen Storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Mauron, P.; Buchter, F.; Friedrichs, O.; Remhof, A.; Bielmann, M.; Zwicky, C.N.; Zuttel, A. Stability and Reversibility of LiBH4. J. Phys. Chem. B 2008, 112, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Martelli, P.; Caputo, R.; Remhof, A.; Mauron, P.; Borgschulte, A.; Züttel, A. Stability and Decomposition of NaBH4. J. Phys. Chem. C 2010, 114, 7173–7177. [Google Scholar] [CrossRef]
- Dornheim, M. Thermodynamics of Metal Hydrides: Tailoring Reaction Enthalpies of Hydrogen Storage Materials; InTech: Rijeka, Croatia, 2011; ISBN 978-953-307-563-1. [Google Scholar]
- Na Ranong, C.; Höhne, M.; Franzen, J.; Hapke, J.; Fieg, G.; Dornheim, M.; Eigen, N.; Bellosta von Colbe, J.M.; Metz, O. Concept, Design and Manufacture of a Prototype Hydrogen Storage Tank Based on Sodium Alanate. Chem. Eng. Technol. 2009, 32, 1154–1163. [Google Scholar] [CrossRef]
- Isobe, S.; Ichikawa, T.; Tokoyoda, K.; Hanada, N.; Leng, H.; Fujii, H.; Kojima, Y. Evaluation of enthalpy change due to hydrogen desorption for lithium amide/imide system by differential scanning calorimetry. Thermochim. Acta 2008, 468, 35–38. [Google Scholar] [CrossRef]
- Kojima, Y.; Kawai, Y. IR characterizations of lithium imide and amide. J. Alloys Compd. 2005, 395, 236–239. [Google Scholar] [CrossRef]
- Ichikawa, T.; Isobe, S.; Hanada, N.; Fujii, H. Lithium nitride for reversible hydrogen storage. J. Alloys Compd. 2004, 365, 271–276. [Google Scholar] [CrossRef]
- Paskevicius, M.; Webb, J.; Pitt, M.P.; Blach, T.P.; Hauback, B.C.; Gray, E.; Buckley, C.E. Mechanochemical synthesis of aluminium nanoparticles and their deuterium sorption properties to 2kbar. J. Alloys Compd. 2009, 481, 595–599. [Google Scholar] [CrossRef]
- Sartori, S.; Opalka, S.M.; Løvvik, O.M.; Guzik, M.N.; Tang, X.; Hauback, B.C. Experimental studies of α-AlD3 and α′-AlD3 versus first-principles modelling of the alane isomorphs. J. Mater. Chem. 2008, 18, 2361. [Google Scholar] [CrossRef]
- Sandrock, G.; Reilly, J.; Graetz, J.; Zhou, W.-M.; Johnson, J.; Wegrzyn, J. Accelerated thermal decomposition of AlH3 for hydrogen-fueled vehicles. Appl. Phys. A 2005, 80, 687–690. [Google Scholar] [CrossRef]
- Ikeda, K.; Ohshita, H.; Kaneko, N.; Zhang, J.; Yonemura, M.; Otomo, T.; Suzuya, K.; Yukawa, H.; Morinaga, M.; Li, H.-W.; et al. Structural and Hydrogen Desorption Properties of Aluminum Hydride. Mater. Trans. 2011, 52, 598–601. [Google Scholar] [CrossRef]
- Finholt, A.E.; Bond, A.C.; Schlesinger, H.I. Lithium Aluminum Hydride, Aluminum Hydride and Lithium Gallium Hydride, and Some of their Applications in Organic and Inorganic Chemistry1. J. Am. Chem. Soc. 1947, 69, 1199–1203. [Google Scholar] [CrossRef]
- Hauback, B.C. Structures of aluminium-based light weight hydrides. Z. Krist. 2009, 223, 636–648. [Google Scholar] [CrossRef]
- Brower, F.M.; Matzek, N.E.; Reigler, P.F.; Rinn, H.W.; Roberts, C.B.; Schmidt, D.L.; Snover, J.A.; Terada, K. Preparation and properties of aluminum hydride. J. Am. Chem. Soc. 1976, 98, 2450–2453. [Google Scholar] [CrossRef]
- Turley, J.W.; Rinn, H.W. Crystal structure of aluminum hydride. Inorg. Chem. 1969, 8, 18–22. [Google Scholar] [CrossRef]
- Brinks, H.W.; Istad-Lem, A.; Hauback, B.C. Mechanochemical Synthesis and Crystal Structure of α′-AlD3 and α-AlD3. J. Phys. Chem. B 2006, 110, 25833–25837. [Google Scholar] [CrossRef] [PubMed]
- Brinks, H.W.; Langley, W.; Jensen, C.M.; Graetz, J.; Reilly, J.J.; Hauback, B.C. Synthesis and crystal structure of β-AlD3. J. Alloys Compd. 2007, 433, 180–183. [Google Scholar] [CrossRef]
- Brinks, H.W.; Brown, C.; Jensen, C.M.; Graetz, J.; Reilly, J.J.; Hauback, B.C. The crystal structure of γ-AlD3. J. Alloys Compd. 2007, 441, 364–367. [Google Scholar] [CrossRef]
- Yartys, V.A.; Denys, R.V.; Maehlen, J.P.; Frommen, C.; Fichtner, M.; Bulychev, B.M.; Emerich, H. Double-Bridge Bonding of Aluminium and Hydrogen in the Crystal Structure of γ-AlH3. Inorg. Chem. 2007, 46, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Konovalov, S.K.; Bulychev, B.M. The P,T-State Diagram and Solid Phase Synthesis of Aluminum Hydride. Inorg. Chem. 1995, 34, 172–175. [Google Scholar] [CrossRef]
- Sartori, S.; Istad-Lem, A.; Brinks, H.W.; Hauback, B.C. Mechanochemical synthesis of alane. Int. J. Hydrogen Energy 2009, 34, 6350–6356. [Google Scholar] [CrossRef]
- Paskevicius, M.; Sheppard, D.A.; Buckley, C.E. Characterisation of mechanochemically synthesised alane (AlH3) nanoparticles. J. Alloys Compd. 2009, 487, 370–376. [Google Scholar] [CrossRef]
- Gupta, S.; Kobayashi, T.; Hlova, I.Z.; Goldston, J.F.; Pruski, M.; Pecharsky, V.K. Solvent-free mechanochemical synthesis of alane, AlH3: Effect of pressure on the reaction pathway. Green Chem. 2014, 16, 4378–4388. [Google Scholar] [CrossRef]
- Dinh, L.V.; Knight, D.A.; Paskevicius, M.; Buckley, C.E.; Zidan, R. Novel methods for synthesizing halide-free alane without the formation of adducts. Appl. Phys. A 2012, 107, 173–181. [Google Scholar] [CrossRef]
- Graetz, J.; Chaudhuri, S.; Lee, Y.; Vogt, T.; Muckerman, J.T.; Reilly, J.J. Pressure-induced structural and electronic changes in α-AlH3. Phys. Rev. B 2006, 74, 214114. [Google Scholar] [CrossRef]
- Graetz, J.; Reilly, J.J. Decomposition Kinetics of the AlH3 Polymorphs. J. Phys. Chem. B 2005, 109, 22181–22185. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ikeda, K.; Li, H.-W.; Yukawa, H.; Morinaga, M.; Orimo, S. Direct Dry Syntheses and Thermal Analyses of a Series of Aluminum Complex Hydrides. Mater. Trans. 2009, 50, 182–186. [Google Scholar] [CrossRef]
- Graetz, J. New approaches to hydrogen storage. Chem. Soc. Rev. 2009, 38, 73. [Google Scholar] [CrossRef] [PubMed]
- Ashby, E.C.; Brendel, G.J.; Redman, H.E. Direct Synthesis of Complex Metal Hydrides. Inorg. Chem. 1963, 2, 499–504. [Google Scholar] [CrossRef]
- Clasen, H. Alanat-Synthese aus den Elementen und ihre Bedeutung. Angew. Chem. 1961, 73, 322–331. [Google Scholar] [CrossRef]
- Balema, V.P.; Balema, L. Missing pieces of the puzzle or about some unresolved issues in solid state chemistry of alkali metal aluminohydrides. Phys. Chem. Chem. Phys. 2005, 7, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Dilts, J.A.; Ashby, E.C. Thermal decomposition of complex metal hydrides. Inorg. Chem. 1972, 11, 1230–1236. [Google Scholar] [CrossRef]
- Arnbjerg, L.M.; Jensen, T.R. New compounds in the potassium-aluminium-hydrogen system observed during release and uptake of hydrogen. Int. J. Hydrogen Energy 2012, 37, 345–356. [Google Scholar] [CrossRef]
- Ares, J.R.; Aguey-Zinsou, K.-F.; Porcu, M.; Sykes, J.M.; Dornheim, M.; Klassen, T.; Bormann, R. Thermal and mechanically activated decomposition of LiAlH4. Mater. Res. Bull. 2008, 43, 1263–1275. [Google Scholar] [CrossRef]
- Block, J.; Gray, A.P. The Thermal Decomposition of Lithium Aluminum Hydride. Inorg. Chem. 1965, 4, 304–305. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Xu, Q.; Takeshita, H.T.; Sakai, T. Reversible Hydrogen Storage via Titanium-Catalyzed LiAlH4 and Li3AlH6. J. Phys. Chem. B 2001, 105, 11214–11220. [Google Scholar] [CrossRef]
- Dymova, T.N.; Aleksandrov, D.P.; Konoplev, V.N.; Silina, T.A.; Sizareva, A.S. Spontaneous and thermal-decomposition of Lithium Tetrahydroaluminate LiAlH4-the promoting effect of mechanochemical action on the process. Koord. Khimiya 1994, 20, 279–285. [Google Scholar]
- Andreasen, A.; Vegge, T.; Pedersen, A.S. Dehydrogenation kinetics of as-received and ball-milled. J. Solid State Chem. 2005, 178, 3672–3678. [Google Scholar] [CrossRef]
- Balema, V.P.; Dennis, K.W.; Pecharsky, V.K. Rapid solid-state transformation of tetrahedral [AlH4]− into octahedral [AlH6]3− in lithium aluminohydride. Chem. Commun. 2000, 1665–1666. [Google Scholar] [CrossRef]
- Balema, V.P.; Wiench, J.W.; Dennis, K.W.; Pruski, M.; Pecharsky, V.K. Titanium catalyzed solid-state transformations in LiAlH4 during high-energy ball-milling. J. Alloys Compd. 2001, 329, 108–114. [Google Scholar] [CrossRef]
- Balema, V.P.; Pecharsky, V.K.; Dennis, K.W. Solid state phase transformations in LiAlH4 during high-energy ball-milling. J. Alloys Compd. 2000, 313, 69–74. [Google Scholar] [CrossRef]
