Hydrogen Generation through Solar Photocatalytic Processes: A Review of the Configuration and the Properties of Effective Metal-Based Semiconductor Nanomaterials
Abstract
:1. Introduction
2. Photocatalyst Properties
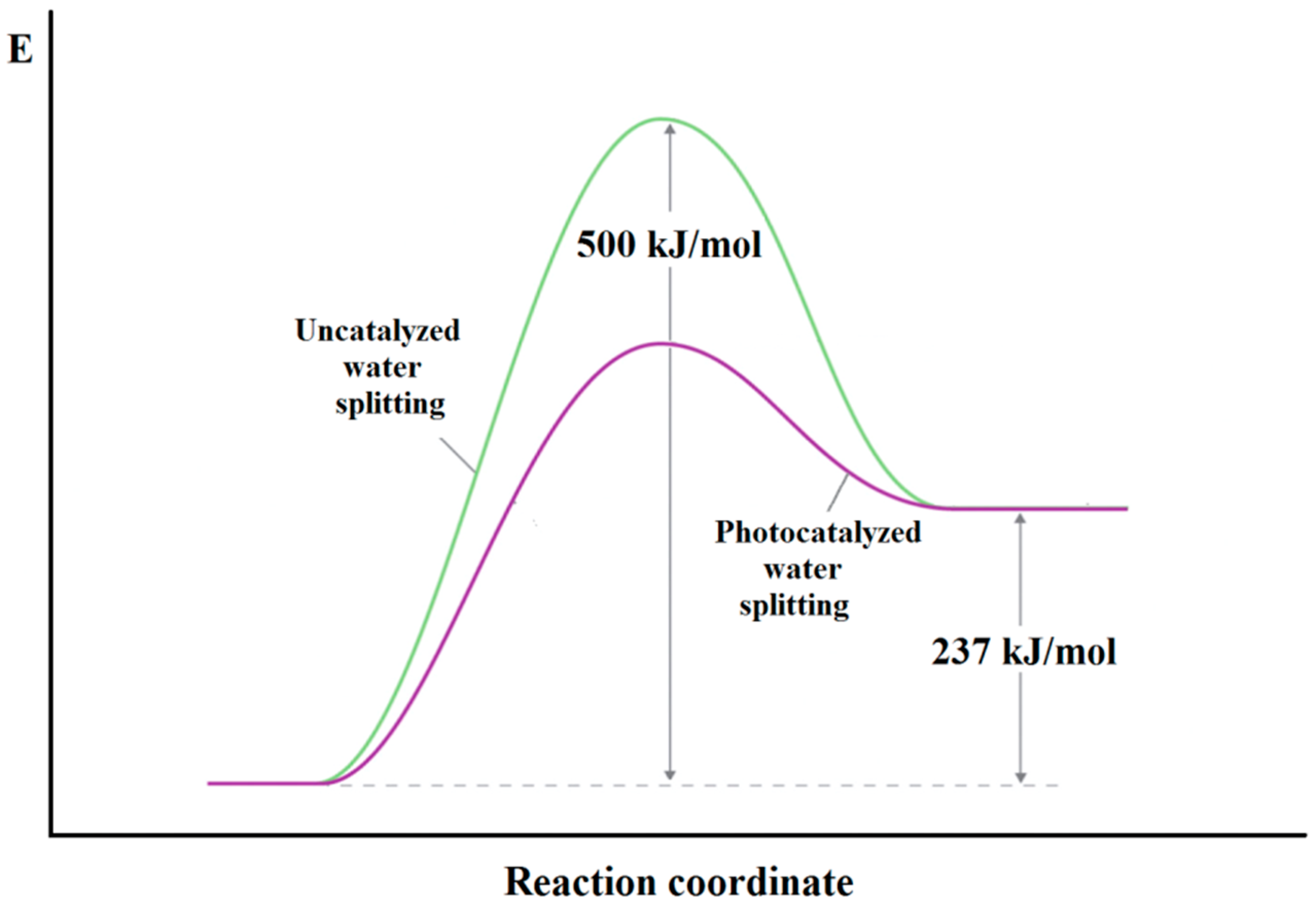
2.1. Thermodynamic and Kinetic Requirements for Water Splitting Reaction
2.2. Hydrogen Generation Efficiency
- size and shape tuning of the photocatalyst particles (water photosplitting and photoreforming);
- band gap engineering of the photocatalysts (water photosplitting and photoreforming);
- use of suitable sacrificial electron donors (photoreforming).
3. Configurations of Photocatalytic Materials for Hydrogen Generation
3.1. Photocatalytic Water Splitting
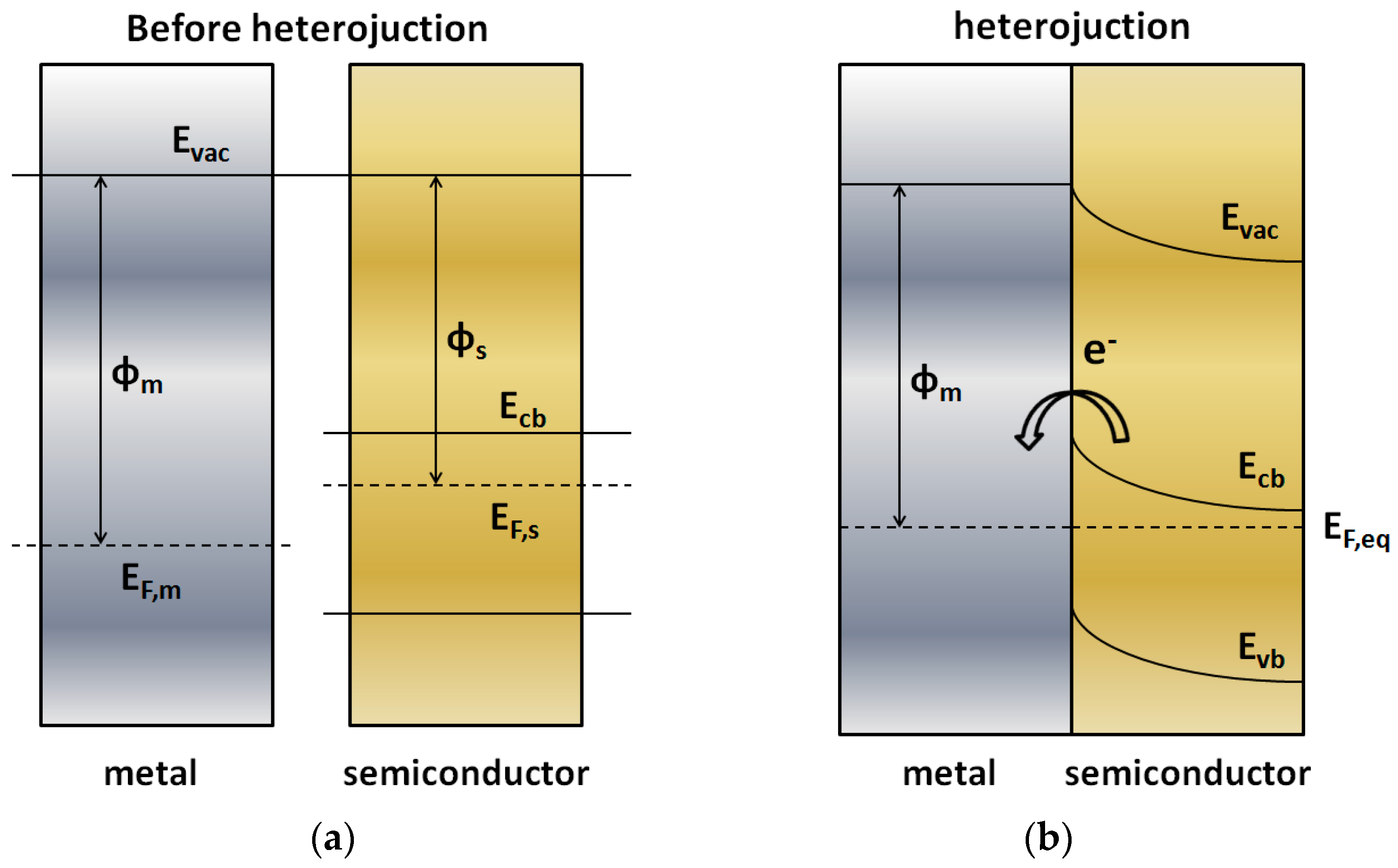
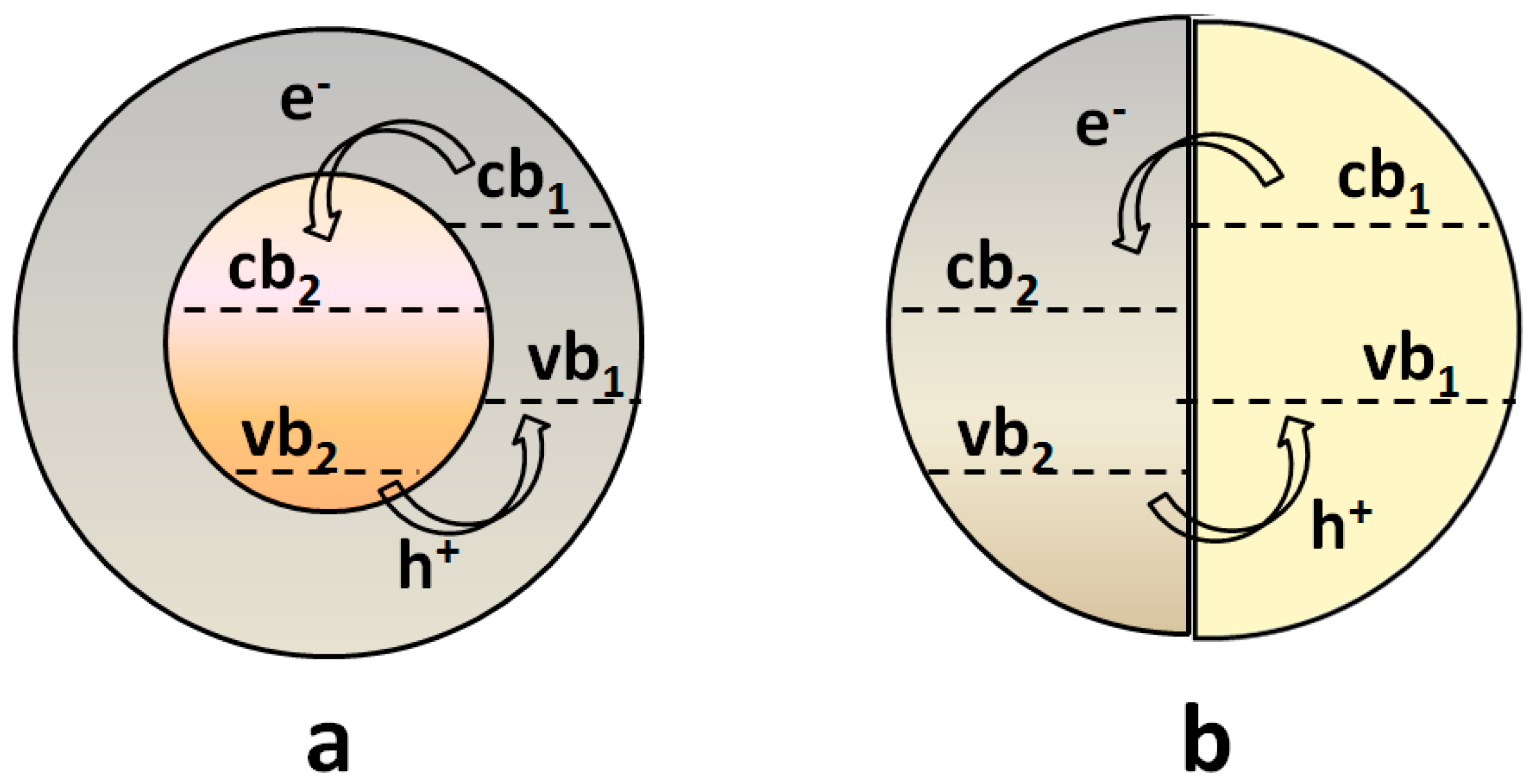
3.1.1. Metal-Semiconductor Heterojunctions
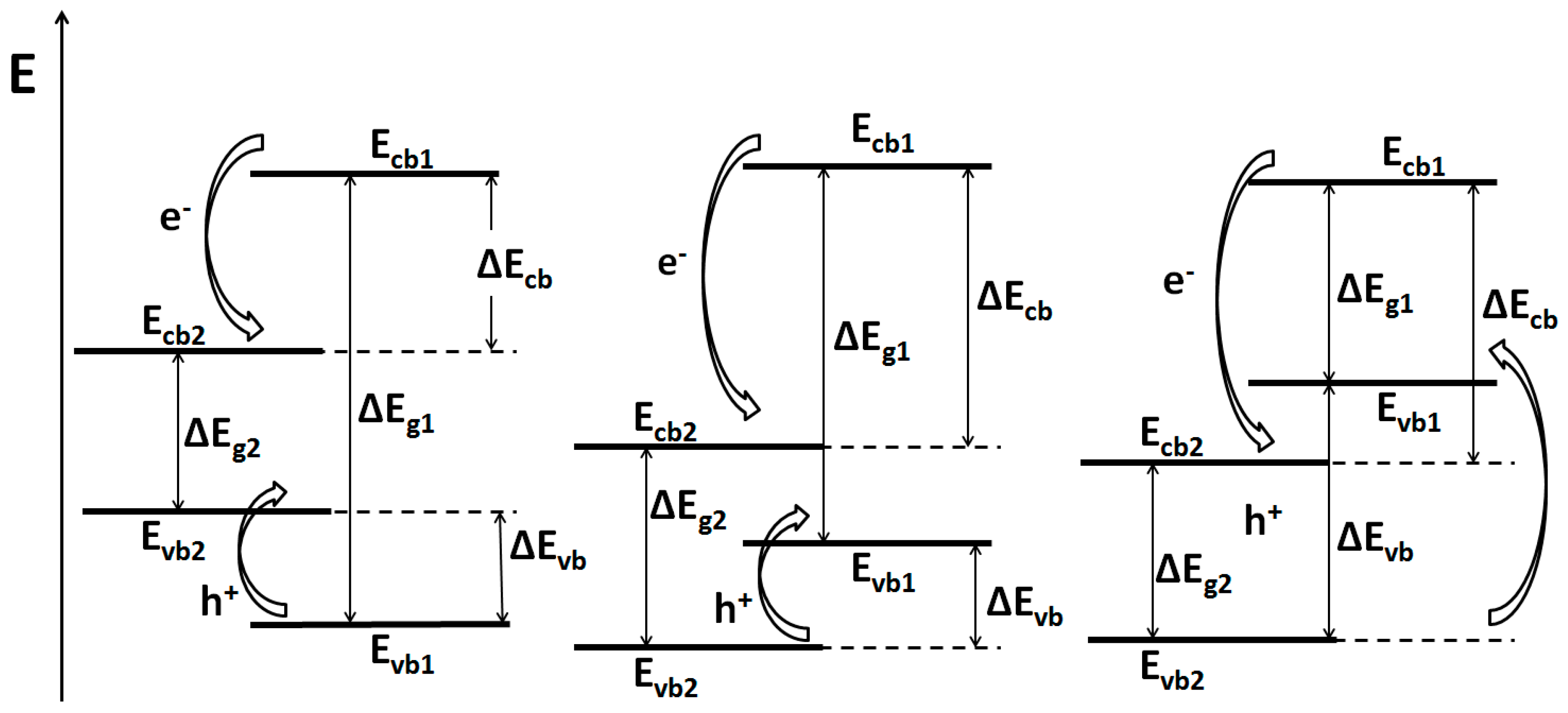
3.1.2. Semiconductor-Semiconductor Junctions
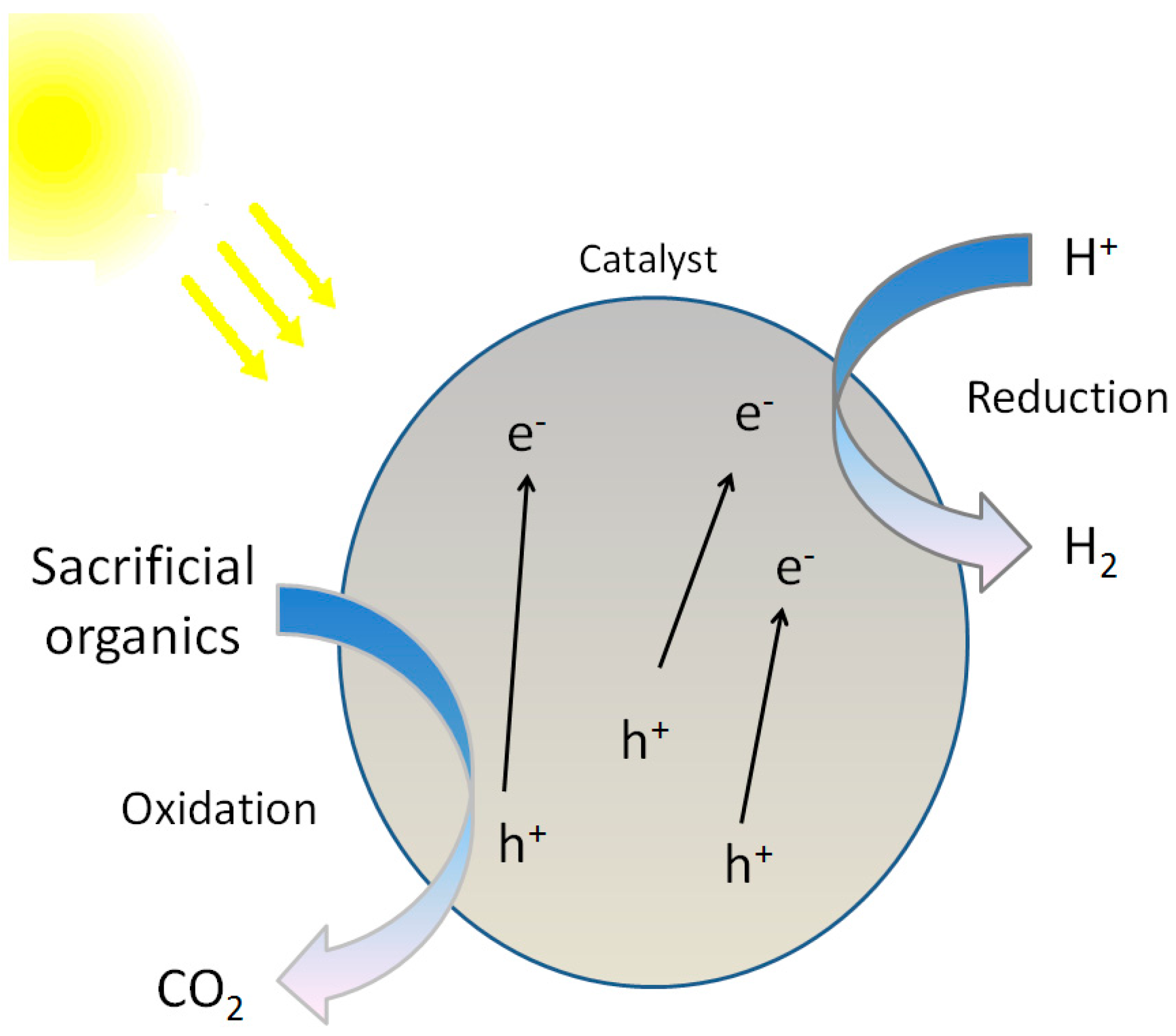
3.2. Photocatalytic Reforming of Organics
3.2.1. General Remarks
3.2.2. Reaction Mechanism
3.2.3. Materials
4. Operating Conditions Affecting Photocatalytic Hydrogen Generation
4.1. Particle Size
4.2. Structure and Morphology
4.3. Surface
4.4. Co-Catalyst
4.5. pH of the Solution
4.6. Operating Temperature
5. Conclusions
- proper band gap energy and band potentials,
- photostability in aqueous solution;
- high crystallinity;
- high specific photoactivity (>104µmoles H2/h·g).
Conflicts of Interest
References
- Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag: Weinheim, Germany, 2016.
- Kothari, R.; Buddhi, D.; Sawhney, R.L. Comparison of environmental and economic aspects of various hydrogen production methods. Renew. Sustain. Energy Rev. 2008, 12, 553–563. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 20–25. [Google Scholar] [CrossRef]
- Centi, G.; Van Santen, R.A. Catalysis for Renewables: From Feedstock to Energy Production; Wiley-VCH Verlag: Weinheim, Germany, 2008. [Google Scholar]
- Steinfeld, A. Solar thermochemical production of hydrogen—A review. Sol. Energy 2005, 78, 603–615. [Google Scholar] [CrossRef]
- Kuang, Y.; Jia, Q.; Ma, G; Hisatomi, T; Minegishi, T.; Nishiyama, H.; Nakabayashi, M.; Shibata, N.; Yamada, T.; Kudo, A.; et al. Ultrastable low-bias water splitting photoanodes via photocorrosion inhibition and in situ catalyst regeneration. Nat. Energy 2017, 2, 16191. [Google Scholar] [CrossRef]
- Crespo-Quesada, M.; Reisner, E. Emerging approaches to stabilise photocorrodible electrodes and catalysts for solar fuel applications. Energy Environ. Sci. 2017, 10, 1116–1127. [Google Scholar] [CrossRef]
- Xu, P.; McCool, N.S.; Mallouk, T.E. Water splitting dye-sensitized solar cells. Nano Today 2017, 14, 42–58. [Google Scholar] [CrossRef]
- Kamat, P.V.; Bisquert, J. Solar fuels. Photocatalytic hydrogen generation. J. Phys. Chem. C 2013, 117, 14873–14875. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, W. Metal–organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2017, 43, 5982–5993. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Roberts, Z.Z.; Naterer, G.F.; Gabriel, K.S. Comparison of thermochemical, electrolytic, photoelectrolytic and photochemical solar-to-hydrogen production technologies. Int. J. Hydrogen Energy 2012, 37, 16287–16301. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Puga, A.V. Photocatalytic production of hydrogen from biomass-derived feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Introductory lecture: Sunlight-driven water splitting and carbon dioxide reduction by heterogeneous semiconductor systems as key processes in artificial photosynthesis. Faraday Discuss. 2017, 198, 11–35. [Google Scholar] [CrossRef] [PubMed]
- Bowker, M. Sustainable hydrogen production by the application of ambient temperature photocatalysis. Green Chem. 2011, 13, 2235–2246. [Google Scholar] [CrossRef]
- Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Qureshi, M.; Takanabe, K. Insights on measuring and reporting heterogeneous photocatalysis: Efficiency definitions and setup examples. Chem. Mater. 2016, 29, 158–167. [Google Scholar] [CrossRef]
- Kitano, M.; Hara, M. Heterogeneous photocatalytic cleavage of water. J. Mater. Chem. 2010, 20, 627–641. [Google Scholar] [CrossRef]
- Khodadadian, F.; Nasalevich, M.; Kapteijn, F.; Stankiewicz, A.I.; Lakerveld, R.; Gascon, J. Photocatalysis: Past Achievements and Future Trends. In Alternative Energy Sources for Green Chemistry; Stefanidis, G., Stankiewicz, A., Eds.; Royal Society of Chemistry: London, UK, 2016. [Google Scholar]
- Kumar, P.; Kumar, A.; Joshi, C.; Boukherroub, R.; Jain, S.L. Graphene–semiconductor hybrid photocatalysts and their application in solar fuel production. In Advanced 2D Materials; Tiwari, A., Syvãjãrvi, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.S.; Kim, H.G.; Lee, J.S. Heterojunction semiconductors: A strategy to develop efficient photocatalytic materials for visible light water splitting. Catal. Today 2012, 185, 270–277. [Google Scholar] [CrossRef]
- Ge, M.; Cai, J.; Iocozzia, J.; Cao, C.; Huang, J.; Zhang, X.; Shen, J.; Wang, S.; Zhang, S.; Zhang, K.Q.; et al. A review of TiO2 nanostructured catalysts for sustainable H2 generation. Int. J. Hydrogen Energy 2017, 42, 8418–8449. [Google Scholar] [CrossRef]
- Wu, F.; Hu, X.; Fan, J.; Liu, E.; Sun, T.; Kang, L.; Hou, W.; Zhu, C.; Liu, H. Photocatalytic Activity of Ag/TiO2 nanotube arrays enhanced by surface plasmon resonance and application in hydrogen evolution by water splitting. Plasmonics 2013, 8, 501–508. [Google Scholar] [CrossRef]
- Liu, E.; Kang, L.; Yang, Y.; Sun, T.; Hu, X.; Zhu, C.; Liu, H.; Wang, Q.; Li, X.; Fan, J. Plasmonic Ag deposited TiO2 nano-sheet film for enhanced photocatalytic hydrogen production by water splitting. Nanotechnology 2014, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.G.; Juarez, R.; Marino, T.; Molinari, R.; Garcia, H. Influence of excitation wavelength (UV or visible light) on the photocatalytic activity of titania containing gold nanoparticles for the generation of hydrogen or oxygen from water. J. Am. Chem. Soc. 2011, 133, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Clarizia, L.; Spasiano, D.; Di Somma, I.; Marotta, R.; Andreozzi, R.; Dionysiou, D.D. Copper modified-TiO2 catalysts for hydrogen generation through photoreforming of organics. A short review. Int. J. Hydrogen Energy 2014, 39, 16812–16831. [Google Scholar] [CrossRef]
- Colon, G. Towards the hydrogen production by photocatalysis. Appl. Catal. A Gen. 2015, 518, 48–59. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Dozzi, M.V.; Selli, E. TiO2-based materials for photocatalytic hydrogen production. J. Energy Chem. 2017, 26, 250–258. [Google Scholar] [CrossRef]
- Yu, J.; Qi, L.; Jaroniec, M. Hydrogen production by photocatalytic water splitting over Pt/TiO2 nanosheets with exposed (001) facets. J. Phys. Chem. C 2010, 114, 13118–13125. [Google Scholar] [CrossRef]
- Li, Q.Y.; Zhao, Z.Y. Interfacial properties of α/β-Bi2O3 homo-junction from first-principles calculations. Phys. Lett. A 2015, 379, 2766–2771. [Google Scholar] [CrossRef]
- Melvin, A.A.; Illath, K.; Das, T.; Raja, T.; Bhattacharyya, S.; Gopinath, C.S. M–Au/TiO2 (M = Ag, Pd, and Pt) nanophotocatalyst for overall solar water splitting: Role of interfaces. Nanoscale 2015, 7, 13477–13488. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Renzas, J.R.; Hsu, B.B.; Somorjai, G.A. Interfacial and chemical properties of Pt/TiO2, Pd/TiO2, and Pt/GaN catalytic nanodiodes influencing hot electron flow. J. Phys. Chem. C 2007, 111, 15331–15336. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P. Catalysis with TiO2/gold nanocomposites: Effect of metal particle size on the Fermi level equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, G.; Yu, J.; Fan, W. Surface plasmon resonance-mediated photocatalysis by noble metal-based composites under visible light. J. Mater. Chem. 2012, 22, 21337–21354. [Google Scholar] [CrossRef]
- Chen, J.J.; Wu, J.C.S.; Wu, P.C.; Tsai, D.P. Plasmonicphotocatalyst for H2 evolution in photocatalytic water splitting. J. Phys. Chem. C 2011, 115, 210–216. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.L.; Liu, R.S.; Tsai, D.P. Plasmonic photocatalysis. Rep.Prog. Phys. 2013, 76, 046401. [Google Scholar] [CrossRef] [PubMed]
- Warren, S.C.; Thimsen, E. Plasmonic solar water splitting. Energy Environ. Sci. 2012, 5, 5133–5146. [Google Scholar] [CrossRef]
- Kitano, M.; Tsujimaru, K.; Anpo, M. Hydrogen production using highly active titanium oxide-based photocatalysts. Top. Catal. 2008, 49, 4–17. [Google Scholar] [CrossRef]
- Bazzo, A.; Urakawa, A. Understanding synergetic effects of Zn and Rh–Cr promotion to wide-bandgap Ga, Ta and Ti oxides in photocatalytic water splitting. Catal. Sci. Technol. 2016, 6, 4243–4253. [Google Scholar] [CrossRef]