- Blanchard, D.; Brinks, H.W.; Hauback, B.C.; Norby, P. Desorption of LiAlH4 with Ti- and V-based additives. Mater. Sci. Eng. B 2004, 108, 54–59. [Google Scholar] [CrossRef]
- Lai, Q.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.; Mao, J.; Huang, Z.; Liu, H.K.; et al. Hydrogen Storage Materials for Mobile and Stationary Applications: Current State of the Art. ChemSusChem 2015, 8, 2789–2825. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; McGrady, G.S.; Langmi, H.W.; Jensen, C.M. Facile Cycling of Ti-Doped LiAlH4 for High Performance Hydrogen Storage. J. Am. Chem. Soc. 2009, 131, 5032–5033. [Google Scholar] [CrossRef] [PubMed]
- Graetz, J.; Wegrzyn, J.; Reilly, J.J. Regeneration of Lithium Aluminum Hydride. J. Am. Chem. Soc. 2008, 130, 17790–17794. [Google Scholar] [CrossRef] [PubMed]
- Pitt, M.P.; Vullum, P.E.; Sørby, M.H.; Emerich, H.; Paskevicius, M.; Buckley, C.E.; Walmsley, J.C.; Holmestad, R.; Hauback, B.C. Hydrogen Absorption Kinetics of the Transition-Metal-Chloride-Enhanced NaAlH4 System. J. Phys. Chem. C 2012, 116, 14205–14217. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Javadian, P.; Polanski, M.; Besenbacher, F.; Bystrzycki, J.; Skibsted, J.; Jensen, T.R. Nanoconfined NaAlH4: Prolific effects from increased surface area and pore volume. Nanoscale 2014, 6, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.K.; Javadian, P.; Polanski, M.; Besenbacher, F.; Bystrzycki, J.; Jensen, T.R. Nanoconfined NaAlH4: Determination of distinct prolific effects from pore size, crystallite size, and surface interactions. J. Phys. Chem. C 2012, 116, 21046–21051. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Polanski, M.; Zasada, D.; Javadian, P.; Besenbacher, F.; Bystrzycki, J.; Skibsted, J.; Jensen, T.R. Improved Hydrogen Storage Kinetics of Nanoconfined NaAlH4 Catalyzed with TiCl3 Nanoparticles. ACS Nano 2011, 5, 4056–4064. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.M.; Gross, K.J. Development of catalytically enhanced sodium aluminum hydride as a hydrogen-storage material. Appl. Phys. A 2001, 72, 213–219. [Google Scholar] [CrossRef]
- Zidan, R.A.; Takara, S.; Hee, A.G.; Jensen, C.M. Hydrogen cycling behavior of zirconium and titanium–zirconium-doped sodium aluminum hydride. J. Alloys Compd. 1999, 285, 119–122. [Google Scholar] [CrossRef]
- Bogdanović, B.; Felderhoff, M.; Pommerin, A.; Schüth, F.; Spielkamp, N. Advanced Hydrogen-Storage Materials Based on Sc-, Ce-, and Pr-Doped NaAlH4. Adv. Mater. 2006, 18, 1198–1201. [Google Scholar] [CrossRef]
- Pitt, M.P.; Paskevicius, M.; Webb, C.J.; Sørby, M.H.; Delleda, S.; Jensen, T.R.; Hauback, B.C.; Buckley, C.E.; Gray, E.M. Nanoscopic Al1−xCex phases in the NaH + Al + 0.02CeCl3 system. Int. J. Hydrogen Energy 2011, 36, 8403–8411. [Google Scholar] [CrossRef]
- Fichtner, M.; Fuhr, O. Synthesis and structures of magnesium alanate and two solvent adducts. J. Alloys Compd. 2002, 345, 286–296. [Google Scholar] [CrossRef]
- Fichtner, M.; Frommen, C.; Fuhr, O. Synthesis and Properties of Calcium Alanate and Two Solvent Adducts. Inorg. Chem. 2005, 44, 3479–3484. [Google Scholar] [CrossRef] [PubMed]
- Pommerin, A.; Wosylus, A.; Felderhoff, M.; Schüth, F.; Weidenthaler, C. Synthesis, Crystal Structures, and Hydrogen-Storage Properties of Eu(AlH4)2 and Sr(AlH4)2 and of Their Decomposition Intermediates, EuAlH5 and SrAlH5. Inorg. Chem. 2012, 51, 4143–4150. [Google Scholar] [CrossRef] [PubMed]
- Løvvik, O.M.; Swang, O.; Opalka, S.M. Modeling alkali alanates for hydrogen storage by density-functional band-structure calculations. J. Mater. Res. 2005, 20, 3199–3213. [Google Scholar] [CrossRef]
- Arroyo y de Dompablo, M.E.; Ceder, G. First principles investigations of complex hydrides AMH4 and A3MH6 (A = Li, Na, K, M = B, Al, Ga) as hydrogen storage systems. J. Alloys Compd. 2004, 364, 6–12. [Google Scholar] [CrossRef]
- Ares, J.R.; Aguey-Zinsou, K.-F.; Leardini, F.; Ferrer, I.J.; Fernandez, J.-F.; Guo, Z.-X.; Sánchez, C. Hydrogen Absorption/Desorption Mechanism in Potassium Alanate (KAlH4) and Enhancement by TiCl3 Doping. J. Phys. Chem. C 2009, 113, 6845–6851. [Google Scholar] [CrossRef]
- Morioka, H.; Kakizaki, K.; Chung, S.-C.; Yamada, A. Reversible hydrogen decomposition of KAlH4. J. Alloys Compd. 2003, 353, 310–314. [Google Scholar] [CrossRef]
- Černý, R.; Schouwink, P. The crystal chemistry of inorganic metal borohydrides and their relation to metal oxides. Acta Crystallogr. B 2015, 71, 619–640. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.; Sudan, P.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloys Compd. 2003, 356–357, 515–520. [Google Scholar] [CrossRef]
- Nakamori, Y.; Orimo, S. Destabilization of Li-based complex hydrides. J. Alloys Compd. 2004, 370, 271–275. [Google Scholar] [CrossRef]
- Hartman, M.R.; Rush, J.J.; Udovic, T.J.; Bowman, R.C., Jr.; Hwang, S.-J. Structure and vibrational dynamics of isotopically labeled lithium borohydride using neutron diffraction and spectroscopy. J. Solid State Chem. 2007, 180, 1298–1305. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Chernyshov, D.; Cerny, R. Lightest Borohydride Probed by Synchrotron X-ray Diffraction: Experiment Calls for a New Theoretical Revision. J. Phys. Chem. C 2008, 112, 10579–10584. [Google Scholar] [CrossRef]
- Eberle, U.; Felderhoff, M.; Schüth, F. Chemical and Physical Solutions for Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 6608–6630. [Google Scholar] [CrossRef] [PubMed]
- Grube, E.; Olesen, C.H.; Ravnsbæk, D.B.; Jensen, T.R. Barium borohydride chlorides: Synthesis, crystal structures and thermal properties. Dalton Trans. 2016, 45, 8291–8299. [Google Scholar] [CrossRef] [PubMed]
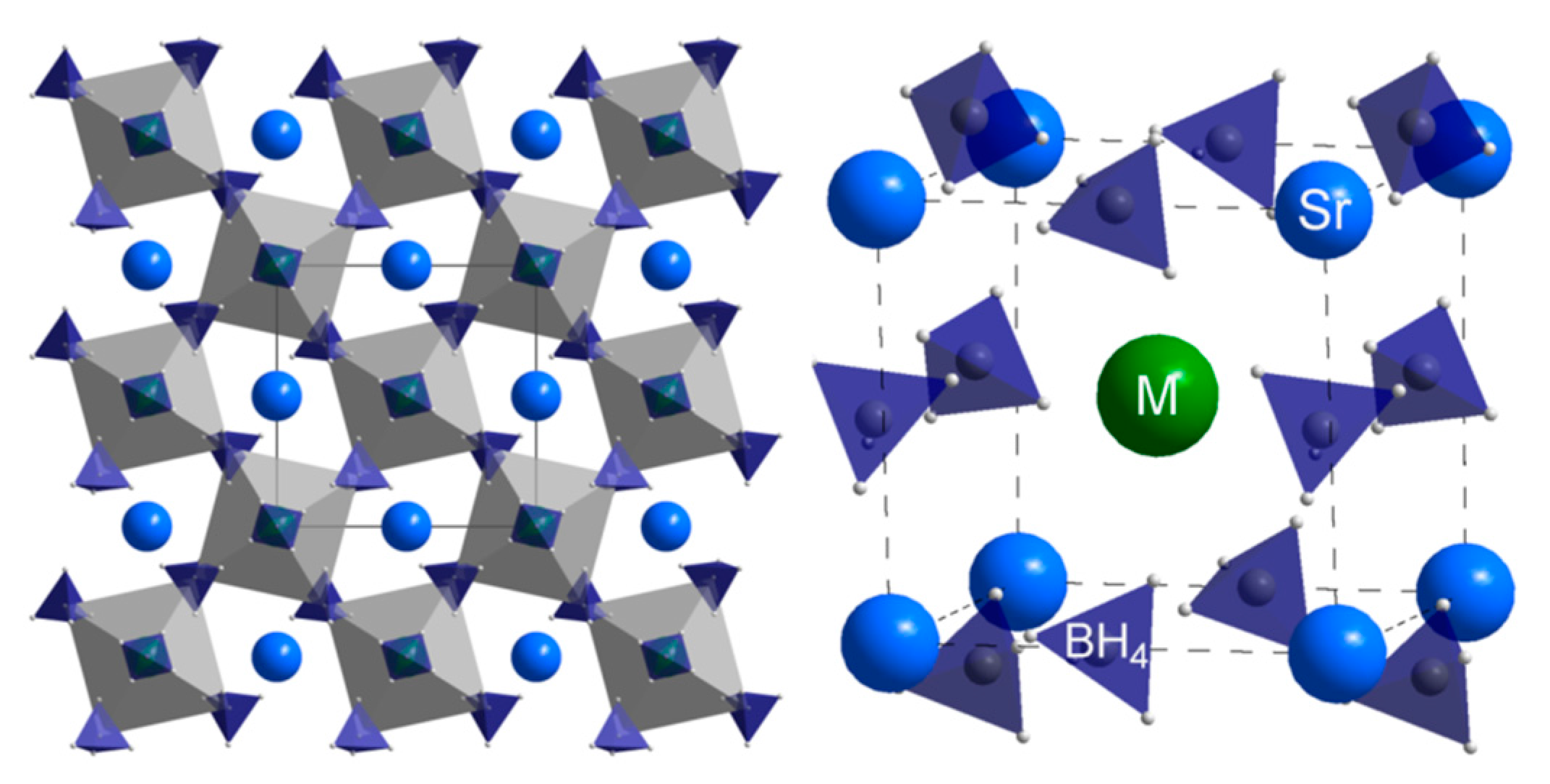
- Ravnsbæk, D.B.; Nickels, E.A.; Černý, R.; Olesen, C.H.; David, W.I.F.; Edwards, P.P.; Filinchuk, Y.; Jensen, T.R. Novel Alkali Earth Borohydride Sr(BH4)2 and Borohydride-Chloride Sr(BH4)Cl. Inorg. Chem. 2013, 52, 10877–10885. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Didelot, E.; Spyratou, A.; Lawson Daku, L.M.; Černý, R.; Hagemann, H. Halide Free M(BH4)2 (M = Sr, Ba, and Eu) Synthesis, Structure, and Decomposition. Inorg. Chem. 2016, 55, 7090–7097. [Google Scholar] [CrossRef] [PubMed]
- Filinchuk, Y.; Richter, B.; Jensen, T.R.; Dmitriev, V.; Chernyshov, D.; Hagemann, H. Porous and Dense Magnesium Borohydride Frameworks: Synthesis, Stability, and Reversible Absorption of Guest Species. Angew. Chem. Int. Ed. 2011, 50, 11162–11166. [Google Scholar] [CrossRef] [PubMed]
- Filinchuk, Y.; Černý, R.; Hagemann, H. Insight into Mg(BH4)2 with Synchrotron X-ray Diffraction: Structure Revision, Crystal Chemistry, and Anomalous Thermal Expansion. Chem. Mater. 2009, 21, 925–933. [Google Scholar] [CrossRef]
- Her, J.-H.; Stephens, P.W.; Gao, Y.; Soloveichik, G.L.; Rijssenbeek, J.; Andrus, M.; Zhao, J.-C. Structure of unsolvated magnesium borohydride Mg(BH4)2. Acta Crystallogr. B 2007, 63, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Pitt, M.P.; Webb, C.J.; Paskevicius, M.; Sheptyakov, D.; Buckley, C.E.; Gray, E.M. In Situ Neutron Diffraction Study of the Deuteration of Isotopic Mg11B2. J. Phys. Chem. C 2011, 115, 22669–22679. [Google Scholar] [CrossRef]
- Paskevicius, M.; Pitt, M.P.; Webb, C.J.; Sheppard, D.A.; Filsø, U.; Gray, E.M.; Buckley, C.E. In-Situ X-ray Diffraction Study of γ-Mg(BH4)2 Decomposition. J. Phys. Chem. C 2012, 116, 15231–15240. [Google Scholar] [CrossRef]
- David, W.I.F.; Callear, S.K.; Jones, M.O.; Aeberhard, P.C.; Culligan, S.D.; Pohl, A.H.; Johnson, S.R.; Ryan, K.R.; Parker, J.E.; Edwards, P.P.; et al. The structure, thermal properties and phase transformations of the cubic polymorph of magnesium tetrahydroborate. Phys. Chem. Chem. Phys. 2012, 14, 11800–11807. [Google Scholar] [CrossRef] [PubMed]
- Amieiro-Fonseca, A.; Ellis, S.R.; Nuttall, C.J.; Hayden, B.E.; Guerin, S.; Purdy, G.; Soulié, J.-P.; Callear, S.K.; Culligan, S.D.; David, W.I.F.; et al. A multidisciplinary combinatorial approach for tuning promising hydrogen storage materials towards automotive applications. Faraday Discuss. 2011, 151, 369. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-W.; Kikuchi, K.; Nakamori, Y.; Miwa, K.; Towata, S.; Orimo, S. Effects of ball milling and additives on dehydriding behaviors of well-crystallized Mg(BH4)2. Scr. Mater. 2007, 57, 679–682. [Google Scholar] [CrossRef]
- Li, H.-W.; Miwa, K.; Ohba, N.; Fujita, T.; Sato, T.; Yan, Y.; Towata, S.; Chen, M.W.; Orimo, S. Formation of an intermediate compound with a B12H12 cluster: Experimental and theoretical studies on magnesium borohydride Mg(BH4)2. Nanotechnology 2009, 20, 204013. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-W.; Matsunaga, T.; Yan, Y.; Maekawa, H.; Ishikiriyama, M.; Orimo, S. Nanostructure-induced hydrogenation of layered compound MgB2. J. Alloys Compd. 2010, 505, 654–656. [Google Scholar] [CrossRef]
- Roedern, E.; Jensen, T.R. Ammine-Stabilized Transition-Metal Borohydrides of Iron, Cobalt, and Chromium: Synthesis and Characterization. Inorg. Chem. 2015, 54, 10477–10482. [Google Scholar] [CrossRef] [PubMed]
- Callini, E.; Szilágyi, P.Á.; Paskevicius, M.; Stadie, N.P.; Réhault, J.; Buckley, C.E.; Borgschulte, A.; Züttel, A. Stabilization of volatile Ti(BH4)3 by nano-confinement in a metal–organic framework. Chem. Sci. 2015, 7, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Rude, L.H.; Corno, M.; Ugliengo, P.; Baricco, M.; Lee, Y.-S.; Cho, Y.W.; Besenbacher, F.; Overgaard, J.; Jensen, T.R. Synthesis and Structural Investigation of Zr(BH4)4. J. Phys. Chem. C 2012, 116, 20239–20245. [Google Scholar] [CrossRef]
- Nickels, E.A.; Jones, M.O.; David, W.I.F.; Johnson, S.R.; Lowton, R.L.; Sommariva, M.; Edwards, P.P. Tuning the Decomposition Temperature in Complex Hydrides: Synthesis of a Mixed Alkali Metal Borohydride. Angew. Chem. Int. Ed. 2008, 47, 2817–2819. [Google Scholar] [CrossRef] [PubMed]
- Roedern, E.; Hansen, B.R.S.; Ley, M.B.; Jensen, T.R. Effect of Eutectic Melting, Reactive Hydride Composites, and Nanoconfinement on Decomposition and Reversibility of LiBH4–KBH4. J. Phys. Chem. C 2015, 119, 25818–25825. [Google Scholar] [CrossRef]
- Jensen, S.R.H.; Jepsen, L.H.; Skibsted, J.; Jensen, T.R. Phase Diagram for the NaBH4–KBH4 System and the Stability of a Na1−xKxBH4 Solid Solution. J. Phys. Chem. C 2015, 119, 27919–27929. [Google Scholar] [CrossRef]
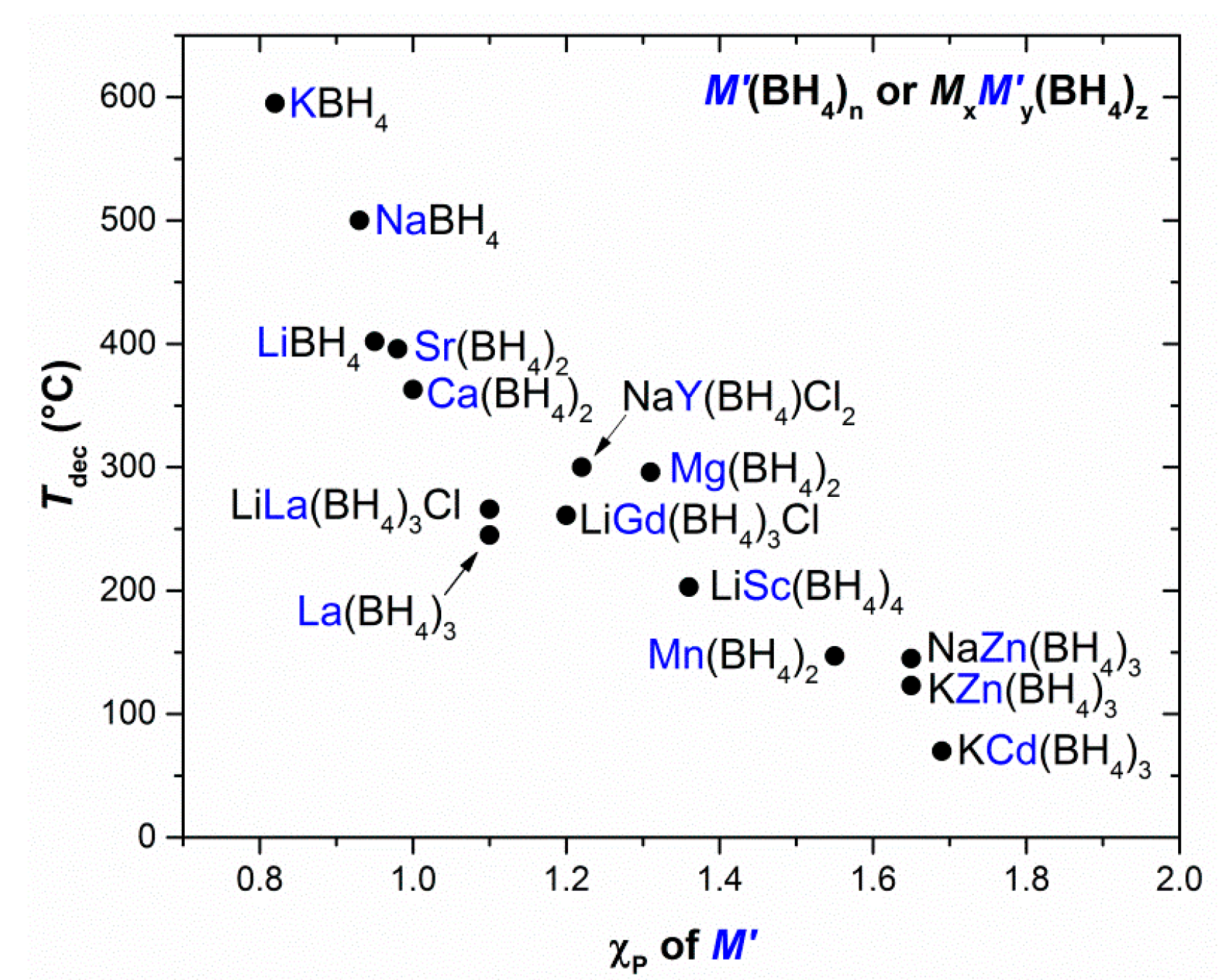
- Nakamori, Y.; Miwa, K.; Ninomiya, A.; Li, H.; Ohba, N.; Towata, S.; Züttel, A.; Orimo, S. Correlation between thermodynamical stabilities of metal borohydrides and cation electronegativites: First-principles calculations and experiments. Phys. Rev. B 2006, 74, 45126. [Google Scholar] [CrossRef]
- Li, H.-W.; Orimo, S.; Nakamori, Y.; Miwa, K.; Ohba, N.; Towata, S.; Züttel, A. Materials designing of metal borohydrides: Viewpoints from thermodynamical stabilities. J. Alloys Compd. 2007, 446–447, 315–318. [Google Scholar] [CrossRef]
- Ravnsbæk, D.; Filinchuk, Y.; Cerenius, Y.; Jakobsen, H.J.; Besenbacher, F.; Skibsted, J.; Jensen, T.R. A Series of Mixed-Metal Borohydrides. Angew. Chem. Int. Ed. 2009, 48, 6659–6663. [Google Scholar] [CrossRef] [PubMed]
- Ravnsbæk, D.B.; Frommen, C.; Reed, D.; Filinchuk, Y.; Sørby, M.; Hauback, B.C.; Jakobsen, H.J.; Book, D.; Besenbacher, F.; Skibsted, J.; et al. Structural studies of lithium zinc borohydride by neutron powder diffraction, Raman and NMR spectroscopy. J. Alloys Compd. 2011, 509, S698–S704. [Google Scholar] [CrossRef]
- Černý, R.; Kim, K.C.; Penin, N.; D’Anna, V.; Hagemann, H.; Sholl, D.S. AZn2(BH4)5 (A = Li, Na) and NaZn(BH4)3: Structural Studies. J. Phys. Chem. C 2010, 114, 19127–19133. [Google Scholar] [CrossRef]
- Ley, M.B.; Ravnsbæk, D.B.; Filinchuk, Y.; Lee, Y.-S.; Janot, R.; Cho, Y.W.; Skibsted, J.; Jensen, T.R. LiCe(BH4)3Cl, a New Lithium-Ion Conductor and Hydrogen Storage Material with Isolated Tetranuclear Anionic Clusters. Chem. Mater. 2012, 24, 1654–1663. [Google Scholar] [CrossRef]
- Møller, K.T.; Ley, M.B.; Schouwink, P.; Černý, R.; Jensen, T.R. Synthesis and thermal stability of perovskite alkali metal strontium borohydrides. Dalton Trans. 2016, 45, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Pezat, M.; Darriet, B. A new ternary hydride: CsCaH3. Rev. Chim. Minérale 1986, 23, 323–328. [Google Scholar]
- Bouamrane, A.; Laval, J.P.; Soulie, J.-P.; Bastide, J.P. Structural characterization of NaMgH2F and NaMgH3. Mater. Res. Bull. 2000, 35, 545–549. [Google Scholar] [CrossRef]
- Pottmaier, D.; Pinatel, E.R.; Vitillo, J.G.; Garroni, S.; Orlova, M.; Baró, M.D.; Vaughan, G.B.M.; Fichtner, M.; Lohstroh, W.; Baricco, M. Structure and Thermodynamic Properties of the NaMgH3 Perovskite: A Comprehensive Study. Chem. Mater. 2011, 23, 2317–2326. [Google Scholar] [CrossRef]
- Ikeda, K.; Kogure, Y.; Nakamori, Y.; Orimo, S. Reversible hydriding and dehydriding reactions of perovskite-type hydride NaMgH3. Scr. Mater. 2005, 53, 319–322. [Google Scholar] [CrossRef]
- Ikeda, K.; Kato, S.; Shinzato, Y.; Okuda, N.; Nakamori, Y.; Kitano, A.; Yukawa, H.; Morinaga, M.; Orimo, S. Thermodynamical stability and electronic structure of a perovskite-type hydride, NaMgH3. J. Alloys Compd. 2007, 446–447, 162–165. [Google Scholar] [CrossRef]
- Schouwink, P.; D’Anna, V.; Ley, M.B.; Daku, L.M.L.; Richter, B.; Jensen, T.R.; Hagemann, H.; Černý, R. Bimetallic Borohydrides in the System M(BH4)2–KBH4 (M = Mg, Mn): On the Structural Diversity. J. Phys. Chem. C 2012, 116, 10829–10840. [Google Scholar] [CrossRef]
- Schouwink, P.; Ley, M.B.; Tissot, A.; Hagemann, H.; Jensen, T.R.; Smrčok, Ľ.; Černý, R. Structure and properties of complex hydride perovskite materials. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Møller, K.T.; Jørgensen, M.; Fogh, A.S.; Jensen, T.R. Perovskite alkali metal samarium borohydrides: Crystal structures and thermal decomposition. Dalton Trans. 2017, 46, 11905–11912. [Google Scholar] [CrossRef] [PubMed]
- Schouwink, P.; Hagemann, H.; Embs, J.P.; D’Anna, V.; Černý, R. Di-hydrogen contact induced lattice instabilities and structural dynamics in complex hydride perovskites. J. Phys. Condens. Matter 2015, 27, 265403. [Google Scholar] [CrossRef] [PubMed]
- Černý, R.; Schouwink, P.; Sadikin, Y.; Stare, K.; Smrčok, L.; Richter, B.; Jensen, T.R. Trimetallic Borohydride Li3MZn5(BH4)15 (M = Mg, Mn) Containing Two Weakly Interconnected Frameworks. Inorg. Chem. 2013, 52, 9941–9947. [Google Scholar] [CrossRef] [PubMed]
- Schouwink, P.; Ley, M.B.; Jensen, T.R.; Černý, R. Borohydrides: From sheet to framework topologies. Dalton Trans. 2014, 43, 7726–7733. [Google Scholar] [CrossRef] [PubMed]
- Brighi, M.; Schouwink, P.; Sadikin, Y.; Černý, R. Fast ion conduction in garnet-type metal borohydrides Li3K3Ce2(BH4)12 and Li3K3La2(BH4)12. J. Alloys Compd. 2016, 662, 388–395. [Google Scholar] [CrossRef]
- Payandeh GharibDoust, S.; Heere, M.; Sørby, M.H.; Ley, M.B.; Ravnsbæk, D.B.; Hauback, B.C.; Černý, R.; Jensen, T.R. Synthesis, structure and properties of new bimetallic sodium and potassium lanthanum borohydrides. Dalton Trans. 2016, 45, 19002–19011. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, L.H.; Ley, M.B.; Filinchuk, Y.; Besenbacher, F.; Jensen, T.R. Tailoring the Properties of Ammine Metal Borohydrides for Solid-State Hydrogen Storage. ChemSusChem 2015, 8, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tan, Y.; Su, J.; Gu, Q.; Černý, R.; Ouyang, L.; Sun, D.; Yu, X.; Zhu, M. Synthesis, structure and dehydrogenation of zirconium borohydride octaammoniate. Chem. Commun. 2015, 51, 2794–2797. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, L.H.; Ley, M.B.; Černý, R.; Lee, Y.-S.; Cho, Y.W.; Ravnsbæk, D.; Besenbacher, F.; Skibsted, J.; Jensen, T.R. Trends in Syntheses, Structures, and Properties for Three Series of Ammine Rare-Earth Metal Borohydrides, M(BH4)3·nNH3 (M = Y, Gd, and Dy). Inorg. Chem. 2015, 54, 7402–7414. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, X.; Sun, W.; Sun, D.; Yang, W. The Hydrogen-Enriched Al–B–N System as an Advanced Solid Hydrogen-Storage Candidate. Angew. Chem. Int. Ed. 2011, 50, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, H.; Zhou, W.; Yu, X. Dehydrogenation Tuning of Ammine Borohydrides Using Double-Metal Cations. J. Am. Chem. Soc. 2011, 133, 4690–4693. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Gao, L.; Guo, Y.; Tan, Y.; Tang, Z.; Wallwork, K.S.; Zhang, F.; Yu, X. Structure and decomposition of zinc borohydride ammonia adduct: Towards a pure hydrogen release. Energy Environ. Sci. 2012, 5, 7590–7600. [Google Scholar] [CrossRef]