- Kim, H.G.; Hwang, D.W.; Lee, J.S. An undoped, single-phase oxide photocatalyst working under visible light. J. Am. Chem. Soc. 2004, 126, 8912–8913. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Yoshimizu, M.; Tanigawa, S.; Ni, L.; Ohtani, B.; Irie, H. Hydrogen and oxygen evolution photocatalysts synthesized from strontium titanate by controlled doping and their performance in two-step overall water splitting under visible light. J. Phys. Chem. C 2012, 116, 17458–17463. [Google Scholar] [CrossRef]
- Wang, Q.; Hisatomi, T.; Ma, S.S.K.; Li, Y.; Domen, K. Core/shell structured La- and Rh-codoped SrTiO3 as a hydrogen evolution photocatalyst in Z-scheme overall water splitting under visible light irradiation. Chem. Mater. 2014, 26, 4144–4150. [Google Scholar] [CrossRef]
- Primo, A.; Marino, T.; Corma, A.; Molinari, R.; Garcia, H. Efficient visible-light photocatalytic water splitting by minute amounts of gold supported on nanoparticulate CeO2 obtained by a biopolymer templating method. J. Am. Chem. Soc. 2012, 133, 6930–6933. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hisatomi, T.; Jia, Q.; Tokudome, H.; Zhong, M.; Wang, C.; Pan, Z.; Takata, T.; Nakabayashi, M.; Shibata, N.; et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 2016, 15, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Zhang, Q.; Su, Z.; Zhao, Z.; Wang, Y.; Li, Y.; Lu, X.; Wei, D.; Feng, G.; Yu, Q.; et al. Efficient solar water-splitting using a nanocrystalline CoO photocatalyst. Nat. Nanotechnol. 2014, 9, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, Y.; Zhang, B. Rational design of semiconductor-based photocatalysts for advanced photocatalytic hydrogen production: The case of cadmium chalcogenides. Inorg. Chem. Front. 2016, 3, 591–615. [Google Scholar] [CrossRef]
- Sun, B.; Vorontsov, A.V.; Smirniotis, P.G. Role of platinum deposited on TiO2 in phenol photocatalytic oxidation. J. Phys. Chem. B 2003, 19, 3151–3156. [Google Scholar]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Weng, Y. Band alignment and controllable electron migration between rutile and anatase TiO2. Sci. Rep. 2015, 5, 11482. [Google Scholar] [CrossRef] [PubMed]
- Deak, P.; Aradi, B.; Frauenheim, T. Band lineup and charge carrier separation in mixed rutile-anatase systems. J. Phys. Chem. C 2011, 115, 3443–3446. [Google Scholar] [CrossRef]
- Zhao, W.N.; Zhu, S.C.; Li, Y.F.; Liu, Z.P. Three-phase junction for modulating electron–hole migration in anatase–rutile photocatalysts. Chem. Sci. 2015, 6, 3483–3494. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Li, M.R.; Shen, S.; Wang, X.L.; Wang, Y.C.; Feng, Z.C.; Shi, Y.J.; Han, H.X.; Li, C. Photocatalytic overall water splitting promoted by anα–β phase junction on Ga2O3. Angew. Chem. Int. Ed. 2012, 51, 13089–13092. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, P.R.; Kostka, A.; Marschall, R.; Wark, M. Control of phase coexistence in calcium tantalate composite photocatalysts for highly efficient hydrogen production. Chem. Mater. 2013, 25, 4739–4745. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Ishchenko, O.M.; Rogé, V.; Lamblin, G.; Lenoble, D. TiO2- and ZnO-Based Materials for Photocatalysis: Material Properties, Device Architecture and Emerging Concepts. Semiconductor Photocatalysis—Materials, Mechanisms and Applications. Wenbin, Cao, Ed.; InTechOpen, 2016. Available online: https://www.intechopen.com/books/semiconductor-photocatalysis-materials-mechanisms-and-applications/tio2-and-zno-based-materials-for-photocatalysis-material-properties-device-architecture-and-emerging (accessed on 13 October 2017).
- Maeda, K. Z-scheme water splitting using two different semiconductor photocatalysts. ACS Catal. 2013, 3, 1486–1503. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Synthesis, characterization and visible light photocatalytic activity of metal based TiO2 monoliths for CO2 reduction. Chem. Eng. J. 2016, 283, 1244–1253. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Zielinska-Jurek, A. Progress, challenge, and perspective of bimetallic TiO2-Based photocatalysts. J. Nanomater. 2014, 1–17. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic water splitting—The untamed dream: A review of recent advances. Molecules 2016, 21, 900–929. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Sayama, K.; Domen, K.; Arakawa, H. A new type of water splitting system composed of two different TiO2photocatalysts (anatase, rutile) and a shuttle redox mediator. Chem. Phys. Lett. 2001, 344, 339–344. [Google Scholar] [CrossRef]
- Kudo, A. Development of photocatalyst materials forwater splitting. Int. J. Hydrogen Energy 2006, 31, 197–202. [Google Scholar] [CrossRef]