- Jepsen, L.H.; Lee, Y.-S.; Černý, R.; Sarusie, R.S.; Cho, Y.W.; Besenbacher, F.; Jensen, T.R. Ammine Calcium and Strontium Borohydrides: Syntheses, Structures, and Properties. ChemSusChem 2015, 8, 3472–3482. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jiang, Y.; Xia, G.; Yu, X. Ammine aluminium borohydrides: An appealing system releasing over 12 wt % pure H2 under moderate temperature. Chem. Commun. 2012, 48, 4408–4410. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhou, W.; Pinkerton, F.E.; Meyer, M.S.; Srinivas, G.; Yildirim, T.; Udovic, T.J.; Rush, J.J. A new family of metal borohydride ammonia borane complexes: Synthesis, structures, and hydrogen storage properties. J. Mater. Chem. 2010, 20, 6550–6556. [Google Scholar] [CrossRef]
- Jepsen, L.H.; Ban, V.; Møller, K.T.; Lee, Y.-S.; Cho, Y.W.; Besenbacher, F.; Filinchuk, Y.; Skibsted, J.; Jensen, T.R. Synthesis, Crystal Structure, Thermal Decomposition, and 11B MAS NMR Characterization of Mg(BH4)2(NH3BH3)2. J. Phys. Chem. C 2014, 118, 12141–12153. [Google Scholar] [CrossRef]
- Dovgaliuk, I.; Le Duff, C.S.; Robeyns, K.; Devillers, M.; Filinchuk, Y. Mild Dehydrogenation of Ammonia Borane Complexed with Aluminum Borohydride. Chem. Mater. 2015, 27, 768–777. [Google Scholar] [CrossRef]
- Luo, J.; Wu, H.; Zhou, W.; Kang, X.; Fang, Z.; Wang, P. LiBH4·NH3BH3: A new lithium borohydride ammonia borane compound with a novel structure and favorable hydrogen storage properties. Int. J. Hydrogen Energy 2012, 37, 10750–10757. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, F.; Gu, Q.; Yu, X. Synthesis, structures and hydrogen storage properties of two new H-enriched compounds: Mg(BH4)2(NH3BH3)2 and Mg(BH4)2·(NH3)2(NH3BH3). Dalton Trans. 2013, 42, 14365–14368. [Google Scholar] [CrossRef] [PubMed]
- Chua, Y.S.; Chen, P.; Wu, G.; Xiong, Z. Development of amidoboranes for hydrogen storage. Chem. Commun. 2011, 47, 5116–5129. [Google Scholar] [CrossRef] [PubMed]
- Dovgaliuk, I.; Jepsen, L.H.; Safin, D.A.; Łodziana, Z.; Dyadkin, V.; Jensen, T.R.; Devillers, M.; Filinchuk, Y. A Composite of Complex and Chemical Hydrides Yields the First Al-Based Amidoborane with Improved Hydrogen Storage Properties. Chem. Eur. J. 2015, 14562–14570. [Google Scholar] [CrossRef] [PubMed]
- Møller, K.T.; Jørgensen, M.; Andreasen, J.G.; Skibsted, J.; Łodziana, Z.; Filinchuk, Y.; Jensen, T.R. Synthesis and Thermal Decomposition of Potassium Tetraamidoboranealuminate, K[Al(NH2BH3)4]. Int. J. Hydrogen Energy 2017. accepted. [Google Scholar]
- Ikoma, M. Ni-Metal Hydride Batteries. In Encyclopedia of Applied Electrochemistry; Kreysa, G., Ota, K., Savinell, R.F., Eds.; Springer: New York, NY, USA, 2014; pp. 1363–1366. ISBN 978-1-4419-6995-8. [Google Scholar]
- Sakai, T.; Miyamura, H.; Kuriyama, N.; Kato, A.; Oguro, K.; Ishikawa, H. Metal Hydride Anodes for Nickel-Hydrogen Secondary Battery. J. Electrochem. Soc. 1990, 137, 795–799. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H. Hydrogen—A sustainable energy carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Oumellal, Y.; Rougier, A.; Nazri, G.A.; Tarascon, J.-M.; Aymard, L. Metal hydrides for lithium-ion batteries. Nat. Mater. 2008, 7, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Zaïdi, W.; Oumellal, Y.; Bonnet, J.-P.; Zhang, J.; Cuevas, F.; Latroche, M.; Bobet, J.-L.; Aymard, L. Carboxymethylcellulose and carboxymethylcellulose-formate as binders in MgH2–carbon composites negative electrode for lithium-ion batteries. J. Power Sources 2011, 196, 2854–2857. [Google Scholar] [CrossRef]
- Hanada, N.; Kamura, A.; Suzuki, H.; Takai, K.; Ichikawa, T.; Kojima, Y. Electrochemical charge and discharge properties for the formation of magnesium and aluminum hydrides. J. Alloys Compd. 2011, 509, S584–S587. [Google Scholar] [CrossRef]
- Brutti, S.; Mulas, G.; Piciollo, E.; Panero, S.; Reale, P. Magnesium hydride as a high capacity negative electrode for lithium ion batteries. J. Mater. Chem. 2012, 22, 14531–14537. [Google Scholar] [CrossRef]
- Ikeda, S.; Ichikawa, T.; Kawahito, K.; Hirabayashi, K.; Miyaoka, H.; Kojima, Y. Anode properties of magnesium hydride catalyzed with niobium oxide for an all solid-state lithium-ion battery. Chem. Commun. 2013, 49, 7174–7176. [Google Scholar] [CrossRef] [PubMed]
- Oumellal, Y.; Zlotea, C.; Bastide, S.; Cachet-Vivier, C.; Léonel, E.; Sengmany, S.; Leroy, E.; Aymard, L.; Bonnet, J.-P.; Latroche, M. Bottom-up preparation of MgH2 nanoparticles with enhanced cycle life stability during electrochemical conversion in Li-ion batteries. Nanoscale 2014, 6, 14459–14466. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Ichikawa, T.; Goshome, K.; Yamaguchi, S.; Miyaoka, H.; Kojima, Y. Anode properties of Al2O3-added MgH2 for all-solid-state lithium-ion batteries. J. Solid State Electrochem. 2015, 12, 3639–3644. [Google Scholar] [CrossRef]
- Meggiolaro, D.; Gigli, G.; Paolone, A.; Reale, P.; Doublet, M.L.; Brutti, S. Origin of the Voltage Hysteresis of MgH2 Electrodes in Lithium Batteries. J. Phys. Chem. C 2015, 119, 17044–17052. [Google Scholar] [CrossRef]
- Zeng, L.; Ichikawa, T.; Kawahito, K.; Miyaoka, H.; Kojima, Y. Bulk-Type All-Solid-State Lithium-Ion Batteries: Remarkable Performances of a Carbon Nanofiber-Supported MgH2 Composite Electrode. ACS Appl. Mater. Interfaces 2017, 9, 2261–2266. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, H.; Hu, R.; Ouyang, L.; Liu, J.; Zhu, M. Electrochemical performances of MgH2 and MgH2-C films for lithium ion battery anode. J. Alloys Compd. 2017, 711, 473–479. [Google Scholar] [CrossRef]
- Oumellal, Y.; Zaïdi, W.; Bonnet, J.-P.; Cuevas, F.; Latroche, M.; Zhang, J.; Bobet, J.-L.; Rougier, A.; Aymard, L. Reactivity of TiH2 hydride with lithium ion: Evidence for a new conversion mechanism. Int. J. Hydrogen Energy 2012, 37, 7831–7835. [Google Scholar] [CrossRef]
- Kawahito, K.; Zeng, L.; Ichikawa, T.; Miyaoka, H.; Kojima, Y. Electrochemical Performance of Titanium Hydride for Bulk-Type All-Solid-State Lithium-Ion Batteries. Mater. Trans. 2016, 57, 755–757. [Google Scholar] [CrossRef]
- Teprovich, J.A.; Zhang, J.; Colón-Mercado, H.; Cuevas, F.; Peters, B.; Greenway, S.; Zidan, R.; Latroche, M. Li-Driven Electrochemical Conversion Reaction of AlH3, LiAlH4, and NaAlH4. J. Phys. Chem. C 2015, 119, 4666–4674. [Google Scholar] [CrossRef]
- Oumellal, Y.; Rougier, A.; Tarascon, J.-M.; Aymard, L. 2LiH + M (M = Mg, Ti): New concept of negative electrode for rechargeable lithium-ion batteries. J. Power Sources 2009, 192, 698–702. [Google Scholar] [CrossRef]
- Huang, L.; Aymard, L.; Bonnet, J.-P. MgH2–TiH2 mixture as an anode for lithium-ion batteries: Synergic enhancement of the conversion electrode electrochemical performance. J. Mater. Chem. A 2015, 3, 15091–15096. [Google Scholar] [CrossRef]
- Zaïdi, W.; Bonnet, J.-P.; Zhang, J.; Cuevas, F.; Latroche, M.; Couillaud, S.; Bobet, J.-L.; Sougrati, M.T.; Jumas, J.-C.; Aymard, L. Reactivity of complex hydrides Mg2FeH6, Mg2CoH5 and Mg2NiH4 with lithium ion: Far from equilibrium electrochemically driven conversion reactions. Int. J. Hydrogen Energy 2013, 38, 4798–4808. [Google Scholar] [CrossRef]
- Aymard, L.; Oumellal, Y.; Bonnet, J.-P. Metal hydrides: An innovative and challenging conversion reaction anode for lithium-ion batteries. Beilstein J. Nanotechnol. 2015, 6, 1821–1839. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.; Cuevas, F.; Latroche, M. Metal hydrides used as negative electrode materials for Li-ion batteries. Appl. Phys. A 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Silvestri, L.; Forgia, S.; Farina, L.; Meggiolaro, D.; Panero, S.; La Barbera, A.; Brutti, S.; Reale, P. Lithium Alanates as Negative Electrodes in Lithium-Ion Batteries. ChemElectroChem 2015, 2, 877–886. [Google Scholar] [CrossRef]