- Zhou, H.; Pan, J.; Ding, L.; Tang, Y.; Ding, J.; Guo, Q.; Fan, T.; Zhang, D. Biomass-derived hierarchical porous CdS/M/TiO2 (M=Au, Ag, pt, pd) ternary heterojunctions for photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2014, 39, 16293–16301. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, S.; Wang, X.; Peng, Q.; Lin, R.; Wang, Y.; Shen, R.; Cao, X.; Zhang, L.; Zhou, G.; et al. Synergetic integration of Cu1.94S-ZnxCd1−xS heteronanorods for enhanced visible-light-driven photocatalytic hydrogen production. J. Am. Chem. Soc. 2016, 138, 4286–4289. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Sun, Z.; Jia, H.; Lu, D.; Du, P. A cocatalyst-free CdS nanorod/ZnS nanoparticle composite for high-performance visible-light-driven hydrogen production from water. J. Mater. Chem. A Mater. Energy Sustain. 2016, 4, 675–683. [Google Scholar] [CrossRef]
- Tang, M.L.; Grauer, D.C.; Lassalle-Kaiser, B.; Yachandra, V.K.; Amirav, L.; Yano, J.; Long, J.R.; Alivisatos, A.P. Structural and electronic study of an amorphous MoS3 hydrogen-generation catalyst on a quantum-controlled photosensitizer. Angew. Chem. Int. Ed. 2011, 50, 10203–10207. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhai, L.; Zhao, X.; Xu, D. Band-Gap engineering of semiconductor nanowires through composition modulation. J. Phys. Chem. B 2005, 109, 7120–7123. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.J.; Chen, D.Q.; Huang, Y.W.; Yu, Z.T.; Zhong, J.S.; Chen, T.T.; Tu, W.G.; Guan, Z.J.; Cao, D.P.; Zou, Z.G. MoS2 nanosheet-modified CuInS2photocatalyst for visible-light-driven hydrogen production from water. ChemSusChem 2016, 9, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Dubale, A.A.; Pan, C.J.; Tamirat, A.G.; Chen, H.M.; Su, W.N.; Chen, C.H.; Rick, J.; Ayele, D.W.; Aragaw, B.A.; Lee, J.F.; et al. Heterostructured Cu2O/CuO decorated with nickel as a highly efficient photocathode for photoelectrochemical water reduction. J. Mater. Chem. A 2015, 3, 12482–12499. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, H.; Zhang, L.; Lu, D.; Du, P. Enhanced photocatalytic H2 production on cadmium sulfide photocatalysts using nickel nitride as a novel cocatalyst. J. Mater. Chemistry A 2016, 4, 13289–13295. [Google Scholar] [CrossRef]
- Maeda, K.; Lu, D.; Domen, K. Solar-driven Z-scheme water splitting using modified BaZrO3−BaTaO2N solid solutions as photocatalysts. ACS Catal. 2013, 3, 1026–1033. [Google Scholar] [CrossRef]
- Ma, S.S.K.; Maeda, K.; Hisatomi, T.; Tabata, M.; Kudo, A.; Domen, K. A redox-mediator-free solar-driven Z-scheme water-splitting system consisting of modified Ta3N5 as an oxygen-evolution photocatalyst. Chem. A Eur. J. 2013, 19, 7480–7486. [Google Scholar] [CrossRef] [PubMed]
- Matoba, T.; Maeda, K.; Domen, K. Activation of BaTaO2N photocatalyst for enhanced non-sacrificial hydrogen evolution from water under visible light by forming a solid solution with BaZrO3. Chem. A Eur. J. 2011, 17, 14731–14735. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Nemoto, H.; Saito, K.; Kudo, A. Solar water splitting using powdered photocatalysts driven by Z-schematic interparticle electron transfer without an electron mediator. J. Phys. Chem. C 2009, 113, 17536–17542. [Google Scholar] [CrossRef]
- Pihosh, Y.; Turkevych, I.; Mawatari, K.; Uemura, J.; Kazoe, Y.; Kosar, S.; Makita, K.; Sugaya, T.; Matsui, T.; Fujita, D.; et al. Photocatalytic generation of hydrogen by core-shell WO3/BiVO4 nanorods with ultimate water splitting efficiency. Sci. Rep. 2015, 5, 11141–11151. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Yin, L.C.; Liu, G. Light irradiation-assisted synthesis of ZnO–CdS/reduced graphene oxide heterostructured sheets for efficient photocatalytic H2 evolution. Chem. Commun. 2014, 50, 3460–3463. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.G.; Wang, Z.; Kan, W.B.; Jiao, S.Q.; Zhu, H.M.; Kumar, R.V. Efficient visible-light-driven photocatalytic hydrogen production using CdS@TaON core–shell composites coupled with graphene oxide nanosheets. J. Mater. Chem. 2012, 22, 7291–7299. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.M.; del Valle, F.; Villoria de la Mano, J.A.; Alvarez-Galvan, M.C.; Fierro, J.L.G. Photocatalytic water splitting under visible light: Concept and catalysts development. Adv. Chem. Eng. Prog. Photocatal. React. Eng. 2009, 36, 111–143. [Google Scholar]
- Luque, R.; Balu, A.M. Producing Fuels and Fine Chemicals from Biomass Using Nanomaterials; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Takata, T.; Domen, K. Defect engineering of photocatalysts by doping of aliovalent metal cations for efficient water splitting. J. Phys. Chem. C 2009, 45, 19386–19388. [Google Scholar] [CrossRef]