- Silvestri, L.; Farina, L.; Meggiolaro, D.; Panero, S.; Padella, F.; Brutti, S.; Reale, P. Reactivity of Sodium Alanates in Lithium Batteries. J. Phys. Chem. C 2015, 119, 28766–28775. [Google Scholar] [CrossRef]
- Cirrincione, L.; Silvestri, L.; Mallia, C.; Stallworth, P.E.; Greenbaum, S.; Brutti, S.; Panero, S.; Reale, P. Investigation of the Effects of Mechanochemical Treatment on NaAlH4 Based Anode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2016, 163, A2628–A2635. [Google Scholar] [CrossRef]
- Silvestri, L.; Paolone, A.; Cirrincione, L.; Stallworth, P.; Greenbaum, S.; Panero, S.; Brutti, S.; Reale, P. NaAlH4 Nanoconfinement in a Mesoporous Carbon for Application in Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A1120–A1125. [Google Scholar] [CrossRef]
- Huen, P.; Peru, F.; Charalambopoulou, G.; Steriotis, T.A.; Jensen, T.R.; Ravnsbæk, D.B. Nanoconfined NaAlH4 Conversion Electrodes for Li Batteries. ACS Omega 2017, 2, 1956–1967. [Google Scholar] [CrossRef]
- Meggiolaro, D.; Farina, L.; Silvestri, L.; Panero, S.; Brutti, S.; Reale, P. Lightweight Borohydrides Electro-Activity in Lithium Cells. Energies 2016, 9, 238. [Google Scholar] [CrossRef] [Green Version]
- Mason, T.H.; Liu, X.; Hong, J.; Graetz, J.; Majzoub, E.H. First-Principles Study of Novel Conversion Reactions for High-Capacity Li-Ion Battery Anodes in the Li–Mg–B–N–H System. J. Phys. Chem. C 2011, 115, 16681–16687. [Google Scholar] [CrossRef]
- Unemoto, A.; Matsuo, M.; Orimo, S. Complex Hydrides for Electrochemical Energy Storage. Adv. Funct. Mater. 2014, 24, 2267–2279. [Google Scholar] [CrossRef]
- Matsuo, M.; Orimo, S. Lithium Fast-Ionic Conduction in Complex Hydrides: Review and Prospects. Adv. Energy Mater. 2011, 1, 161–172. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Blanchard, D.; Matsuo, M.; Udovic, T.J.; Orimo, S. Complex hydrides as room-temperature solid electrolytes for rechargeable batteries. Appl. Phys. A 2016, 122, 251. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, M.; Nakamori, Y.; Orimo, S.; Maekawa, H.; Takamura, H. Lithium superionic conduction in lithium borohydride accompanied by structural transition. Appl. Phys. Lett. 2007, 91, 224103. [Google Scholar] [CrossRef]
- Maekawa, H.; Matsuo, M.; Takamura, H.; Ando, M.; Noda, Y.; Karahashi, T.; Orimo, S. Halide-Stabilized LiBH4, a Room-Temperature Lithium Fast-Ion Conductor. J. Am. Chem. Soc. 2009, 131, 894–895. [Google Scholar] [CrossRef] [PubMed]
- Rude, L.H.; Groppo, E.; Arnbjerg, L.M.; Ravnsbæk, D.B.; Malmkjær, R.A.; Filinchuk, Y.; Baricco, M.; Besenbacher, F.; Jensen, T.R. Iodide substitution in lithium borohydride, LiBH4-LiI. J. Alloys Compd. 2011, 509, 8299–8305. [Google Scholar] [CrossRef]
- Mosegaard, L.; Møller, B.; Jørgensen, J.-E.; Filinchuk, Y.; Cerenius, Y.; Hanson, J.C.; Dimasi, E.; Besenbacher, F.; Jensen, T.R. Reactivity of LiBH4: In Situ Synchrotron Radiation Powder X-ray Diffraction Study. J. Phys. Chem. C 2008, 112, 1299–1303. [Google Scholar] [CrossRef]
- Mohtadi, R.; Matsui, M.; Arthur, T.S.; Hwang, S.-J. Magnesium Borohydride: From Hydrogen Storage to Magnesium Battery. Angew. Chem. Int. Ed. 2012, 51, 9780–9783. [Google Scholar] [CrossRef] [PubMed]
- Roedern, E.; Kühnel, R.-S.; Remhof, A.; Battaglia, C. Magnesium Ethylenediamine Borohydride as Solid-State Electrolyte for Magnesium Batteries. Sci. Rep. 2017, 7, 46189. [Google Scholar] [CrossRef] [PubMed]
- Higashi, S.; Miwa, K.; Aoki, M.; Takechi, K. A novel inorganic solid state ion conductor for rechargeable Mg batteries. Chem. Commun. 2014, 50, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Udovic, T.J.; Matsuo, M.; Tang, W.S.; Wu, H.; Stavila, V.; Soloninin, A.V.; Skoryunov, R.V.; Babanova, O.A.; Skripov, A.V.; Rush, J.J.; et al. Exceptional Superionic Conductivity in Disordered Sodium Decahydro-closo-decaborate. Adv. Mater. 2014, 26, 7622–7626. [Google Scholar] [CrossRef] [PubMed]
- Udovic, T.J.; Matsuo, M.; Unemoto, A.; Verdal, N.; Stavila, V.; Skripov, A.V.; Rush, J.J.; Takamura, H.; Orimo, S. Sodium superionic conduction in Na2B12H12. Chem. Commun. 2014, 50, 3750. [Google Scholar] [CrossRef] [PubMed]
- Ravnsbæk, D.B.; Sørensen, L.H.; Filinchuk, Y.; Reed, D.; Book, D.; Jakobsen, H.J.; Besenbacher, F.; Skibsted, J.; Jensen, T.R. Mixed-Anion and Mixed-Cation Borohydride KZn(BH4)Cl2: Synthesis, Structure and Thermal Decomposition. Eur. J. Inorg. Chem. 2010, 2010, 1608–1612. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Ley, M.B.; Lee, Y.-S.; Hagemann, H.; D’Anna, V.; Cho, Y.W.; Filinchuk, Y.; Jensen, T.R. A mixed-cation mixed-anion borohydride NaY(BH4)2Cl2. Int. J. Hydrogen Energy 2012, 37, 8428–8438. [Google Scholar] [CrossRef]
- Jaroń, T.; Grochala, W. Probing Lewis acidity of Y(BH4)3 via its reactions with MBH4 (M = Li, Na, K, NMe4). Dalton Trans. 2011, 40, 12808–12817. [Google Scholar] [CrossRef] [PubMed]
- Černý, R.; Ravnsbæk, D.B.; Schouwink, P.; Filinchuk, Y.; Penin, N.; Teyssier, J.; Smrčok, Ľ.; Jensen, T.R. Potassium Zinc Borohydrides Containing Triangular [Zn(BH4)3]− and Tetrahedral [Zn(BH4)xCl4−x]2− Anions. J. Phys. Chem. C 2012, 116, 1563–1571. [Google Scholar] [CrossRef]
- Rude, L.H.; Filsø, U.; D’Anna, V.; Spyratou, A.; Richter, B.; Hino, S.; Zavorotynska, O.; Baricco, M.; Sørby, M.H.; Hauback, B.C.; et al. Hydrogen–fluorine exchange in NaBH4–NaBF4. Phys. Chem. Chem. Phys. 2013, 15, 18185–18194. [Google Scholar] [CrossRef] [PubMed]
- Richter, B.; Ravnsbæk, D.B.; Sharma, M.; Spyratou, A.; Hagemann, H.; Jensen, T.R. Fluoride substitution in LiBH4: Destabilization and decomposition. Phys. Chem. Chem. Phys. 2017, in press. [Google Scholar] [CrossRef]
- Ley, M.B.; Boulineau, S.; Janot, R.; Filinchuk, Y.; Jensen, T.R. New Li Ion Conductors and Solid State Hydrogen Storage Materials: LiM(BH4)3Cl, M = La, Gd. J. Phys. Chem. C 2012, 116, 21267–21276. [Google Scholar] [CrossRef]
- Frommen, C.; Sørby, M.H.; Ravindran, P.; Vajeeston, P.; Fjellvåg, H.; Hauback, B.C. Synthesis, Crystal Structure, and Thermal Properties of the First Mixed-Metal and Anion-Substituted Rare Earth Borohydride LiCe(BH4)3Cl. J. Phys. Chem. C 2011, 115, 23591–23602. [Google Scholar] [CrossRef] [Green Version]
- Olsen, J.E.; Frommen, C.; Jensen, T.R.; Riktor, M.D.; Sørby, M.H.; Hauback, B.C. Structure and thermal properties of composites with RE-borohydrides (RE = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Er, Yb or Lu) and LiBH4. RSC Adv. 2013, 4, 1570–1582. [Google Scholar] [CrossRef]
- Skripov, A.V.; Soloninin, A.V.; Ley, M.B.; Jensen, T.R.; Filinchuk, Y. Nuclear Magnetic Resonance Studies of BH4 Reorientations and Li Diffusion in LiLa (BH4)3Cl. J. Phys. Chem. C 2013, 117, 14965–14972. [Google Scholar] [CrossRef]
- Lundén, A. On the Paddle -Wheel Mechanism for Cation Conduction in Lithium Sulphate. Z. Naturforschung A 2014, 50, 1067–1076. [Google Scholar] [CrossRef]
- Payandeh GharibDoust, S.; Brighi, M.; Sadikin, Y.; Ravnsbæk, D.B.; Černý, R.; Skibsted, J.; Jensen, T.R. Synthesis, Structure, and Li-Ion Conductivity of LiLa(BH4)3X, X = Cl, Br, I. J. Phys. Chem. C 2017, 121, 19010–19021. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Ley, M.B.; Jensen, T.R.; Cho, Y.W. Lithium Ion Disorder and Conduction Mechanism in LiCe(BH4)3Cl. J. Phys. Chem. C 2016, 120, 19035–19042. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Filinchuk, Y.; Lee, H.-S.; Suh, J.-Y.; Kim, J.W.; Yu, J.-S.; Cho, Y.W. On the Formation and the Structure of the First Bimetallic Borohydride Borate, LiCa3(BH4)(BO3)2. J. Phys. Chem. C 2011, 115, 10298–10304. [Google Scholar] [CrossRef]
- Riktor, M.D.; Filinchuk, Y.; Vajeeston, P.; Bardají, E.G.; Fichtner, M.; Fjellvåg, H.; Sørby, M.H.; Hauback, B.C. The crystal structure of the first borohydride borate, Ca3(BD4)3(BO3). J. Mater. Chem. 2011, 21, 7188. [Google Scholar] [CrossRef]
- Paskevicius, M.; Hansen, B.R.S.; Jørgensen, M.; Richter, B.; Jensen, T.R. Multifunctionality of Silver closo-Boranes. Nat. Commun. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.R.S.; Paskevicius, M.; Jørgensen, M.; Jensen, T.R. Halogenated Sodium-closo-Dodecaboranes as Solid-State Ion Conductors. Chem. Mater. 2017, 29, 3423–3430. [Google Scholar] [CrossRef]
- Bukovsky, E.V.; Peryshkov, D.V.; Wu, H.; Zhou, W.; Tang, W.S.; Jones, W.M.; Stavila, V.; Udovic, T.J.; Strauss, S.H. Comparison of the Coordination of B12F122−, B12Cl122−, and B12H122− to Na+ in the Solid State: Crystal Structures and Thermal Behavior of Na2(B12F12), Na2(H2O)4(B12F12), Na2(B12Cl12), and Na2(H2O)6(B12Cl12). Inorg. Chem. 2017, 56, 4369–4379. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.S.; Unemoto, A.; Zhou, W.; Stavila, V.; Matsuo, M.; Wu, H.; Orimo, S.; Udovic, T.J. Unparalleled lithium and sodium superionic conduction in solid electrolytes with large monovalent cage-like anions. Energy Environ. Sci. 2015, 8, 3637–3645. [Google Scholar] [CrossRef] [PubMed]
- Sandrock, G.; Thomas, G. The IEA/DOE/SNL on-line hydride databases. Appl. Phys. A 2001, 72, 153–155. [Google Scholar] [CrossRef]
- Johnson, J.R. Reaction of hydrogen with the high temperature (C14) form of TiCr2. J. Common Met. 1980, 73, 345–354. [Google Scholar] [CrossRef]
- Manchester, F.D. Phase Diagrams of Binary Hydrogen Alloys; Monograph Series on Alloy Phase Diagrams, 13; ASM International: Materials Park, OH, USA, 2000; ISBN 0-87170-587-7. [Google Scholar]
- Sandrock, G.; Suda, S.; Schlapbach, L. Hydrogen in Intermetallic Compounds II, Topics in Applied Physics; Springer: Berlin/Heidelberg, Germany, 1992; Volume 67. [Google Scholar]
- Dantzer, P. Properties of intermetallic compounds suitable for hydrogen storage applications. Mater. Sci. Eng. A 2002, 329–331, 313–320. [Google Scholar] [CrossRef]
- Sandrock, G.; Bowman, R.C. Gas-based hydride applications: Recent progress and future needs. J. Alloys Compd. 2003, 356, 794–799. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Yartys, V.A.; Pollet, B.G.; Bowman, R.C., Jr. Metal hydride hydrogen compressors: A review. Int. J. Hydrogen Energy 2014, 39, 5818–5851. [Google Scholar] [CrossRef]
- SunShot Vision Study. Chapter 5: Concentrating Solar Power: Technologies, Cost, and Performance; US Department of Energy: Washington, DC, USA, 2012. [Google Scholar]
- Rönnebro, E.C.E.; Whyatt, G.; Powell, M.; Westman, M.; Zheng, F.; Fang, Z.Z. Metal Hydrides for High-Temperature Power Generation. Energies 2015, 8, 8406–8430. [Google Scholar] [CrossRef]
- Sheppard, D.A.; Paskevicius, M.; Humphries, T.D.; Felderhoff, M.; Capurso, G.; von Colbe, J.B.; Dornheim, M.; Klassen, T.; Ward, P.A.; Teprovich, J.A.; et al. Metal hydrides for concentrating solar thermal power energy storage. Appl. Phys. A 2016, 122, 395. [Google Scholar] [CrossRef]
- Sheppard, D.A.; Humphries, T.D.; Buckley, C.E. Sodium-based hydrides for thermal energy applications. Appl. Phys. A 2016, 122, 406. [Google Scholar] [CrossRef]
- Fellet, M.; Buckley, C.E.; Paskevicius, M.; Sheppard, D.A. Research on metal hydrides revived for next-generation solutions to renewable energy storage. MRS Bull. 2013, 38, 1012–1013. [Google Scholar] [CrossRef]
- Ward, P.A.; Corgnale, C.; Teprovich, J.A.; Motyka, T.; Hardy, B.; Sheppard, D.; Buckley, C.; Zidan, R. Technical challenges and future direction for high-efficiency metal hydride thermal energy storage systems. Appl. Phys. A 2016, 122, 462. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef] [Green Version]
- Corgnale, C.; Hardy, B.; Motyka, T.; Zidan, R.; Teprovich, J.; Peters, B. Screening analysis of metal hydride based thermal energy storage systems for concentrating solar power plants. Renew. Sustain. Energy Rev. 2014, 38, 821–833. [Google Scholar] [CrossRef]
- Harries, D.N.; Paskevicius, M.; Sheppard, D.A.; Price, T.E.C.; Buckley, C.E. Concentrating Solar Thermal Heat Storage Using Metal Hydrides. Proc. IEEE 2012, 100, 539–549. [Google Scholar] [CrossRef]
- Sheppard, D.A.; Humphries, T.D.; Buckley, C.E. What is old is new again. Mater. Today 2015, 8, 414–415. [Google Scholar] [CrossRef]
- Felderhoff, M.; Urbanczyk, R.; Peil, S. Thermochemical heat storage for high temperature applications—A review. Green 2013, 3, 113–123. [Google Scholar] [CrossRef]
- Paskevicius, M.; Sheppard, D.A.; Williamson, K.; Buckley, C.E. Metal hydride thermal heat storage prototype for concentrating solar thermal power. Energy 2015, 88, 469–477. [Google Scholar] [CrossRef]
- Bogdanović, B.; Spliethoff, B.; Ritter, A. The magnesium hydride system for heat storage and cooling. Z. Phys. Chem. 1989, 164, 1497–1508. [Google Scholar] [CrossRef]
- Groll, M.; Isselhorst, A.; Wierse, M. Metal hydride devices for environmentally clean energy technology. Int. J. Hydrogen Energy 1994, 19, 507–515. [Google Scholar] [CrossRef]
- Bogdanović, B.; Ritter, A.; Spliethoff, B.; Straβburger, K. A process steam generator based on the high temperature magnesium hydride/magnesium heat storage system. Int. J. Hydrogen Energy 1995, 20, 811–822. [Google Scholar] [CrossRef]
- Dong, D.; Humphries, T.D.; Sheppard, D.A.; Stansby, B.; Paskevicius, M.; Sofianos, M.V.; Chaudhary, A.-L.; Dornheim, M.; Buckley, C.E. Thermal optimisation of metal hydride reactors for thermal energy storage applications. Sustain. Energy Fuels 2017. [Google Scholar] [CrossRef]
- Humphries, T.D.; Sheppard, D.A.; Rowles, M.R.; Sofianos, M.V.; Buckley, C.E. Fluoride substitution in sodium hydride for thermal energy storage applications. J. Mater. Chem. A 2016, 4, 12170–12178. [Google Scholar] [CrossRef]
- Sheppard, D.A.; Paskevicius, M.; Buckley, C.E. Thermodynamics of Hydrogen Desorption from NaMgH3 and Its Application as a Solar Heat Storage Medium. Chem. Mater. 2011, 23, 4298–4300. [Google Scholar] [CrossRef]
- Sheppard, D.A.; Corgnale, C.; Hardy, B.; Motyka, T.; Zidan, R.; Paskevicius, M.; Buckley, C.E. Hydriding characteristics of NaMgH2F with preliminary technical and cost evaluation of magnesium-based metal hydride materials for concentrating solar power thermal storage. RSC Adv. 2014, 4, 26552–26562. [Google Scholar] [CrossRef]
- Javadian, P.; Sheppard, D.A.; Jensen, T.R.; Buckley, C.E. Destabilization of lithium hydride and the thermodynamic assessment of the Li–Al–H system for solar thermal energy storage. Rsc Adv. 2016, 6, 94927–94933. [Google Scholar] [CrossRef]
- Doi, K.; Hino, S.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Hydrogen storage properties of lithium silicon alloy synthesized by mechanical alloying. J. Power Sources 2011, 196, 504–507. [Google Scholar] [CrossRef]
- Vajo, J.J.; Mertens, F.; Ahn, C.C.; Robert, C.B.; Fultz, B. Altering Hydrogen Storage Properties by Hydride Destabilization through Alloy Formation: LiH and MgH2 Destabilized with Si. J. Phys. Chem. B 2004, 108, 13977–13983. [Google Scholar] [CrossRef]
- Veleckis, E. Application of the hydrogen titration method to a thermodynamic investigation of solid Al-Ca alloys. J. Common Met. 1981, 80, 241–255. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Reaction of hydrogen with alloys of magnesium and nickel and the formation of Mg2NiH4. Inorg. Chem. 1968, 7, 2254–2256. [Google Scholar] [CrossRef]
- Reiser, A.; Bogdanović, B.; Schlichte, K. The application of Mg-based metal-hydrides as heat energy storage systems. Int. J. Hydrogen Energy 2000, 25, 425–430. [Google Scholar] [CrossRef]
- Bogdanović, B.; Reiser, A.; Schlichte, K.; Spliethoff, B.; Tesche, B. Thermodynamics and dynamics of the Mg–Fe–H system and its potential for thermochemical thermal energy storage. J. Alloys Compd. 2002, 345, 77–89. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Sheppard, D.A.; Buckley, C.E. Lithium imide systems for high temperature heat storage in concentrated solar thermal systems. J. Alloys Compd. 2017, 716, 291–298. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.; Qu, X. Investigation on LiBH4-CaH2 composite and its potential for thermal energy storage. Sci. Rep. 2017, 7, 41754. [Google Scholar] [CrossRef] [PubMed]
- El Kharbachi, A.; Pinatel, E.; Nuta, I.; Baricco, M. A thermodynamic assessment of LiBH4. Calphad 2012, 39, 80–90. [Google Scholar] [CrossRef]
- HSC Chemistry, version 6.12; Outotec Research Oy: Pori, Finland, 2006.