- Boumaza, S.; Boudjemaa, A.; Bouguelia, A.; Bouarab, R.; Trari, M. Visible light induced hydrogen evolution on new hetero-system ZnFe2O4/SrTiO3. Appl. Energy 2010, 87, 2230–2236. [Google Scholar] [CrossRef]
- Li, Y.; Chen, G.; Wang, Q.; Wang, X.; Zhou, A.; Shen, Z. Hierarchical ZnS-In2S3-CuS nanospheres with nanoporous structure: Facile synthesis, growth mechanism, and excellent photocatalytic activity. Adv. Funct. Mater. 2010, 20, 3390–3398. [Google Scholar] [CrossRef]
- Bamwenda, G.R.; Arakawa, H. The photoinduced evolution of suspension O2 and H2 from a WO3 aqueous suspension in the presence of Ce4+/Ce3+. Sol. Energy Mater. Sol. Cells 2001, 70, 1–14. [Google Scholar] [CrossRef]
- Koca, A.; Sahin, M. Photocatalytic hydrogen production by direct sun light from sulfide/sulfite solution. Int. J. Hydrogen Energy 2002, 27, 363–367. [Google Scholar] [CrossRef]
- Lee, K.; Nam, W.S.; Han, G.Y. Photocatalytic water-splitting in alkaline solution using redox mediator. 1:Parameter study. Int. J. Hydrogen Energy 2004, 29, 1343–1347. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Tee, S.Y.; Win, K.Y.; Teo, W.S.; Koh, L.D.; Liu, S.; Teng, C.P.; Han, M.Y. Recent progress in energy-driven water splitting. Adv. Sci. 2017, 4, 1600337. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Huang, C.W.; Wu, J.C.S. Hydrogen production from semiconductor-based photocatalysis via water splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, D.W.; Kim, H.G.; Bae, S.W.; Lee, J.S.; Li, W.; Oh, S.H. Highly efficient overall water splitting through optimization of preparation and operation conditions of layered perovskite photocatalysts. Top. Catal. 2005, 35, 295–303. [Google Scholar] [CrossRef]
- Kato, H.; Asakura, K.; Kudo, A. Highly efficient water splitting into H2 and O2 over lanthanum-doped NaTaO3 photocatalysts with high crystallinity and surface nanostructure. J. Am. Chem. Soc. 2003, 125, 3082–3089. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Guo, L.; Zhao, L.; Zhang, X.; Liu, H.; Li, M.; Shen, S.; Liu, G.; Hu, X.; Zhang, X.; et al. Efficient solar hydrogen production by photocatalytic water splitting: From fundamental study to pilot demonstration. Int. J. Hydrogen Energy 2010, 35, 7087–7097. [Google Scholar] [CrossRef]
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.C.; Kroposki, B.; Ghirardi, M.; Evans, R.J.; Blake, D. Renewable hydrogen production. Int. J. Energy Res. 2007, 32, 379–407. [Google Scholar] [CrossRef]
- Bowker, M. Photocatalytic hydrogen production and oxygenate photoreforming. Catal. Lett. 2012, 142, 923–929. [Google Scholar] [CrossRef]
- Yasuda, M.; Matsumoto, T.; Yamashita, T. Sacrificial hydrogen production over TiO2-based photocatalysts: Polyols, carboxylic acids, and saccharides. Renew. Sustain. Energy Rev. 2017, in press. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Luque, R. Heterogeneous photocatalytic nanomaterials: Prospects and challenges in selective transformations of biomass-derived compounds. Chem. Soc. Rev. 2014, 43, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Colmenares, J.C.; Magdziarz, A.; Aramendia, M.A.; Marinas, A.; Marinas, J.M.; Urbano, F.J.; Navio, J.A. Influence of the strong metal support interaction effect (SMSI) of Pt/TiO2 and Pd/TiO2 systems in the photocatalytic biohydrogen production from glucose solution. Catal. Commun. 2011, 16, 1–6. [Google Scholar] [CrossRef]
- Ilie, M.; Cojocaru, B.; Parvulescu, V.I.; Garcia, H. Improving TiO2 activity in photo-production of hydrogen from sugar industry wastewaters. Int. J. Hydrogen Energy 2011, 36, 15509–15518. [Google Scholar] [CrossRef]
- Pilkenton, S.; Hwang, S.J.; Raftery, D. Ethanol photocatalysis on TiO2-coated optical microfiber, supported monolayer, and powdered catalysts: An in situ NMR study. J. Phys. Chem. B 1999, 103, 11152–11160. [Google Scholar] [CrossRef]
- Xu, W.; Raftery, D. Photocatalytic oxidation of 2-propanol on TiO2 powder and TiO2 monolayer catalysts studied by solid-state NMR. J. Phys. Chem. B 2001, 105, 4343–4349. [Google Scholar] [CrossRef]
- Fu, X.; Wang, X.; Leung, D.Y.C.; Gu, Q.; Chen, S.; Huang, H. Photocatalytic reforming of C3-polyols for H2 production: Part (I). Role of their OH groups.Appl. Catal. B 2011, 106, 681–688. [Google Scholar] [CrossRef]
- Wang, C.Y.; Rabani, J.; Bahnemann, D.W.; Dohrmann, J.K. Photonic efficiency and quantum yield of formaldehyde formation from methanol in the presence of various TiO2 photocatalysts. J. Photochem. Photobiol. A 2002, 148, 169–176. [Google Scholar] [CrossRef]
- Du, M.; Feng, J.; Zhang, S.B. Photo-oxidation of polyhydroxyl molecules on TiO2 surfaces: From hole scavenging to light-induced self-assembly of TiO2-cyclodextrin wires. Phys. Rev. Lett. 2007, 98, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Long, J.; Wang, X.; Leung, D.Y.C.; Ding, Z.; Wu, L.; Zhang, Z.; Li, Z.; Fu, X. Photocatalytic reforming of biomass: A systematic study of hydrogen evolution from glucose solution. Int. J. Hydrogen Energy 2008, 33, 6484–6491. [Google Scholar] [CrossRef]
- Balducci, G. The adsorption of glucose at the surface of anatase: A computational study. Chem. Phys. Lett. 2010, 494, 54–59. [Google Scholar] [CrossRef]
- Jia, L.; Li, J.; Fang, W. Effect of H2/CO2 mixture gas treatment temperature on the activity of LaNiO3 catalyst for hydrogen production from formaldehyde aqueous solution under visible light. J. Alloy. Compd. 2010, 489, L13–L16. [Google Scholar] [CrossRef]
- Patsoura, A.; Kondarides, D.I.; Verykios, X.E. Photocatalytic degradation of organic pollutants with simultaneous production of hydrogen. Catal. Today 2007, 124, 94–102. [Google Scholar] [CrossRef]
- Shen, S.; Guo, L. Hydrothermal synthesis, characterization, and photocatalytic performances of Cr incorporated, and Cr and Ti co-incorporated MCM-41 as visible light photocatalysts for water splitting. Catal. Today 2007, 129, 414–420. [Google Scholar] [CrossRef]
- Zheng, X.J.; Wei, L.F.; Zhang, Z.H.; Jiang, Q.J.; Wei, Y.J.; Xie, B.; Wei, M.B. Research on photocatalytic H2 production from acetic acid solution by Pt/TiO2 nanoparticles under UV irradiation. Int. J. Hydrogen Energy 2009, 34, 9033–9041. [Google Scholar] [CrossRef]
- Caravaca, A.; Jones, W.; Hardacre, C.; Bowker, M. H2production by the photocatalytic reforming of cellulose and raw biomass using Ni, Pd, Pt and Au on titania. Proc. R. Soc. 2016, 472, 20160054. [Google Scholar] [CrossRef] [PubMed]
- Patsoura, A.; Kondarides, D.I.; Verykios, X.E. Enhancement of photoinduced hydrogen production from irradiated Pt/TiO2 suspensions with simultaneous degradation of azodyes. Appl. Catal. B 2006, 64, 171–179. [Google Scholar] [CrossRef]
- Sakata, T.; Kawai, T.; Hashimoto, K. Heterogeneous photocatalytic reactions of organic acids and water. New reaction paths besides the photo-Kolbe reaction. J. Phys. Chem. 1984, 88, 2344–2350. [Google Scholar] [CrossRef]
- Al-Azri, Z.H.N.; Chen, W.T.; Chan, A.; Jovic, V.; Ina, T.; Idriss, H.; Waterhouse, G.I.N. The roles of metal co-catalysts and reaction media in photocatalytic hydrogen production: Performance evaluation of M/TiO2 photocatalysts (M=Pd, Pt, Au) in different alcohol–water mixtures. J. Catal. 2015, 329, 355–367. [Google Scholar] [CrossRef]
- Sadanandam, G.; Lalitha, K.; Kumari, V.D.; Shankar, M.V.; Subrahmanyam, M. Cobalt doped TiO2: A stable and efficient photocatalyst for continuous hydrogen production from glycerol: Water mixtures under solar light irradiation. Int. J. Hydrogen Energy 2013, 38, 9655–9664. [Google Scholar] [CrossRef]
- Jing, D.; Zhang, Y.; Guo, L. Study on the synthesis of Ni doped mesoporous TiO2 and its photocatalytic activity for hydrogen evolution in aqueous methanol solution. Chem. Phys. Lett. 2005, 415, 74–78. [Google Scholar] [CrossRef]
- Clarizia, L.; Vitiello, G.; Luciani, G.; Di Somma, I.; Andreozzi, R.; Marotta, R. In-situ photodeposited nanoCu on TiO2 as a catalyst for hydrogen production under UV/visible radiation. Appl. Catal. A Gen. 2016, 518, 142–149. [Google Scholar] [CrossRef]
- Clarizia, L.; Di Somma, I.; Marotta, R.; Minutolo, P.; Villamaina, R.; Andreozzi, R. Photocatalytic reforming of formic acid for hydrogen production in aqueous solutions containing cupric ions and TiO2 suspended nanoparticles under UV-simulated solar radiation. Appl. Catal. A Gen. 2016, 518, 181–188. [Google Scholar] [CrossRef]
- Lucchetti, R.; Onotri, L.; Clarizia, L.; Di Natale, F.; Di Somma, I.; Andreozzi, R.; Marotta, R. Removal of nitrate and simultaneous hydrogen generation through photocatalytic reforming of glycerol over “in situ” prepared zero-valent nano copper/P25. Appl. Catal. B Environ. 2017, 202, 539–549. [Google Scholar] [CrossRef]
- Kondarides, D.I.; Daskalaki, V.M.; Patsoura, A.; Verykios, X.E. Hydrogen production by photo-induced reforming of biomass components and derivatives at ambient conditions. Catal.Lett. 2008, 122, 26–32. [Google Scholar] [CrossRef]