- Javadian, P.; GharibDoust, S.P.; Li, H.-W.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Reversibility of LiBH4 Facilitated by the LiBH4-Ca(BH4)2 Eutectic. J. Phys. Chem. C 2017. [Google Scholar] [CrossRef]
- Bogdanović, B.; Brand, R.A.; Marjanović, A.; Schwickardi, M.; Tölle, J. Metal-doped sodium aluminium hydrides as potential new hydrogen storage materials. J. Alloys Compd. 2000, 302, 36–58. [Google Scholar] [CrossRef]
- Fossdal, A.; Brinks, H.W.; Fonneløp, J.E.; Hauback, B.C. Pressure-composition isotherms and thermodynamic properties of TiF3-enhanced Na2LiAlH6. J. Alloys Compd. 2005, 397, 135–139. [Google Scholar] [CrossRef]
- Graetz, J.; Lee, Y.; Reilly, J.J.; Park, S.; Vogt, T. Structures and thermodynamics of the mixed alkali alanates. Phys. Rev. B 2005, 71, 184115. [Google Scholar] [CrossRef]
- Sørby, M.H.; Brinks, H.W.; Fossdal, A.; Thorshaug, K.; Hauback, B.C. The crystal structure and stability of K2NaAlH6. J. Alloys Compd. 2006, 415, 284–287. [Google Scholar] [CrossRef]
- Mamatha, M.; Weidenthaler, C.; Pommerin, A.; Felderhoff, M.; Schüth, F. Comparative studies of the decomposition of alanates followed by in situ XRD and DSC methods. J. Alloys Compd. 2006, 416, 303–314. [Google Scholar] [CrossRef]
- Varin, R.A.; Jang, M. The effects of graphite on the reversible hydrogen storage of nanostructured lithium amide and lithium hydride (LiNH2 + 1.2LiH) system. J. Alloys Compd. 2011, 509, 7143–7151. [Google Scholar] [CrossRef]
- Paskevicius, M.; Sheppard, D.A.; Buckley, C.E. Thermodynamic Changes in Mechanochemically Synthesized Magnesium Hydride Nanoparticles. J. Am. Chem. Soc. 2010, 132, 5077–5083. [Google Scholar] [CrossRef] [PubMed]
- Kenisarin, M.M. High-temperature phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2010, 14, 955–970. [Google Scholar] [CrossRef]
- Wagman, D.D.; Evans, W.H.; Parker, V.B.; Schumm, R.H.; Halow, I.; Bailey, S.M.; Churney, K.L.; Nuttall, R.L. The NBS tables of chemical thermodynamic properties. Selected values for inorganic and C1 and C2 organic substances in SI units. J. Phys. Chem. Ref. Data 1982, 11 (Suppl. 2). [Google Scholar] [CrossRef]
- Gavrichev, K.S. Heat Capacity and Thermodynamic Properties of Inorganic Compounds Containing Tetrahedral Anions (BH-4, AlH-4, GaH-4, BF-4, ClO-4, BrO-4, and IO-4). Inorg. Mater. 2003, 39, S89–S112. [Google Scholar] [CrossRef]
- Juza, R.; Opp, K. Metallamide und Metallnitride, 24. Mitteilung. Die Kristallstruktur des Lithiumamides. Z. Anorg. Allg. Chem. 1951, 266, 313–324. [Google Scholar] [CrossRef]
- Stasinevich, D.S.; Egorenko, G.A. Thermographic investigation of alkali metal and magnesium tetrahydroborates at pressures up to 10 atm. Russ. J. Inorg. Chem. 1968, 13, 341–343. [Google Scholar]
- Paskevicius, M.; Ley, M.B.; Sheppard, D.A.; Jensen, T.R.; Buckley, C.E. Eutectic melting in metal borohydrides. Phys. Chem. Chem. Phys. 2013, 15, 19774–19789. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-K.; Zhong, Y.; Schlom, D.G.; Xi, X.X.; Li, Q. Computational thermodynamic modeling of the Mg-B system. Calphad 2001, 25, 299–303. [Google Scholar] [CrossRef]
- Bogdanović, B.; Bohmhammel, K.; Christ, B.; Reiser, A.; Schlichte, K.; Vehlen, R.; Wolf, U. Thermodynamic investigation of the magnesium–hydrogen system. J. Alloys Compd. 1999, 282, 84–92. [Google Scholar] [CrossRef]
- Bogdanović, B.; Hofmann, H.; Neuy, A.; Reiser, A.; Schlichte, K.; Spliethoff, B.; Wessel, S. Ni-doped versus undoped Mg–MgH2 materials for high temperature heat or hydrogen storage. J. Alloys Compd. 1999, 292, 57–71. [Google Scholar] [CrossRef]
- Marcus, Y. Chapter 3—High Melting Salts in Ionic Liquid Properties: From Molten Salts to RTILs; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Sohal, M.S.; Ebner, M.A.; Sabharwall, P.; Sharpe, P. Engineering Database of Liquid Salt Thermophysical and Thermochemical Properties; Idaho National Laboratory (INL): Idaho Falls, ID, USA, 2010. [Google Scholar]
- Lehman, P.A.; Chamberlin, C.E. Design of a photovoltaic-hydrogen-fuel cell energy system. Int. J. Hydrogen Energy 1991, 16, 349–352. [Google Scholar] [CrossRef]
- Vanhanen, J.P.; Lund, P.D.; Hagström, M.T. Feasibility study of a metal hydride hydrogen store for a self-sufficient solar hydrogen energy system. Int. J. Hydrogen Energy 1996, 21, 213–221. [Google Scholar] [CrossRef]
- Gray, E.M.; Webb, C.J.; Andrews, J.; Shabani, B.; Tsai, P.J.; Chan, S.L.I. Hydrogen storage for off-grid power supply. Int. J. Hydrogen Energy 2011, 36, 654–663. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Tolj, I.; Pickering, L.; Sita, C.; Barbir, F.; Yartys, V. The use of metal hydrides in fuel cell applications. Prog. Nat. Sci. Mater. Int. 2017, 27, 3–20. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Khalil, Y.F.; Opalka, S.M.; Laube, B.L. Experimental and theoretical investigations for mitigating NaAlH4 reactivity risks during postulated accident scenarios involving exposure to air or water. Process Saf. Environ. Prot. 2013, 91, 463–475. [Google Scholar] [CrossRef]
- Chaise, A.; de Rango, P.; Marty, P.; Fruchart, D.; Miraglia, S.; Olivès, R.; Garrier, S. Enhancement of hydrogen sorption in magnesium hydride using expanded natural graphite. Int. J. Hydrogen Energy 2009, 34, 8589–8596. [Google Scholar] [CrossRef]
- Maleki, H.; Howard, J.N. Effects of overdischarge on performance and thermal stability of a Li-ion cell. J. Power Sources 2006, 160, 1395–1402. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Soloveichik, G.L. Battery Technologies for Large-Scale Stationary Energy Storage. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Zaghib, K.; Dontigny, M.; Guerfi, A.; Charest, P.; Rodrigues, I.; Mauger, A.; Julien, C.M. Safe and fast-charging Li-ion battery with long shelf life for power applications. J. Power Sources 2011, 196, 3949–3954. [Google Scholar] [CrossRef]
- Uchida, H.; Naragaki, Y. Influence of the Cyclic Hydriding-Dehydriding Treatment on Pressure-Composition-Temperature Relations of the LaNi5—H System. Z. Phys. Chem. 1993, 179, 93–101. [Google Scholar] [CrossRef]
- Bershadsky, E.; Josephy, Y.; Ron, M. Investigation of kinetics and structural changes in TiFe0.8Ni0.2 after prolonged cycling. J. Common Met. 1991, 172, 1036–1043. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Urbanczyk, R.; Bathen, D.; Felderhoff, M.; Burfeind, J. HT-PEM fuel cell system with integrated complex hydride storage tank. In Parallel Sessions Book 4: Storage Systems/Policy Perspectives, Initiatives and Co-operations, Proceedings of the 18th World Hydrogen Energy Conference 2010 (WHEC2010), Essen, Germany, 16–21 May 2010; Stolten, D., Grube, T., Eds.; Forschungszentrum Jülich GmbH: Jülich, Germany, 2010; pp. 177–183. [Google Scholar]
- Farnes, J.; Vik, A.; Bokach, D.; Svendsen, T.; Schautz, M.; Geneste, X. Recent developments of regenerative fuel cell systems for satellites. In Proceedings of the 10th European Space Power Conference, Noordwijkerhout, The Netherlands, 13–17 April 2014; Available online: http://www.esa-tec.eu/workspace/assets/files/tdo0157-paper-regenerative-f-5504a3929eb6a.pdf (accessed on 16 October 2017).