- Gholipour, M.R.; Dinh, C.T.; Béland, F.; Do, T.O. Nanocomposite heterojunctions as sunlight-drivenphotocatalysts for hydrogen production from water splitting. Nanoscale 2015, 7, 8187–8208. [Google Scholar] [CrossRef] [PubMed]
- Domen, K.; Kondo, J.N.; Hara, M.; Takata, T. Photo- and mechano-catalytic overall water splitting reactions to form hydrogen and oxygen on heterogeneous catalysts. Bull. Chem. Soc. Jpn. 2000, 73, 1307–1331. [Google Scholar] [CrossRef]
- Li, Y.; Chen, G.; Zhang, H.; Li, Z.; Sun, J. Electronic structure and photocatalytic properties of ABi2Ta2O9 (A=Ca, Sr, Ba). J. Solid State Chem. 2008, 181, 2653–2659. [Google Scholar] [CrossRef]
- Simon, T.; Bouchonville, N.; Berr, M.J.; Vaneski, A.R.; Adrović, A.; Volbers, D.; Wyrwich, R.; Döblinger, M.; Susha, A.S.; Rogach, A.L.; et al. Redox shuttle mechanism enhances photocatalytic H2 generation on Ni-decorated CdS nanorods. Nat. Mater. 2014, 13, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, J.R. Highly efficient visible-light-driven photocatalytic hydrogen production of CdS-cluster-decorated graphene nanosheets. J. Am. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef] [PubMed]
- Amirav, L.; Alivisatos, A.P. Photocatalytic hydrogen production with tunable nanorod heterostructures. J. Phys. Chem. Lett. 2010, 1, 1051–1054. [Google Scholar] [CrossRef]
- Parida, K.M.; Martha, S.; Das, D.P.; Biswal, N. Facile fabrication of hierarchical N-doped GaZn mixed oxides for water splitting reactions. J. Mater. Chem. 2010, 20, 7144–7149. [Google Scholar] [CrossRef]
- Fontelles-Carceller, O.; Muñoz-Batista, M.J.; Rodríguez-Castellón, E.; Conesa, J.C.; Fernández-García, M.; Kubacka, A. Measuring and interpreting quantum efficiency for hydrogen photoproduction using Pt-titania catalysts. J. Catal. 2017, 347, 157–169. [Google Scholar] [CrossRef]
- Meshram, S.P.; Adhayak, P.V.; Mulik, U.P.; Amalnerkar, D.P. Facile synthesis of CuO nanomorphs and their morphology dependent sunlight driven photocatalytic properties. Chem. Eng. J. 2012, 204–206, 158–168. [Google Scholar] [CrossRef]
- Zhang, Z.; Maggard, P.A. Investigation of photocatalytically-active hydrated forms of amorphous titania, TiO2·nH2O. J. Photochem. Photobiol. A Chem. 2007, 186, 8–13. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.C.; Zakaria, R.; Ying, J.Y. Role of particle size in nanocrystalline TiO2-based photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Muller, U. Symmetry. In Inorganic Structural Chemistry; John Wiley and Sons, Ltd.: Chichester, UK, 2007. [Google Scholar]
- Tan, Y.N.; Wong, C.L.; Mohamed, A.R. An overview on the photocatalytic activity of nano-doped-TiO2 in the degradation of organic pollutants. ISRN Mater. Sci. 2011, 2011, 1–18. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Osterloh, F.E. Nanoscale effects in water splitting photocatalysis. Top. Curr. Chem. 2015, 371, 105–142. [Google Scholar]
- Olshansky, J.H.; Ding, T.X.; Lee, Y.V.; Leone, S.R.; Alivisatos, A.P. Hole transfer from photoexcited quantum dots: The relationship between driving force and rate. J. Am. Chem. Soc. 2015, 137, 15567–15575. [Google Scholar] [CrossRef] [PubMed]
- Sachs, M.; Pastor, E.; Kafizas, A.; Durrant, J.R. Evaluation of surface state mediated charge recombination in anatase and rutile TiO2. J. Phys. Chem. Lett. 2016, 7, 3742–3746. [Google Scholar] [CrossRef] [PubMed]
- Mijoe, J. Fundamentals of Semiconductor Physics; Anchor Academic Publishing: Hamburg, Germany, 2015. [Google Scholar]
- Kim, W.D.; Kim, J.H.; Lee, S.; Lee, S.; Woo, J.Y.; Lee, K.; Chae, W.S.; Jeong, S.; Bae, W.K.; McGuire, J.A.; et al. Role of surface states in photocatalysis: Study of chlorine-passivated CdSe nanocrystals for photocatalytic hydrogen generation. Chem. Mater. 2016, 28, 962–968. [Google Scholar] [CrossRef]
- Sreethawong, T.; Yoshikawa, S. Comparative investigation on photocatalytic hydrogen evolution over Cu-, Pd-, and Au-loaded mesoporous TiO2 photocatalysts. Catal. Commun. 2005, 6, 661–665. [Google Scholar] [CrossRef]
- Peng, Q.; Xiong, R.; Sa, B.; Zhou, J.; Wen, C.; Wu, B.; Anpo, M.; Sun, Z. Computational mining of photocatalysts for water splitting hydrogen production: Two-dimensional InSe-family monolayers. Catal. Sci. Technol. 2017, 7, 2744–2752. [Google Scholar] [CrossRef]
- Du, Y.A.; Chen, Y.W.; Kuo, J.L. First principles studies on the redox ability of (Ga1−xZnx)N1−xOx solid solutions and thermal reactions for H2 and O2 production on their surfaces. Phys. Chem. Chem. Phys. 2013, 15, 19807–19818. [Google Scholar] [CrossRef] [PubMed]