- Reissner, A.; Pawelke, R.H.; Hummel, S.; Cabelka, D.; Gerger, J.; Farnes, J.; Vik, A.; Wernhus, I.; Svendsen, T.; Schautz, M.; et al. Metal hydride hydrogen and heat storage systems as enabling technology for spacecraft applications. J. Alloys Compd. 2015, 645, S9–S13. [Google Scholar] [CrossRef]
- Pfeifer, P.; Wall, C.; Jensen, O.; Hahn, H.; Fichtner, M. Thermal coupling of a high temperature PEM fuel cell with a complex hydride tank. Int. J. Hydrogen Energy 2009, 34, 3457–3466. [Google Scholar] [CrossRef]
- Nasri, M.; Dickenson, D. Thermal Management of Fuel Cell-driven Vehicles using HT-PEM and Hydrogen Storage. In Proceedings of the Ninth International Conference on Ecological Vehicles and Renewable Energies, Monte-Carlo, Monaco, 25–27 March 2014; pp. 1–6. [Google Scholar]
- Reddy, E.H.; Jayanti, S. Thermal Coupling Studies of a High Temperature Proton Exchange Membrane Fuel Cell Stack and a Metal Hydride Hydrogen Storage System. Energy Procedia 2012, 29, 254–264. [Google Scholar] [CrossRef]
- Hu, J.; Fichtner, M.; Baricco, M. Preparation of Li-Mg-NH hydrogen storage materials for an auxiliary power unit. Int. J. Hydrogen Energy 2017, 42, 17144–17148. [Google Scholar] [CrossRef]
- Delhomme, B.; Lanzini, A.; Ortigoza-Villalba, G.A.; Nachev, S.; de Rango, P.; Santarelli, M.; Marty, P.; Leone, P. Coupling and thermal integration of a solid oxide fuel cell with a magnesium hydride tank. Int. J. Hydrogen Energy 2013, 38, 4740–4747. [Google Scholar] [CrossRef]
- Garrier, S.; Chaise, A.; de Rango, P.; Marty, P.; Delhomme, B.; Fruchart, D.; Miraglia, S. MgH2 intermediate scale tank tests under various experimental conditions. Int. J. Hydrogen Energy 2011, 36, 9719–9726. [Google Scholar] [CrossRef]
- Parra, D.; Gillott, M.; Walker, G.S. Design, testing and evaluation of a community hydrogen storage system for end user applications. Int. J. Hydrogen Energy 2016, 41, 5215–5229. [Google Scholar] [CrossRef]
- Gkanas, E.I.; Makridis, S.S. Effective thermal management of a cylindrical MgH2 tank including thermal coupling with an operating SOFC and the usage of extended surfaces during the dehydrogenation process. Int. J. Hydrogen Energy 2016, 41, 5693–5708. [Google Scholar] [CrossRef]
- Yiotis, A.G.; Kainourgiakis, M.E.; Kosmidis, L.I.; Charalambopoulou, G.C.; Stubos, A.K. Thermal coupling potential of Solid Oxide Fuel Cells with metal hydride tanks: Thermodynamic and design considerations towards integrated systems. J. Power Sources 2014, 269, 440–450. [Google Scholar] [CrossRef]
- Schouwink, P.; Didelot, E.; Lee, Y.-S.; Mazet, T.; Černý, R. Structural and magnetocaloric properties of novel gadolinium borohydrides. J. Alloys Compd. 2016, 664, 378–384. [Google Scholar] [CrossRef]
- Hansen, B.R.S.; Paskevicius, M.; Li, H.-W.; Akiba, E.; Jensen, T.R. Metal boranes: Progress and applications. Coord. Chem. Rev. 2016, 323, 60–70. [Google Scholar] [CrossRef]
- McPhy Energy McPhy Energy. Available online: www.mcphy.com (accessed on 16 October 2017).
- Huiberts, J.N.; Griessen, R.; Rector, J.H.; Wijngaarden, R.J.; Dekker, J.P.; de Groot, D.G.; Koeman, N.J. Yttrium and lanthanum hydride films with switchable optical properties. Nature 1996, 380, 231–234. [Google Scholar] [CrossRef]
| M (g/mol) | ρ (g/mL) | ρm (wt % H2) | ρV (g H2/L) | ΔHdec (kJ/mol) | T(1 bar) (°C) | Tdec (°C) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| LiBH4 | 21.78 | 0.66 | 18.4 | 122.5 | 74 | 370 | ~400 | [25] |
| NaBH4 | 37.83 | 1.07 | 10.8 | 115.6 | 108 | 534 | ~500 | [26] |
| LiAlH4 | 37.95 | 0.92 | 10.6 | 97.5 | −10 | - | ~150 a | [27] |
| Li3AlH6 | 53.85 | 1.02 | 11.2 | 114.2 | 25 | −81 c | ~200 a | [27] |
| NaAlH4 | 54.00 | 1.28 | 7.3 | 93.4 | 33.1 | 18 | ~230 a | [28] |
| Na3AlH6 | 102.00 | 1.45 | 5.9 | 85.6 | 49.0 | 103 | ~275 a | [28] |
| LiNH2 | 22.96 | 1.18 | 8.8 | 103.6 | 67 b | - | ~300 | [29,30,31] |
| Hydride Materials | Theoretical H2 Capacity (wt %) | ΔHdes/ΔHabs (kJ/mol·H2) | kJ/kg | * kJ/L | # Operating Temperature Range (°C) |
|---|---|---|---|---|---|
| NaAlH4 ↔ ⅓Na3AlH6 + ⅔Al + H2(g) | 3.73 | 38.4/−35.2 [243] | 651.8 | 808.3 | 25 a–202 b |
| Na3AlH6 ↔ 3NaH + Al + 3/2H2(g) | 2.96 | 47.6/−46.1 [243] | 678.0 | 983.0 | 100–290 |
| LiNa2AlH6 ↔ 2NaH + LiH + Al + 3/2H2(g) | 3.52 | 54.95/n.a. [244,245] | 959.0 | 1371.4 | 135–315 |
| NaK2AlH6 ↔ 2KH + NaH + Al + 3/2H2(g) | 2.25 | 98.0/−98.0 [246] | 1095.3 | 1818.1 | 380–600 c |
| KAlH4 + LiCl↔KCl + LiH + Al + 3/2H2(g) [247] | 2.69 | 37.6/−37.6 d | 501.2 | 728.2 | 111–396 |
| KAlH4 + NaCl ↔ KCl + NaH + Al + 3/2H2(g) [247] | 2.35 | 62.3/−62.3 d | 726.6 | 1111.8 | 334–574 e |
| Mg(NH2)2 + 2LiH ↔ Li2Mg(NH)2 + 2H2(g) | 5.58 | 38.9/n.a. [14] | 2086.3 | 2166.5 | 75–280 |
| LiNH2 + LiH ↔ Li2NH + H2(g) | 6.52 | 64.5/n.a. [30,248] | 912.6 | 2086.1 | 270–375 f |
| CaNH + CaH2 ↔ Ca2NH + H2(g) | 2.07 | n.a./−88.7 [7] | 1077.8 | 1257.8 | 590–780 g |
| LiBH4(l) ↔ LiH + B + 3/2H2(g) | 13.88 | 57.3/−57.3[240] | 3945.7 | 2634.9 | 460–688 h |
| LiBH4(l) ↔ LiH(l) + B + 3/2H2(g) | 13.88 | 71.7/−71.7 [240] | 4936.2 | 3296.3 | 688 h–1000+ |
| NaBH4 ↔ Na(l) + B + 2H2(g) | 10.66 | 97.9/−97.9 [241] | 5176.0 | 5559.1 | ~507.5 i |
| KBH4 ↔ K(l) + B + 2H2(g) | 7.47 | 114.6/−114.6 [241] | 4250.1 | 4985.9 | ~615.5 j |
| 2LiBH4 + MgH2 ↔ 2LiH + MgB2 + 4H2(g) | 11.54 | 48.3/−48.3 k | 2766.6 | 2308.0 | 205–467 l |
| NaH ↔ Na(l) + 1/2H2(g) | 4.20 | 116.8/−116.8 m | 2433.5 | 2355.7 | 427–638 m |
| MgH2 ↔ Mg + H2(g) | 7.66 | 74.1/−74.1 [249] | 2813.2 | 3994.7 | 282–534 n |
| Mg2NiH4 ↔ Mg2Ni + 2H2(g) | 3.62 | 64.6/n.a. [235] | 1159.7 | 3142.7 | 253–523 |
| Mg2FeH6 ↔ 2Mg + Fe + 3H2(g) | 5.47 | 77.4/−77.4 [237] | 2101.1 | 5757.0 | 300–566 |
| TiH1.6 ↔ TiH1.0 + 0.3H2(g) | 1.22 | n.a./−165.5 o | 1003.2 | 3772.0 | 645–921 |
| Other Thermochemical Materials (TCM) | ΔHdes/ΔHabs (kJ/mol Gas Species) | kJ/kg | kJ/L | Temperature Range (°C) | |
| CaCO3 ↔ CaO + CO2(g) | 178/−178 [218] | 1764 | 4982.4 | 700–1000 p | |
| Ca(OH)2 ↔ CaO + H2O(g) | 104/−104 [218] | 1404 | 3146.4 | 350–900 q | |
| 2Co3O4 ↔ 6CoO + O2(g) | 205/−205 [218] | 864 | 2124 | 700–850 r | |
| 2BaO2 ↔ 2BaO + O2(g) | 77/−77 [218] | 468 | 2361.6 | 400–1025 s | |
| Phase Change Materials (PCM) | ΔHmelt/ΔHfusion (kJ/mol) | kJ/kg | kJ/L | Melting Point (°C) | |
| Al ↔ Al(l) | 10.7/−10.7 [241] | 397 | 1071.9 | 660 | |
| LiF ↔ LiF(l) | 27.0/−27.0 [250] | 1041 | 2747.2 | 849 | |
| LiCl ↔ LiCl(l) | 19.9/−19.9 [241] | 469.3 | 969.6 | 610 | |
| NaCl ↔ NaCl(l) | 28.2/−28.2 [250] | 482 | 1033.4 | 801 | |
| Sensible Heat Materials (SHM) | Specific Heat (kJ/kg·K) | kJ/kg | kJ/L | Temperature Range (°C) | |
| 60 wt % NaNO3(l), 40 wt % KNO3(l) [212] | 1.59 t | 436.3 | 958.6 (802.8 u) | 290–565 | |
| 60 wt % NaNO3(l), 40 wt % KNO3(l) [212] | 1.59 t | 174.5 | 383.4 (321.1 u) | 290–400 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Møller, K.T.; Sheppard, D.; Ravnsbæk, D.B.; Buckley, C.E.; Akiba, E.; Li, H.-W.; Jensen, T.R. Complex Metal Hydrides for Hydrogen, Thermal and Electrochemical Energy Storage. Energies 2017, 10, 1645. https://doi.org/10.3390/en10101645
Møller KT, Sheppard D, Ravnsbæk DB, Buckley CE, Akiba E, Li H-W, Jensen TR. Complex Metal Hydrides for Hydrogen, Thermal and Electrochemical Energy Storage. Energies. 2017; 10(10):1645. https://doi.org/10.3390/en10101645
Chicago/Turabian StyleMøller, Kasper T., Drew Sheppard, Dorthe B. Ravnsbæk, Craig E. Buckley, Etsuo Akiba, Hai-Wen Li, and Torben R. Jensen. 2017. "Complex Metal Hydrides for Hydrogen, Thermal and Electrochemical Energy Storage" Energies 10, no. 10: 1645. https://doi.org/10.3390/en10101645
APA StyleMøller, K. T., Sheppard, D., Ravnsbæk, D. B., Buckley, C. E., Akiba, E., Li, H.-W., & Jensen, T. R. (2017). Complex Metal Hydrides for Hydrogen, Thermal and Electrochemical Energy Storage. Energies, 10(10), 1645. https://doi.org/10.3390/en10101645