- Gurdal, Y.; Iannuzzi, M. DFT-based Theoretical simulations for photocatalytic applications using TiO2. In Titanium Dioxide; Janus, M., Ed.; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Garcia-Esparza, A.T.; Takanabe, K. A simplified theoretical guideline for overall water splitting using photocatalyst particles. J. Mater. Chem. 2016, 4, 2894–2908. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Xu, Y.; Niu, X.; Zheng, L.; Ding, X. The preparation of Zn2+-doped TiO2 nanoparticles by sol–gel and solid phase reaction methods respectively and their photocatalytic activities. Chemosphere 2005, 59, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhang, J.; Xiao, C.; Si, Z.; Tan, X. Solar photocatalytic degradation of methylene blue in carbon-doped TiO2 nanoparticles suspension. Sol. Energy 2008, 82, 706–713. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Bak, T.; Nowotny, J.; Rekas, M.; Sorrell, C.C. Photo-electrochemical hydrogen generation from water using solar energy. Materials-related aspects. Int. J. Hydrogen Energy 2002, 27, 991–1022. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibanez, P.; Di Somma, I. Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl. Catal. B Environ. 2015, 170, 90–123. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Kosmulski, M. Isoelectric points and points of zero charge of metal (hydr)oxides: 50 years after Parks’ review. Adv. Colloid Interface Sci. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN:ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting. J. Am. Chem. Soc. 2005, 127, 8286–8297. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Saito, N.; Lu, D.; Inoue, Y.; Domen, K. Photocatalytic properties of RuO2-loaded β-Ge3N4 for overall water splitting. J. Phys. Chem. C 2007, 111, 4749–4755. [Google Scholar] [CrossRef]
- Takata, T.; Shinohara, S.; Tanaka, A.; Hara, M.; Kondo, J.N.; Domen, K. A highly active photocatalyst for overall water splitting with a hydrated layered perovskite structure. J. Photochem. Photobiol. A Chem. 1997, 106, 45–49. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Water splitting into H2 and O2 on alkali tantalite photocatalysts ATaO3 (A=Li, Na and K). J. Phys. Chem. B 2001, 105, 4285–4292. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Masuda, H.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Efficient overall water splitting under visible-light irradiation on (Ga1−xZnx)(N1−xOx) dispersed with Rh−Cr mixed-oxide nanoparticles: Effect of reaction conditions on photocatalytic activity. J. Phys. Chem. B 2006, 110, 13107–13112. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.A.; Hamed, H.A.; Barakat, M.H.; Mohamed, N.R.; Veziroglu, T.N. Enhancement of photocatalytic hydrogen production rate using photosensitized TiO2/RuO2–MV2+. Int. J. Hydrogen Energy 2008, 33, 3264–3269. [Google Scholar] [CrossRef]
- Ismail, A.A. MesoporousPdO–TiO2nanocomposites with enhanced photocatalytic activity. Appl. Catal. B Environ. 2012, 117–118, 67–72. [Google Scholar] [CrossRef]
- Lalitha, K.; Reddy, J.K.; Sharma, M.V.P.; Kumari, V.D.; Subrahmanyam, M. Continuous hydrogen production activity over finely dispersed Ag2O/TiO2 catalysts from methanol:water mixture under solar irradiation: A structure–activity correlation. Int. J. Hydrogen Energy 2010, 35, 3991–4001. [Google Scholar] [CrossRef]
- Rosseler, O.; Shankar, M.V.; Karkmaz-Le Du, M.; Schmidlin, L.; Keller, N.; Keller, V. Solar light photocatalytic hydrogen production from water over Pt and Au/TiO2 (anatase/rutile) photo-catalysts: Influence of noble metal and porogen promotion. J. Catal. 2010, 269, 179–190. [Google Scholar] [CrossRef]
- Hisatomi, T.; Maeda, K.; Takanabe, K.; Kubota, J.; Domen, K. Aspects of the water splitting mechanism on (Ga1−xZnx)(N1−xOx) photocatalyst modified with Rh2−yCryO3 cocatalyst. J. Phys. Chem. C 2009, 113, 21458–21466. [Google Scholar] [CrossRef]
- Hisatomi, T.; Miyazaki, K.; Takanabe, K.; Maeda, K.; Kubota, J.; Sakata, Y.; Domen, K. Isotopic and kinetic assessment of photocatalytic water splitting on Zn-added Ga2O3 photocatalyst loaded with Rh2−yCryO3cocatalyst. Chem. Phys. Lett. 2010, 486, 144–146. [Google Scholar] [CrossRef]


| Photocatalyst | Band Gap (eV) | Wavelength (nm) | Ref. |
|---|---|---|---|
| TiO2 (anatase)–TiO2 (rutile) | 2.78 | λ > 300 | [50,66] |
| Tantalates–NiO | 3.6–4.0 | λ > 310 | [67] |
| Perovskites–NiOx | 3.2–4.7 | λ < 350 | [22] |
| Noble metal/TiO2–CdS | N/A | λ > 400 | [68] |
| (Ga0.88Zn0.12)(N0.88O0.12)–Rh2-xCrxO3 | 2.6 | λ > 400 | [22] |
| Cu1.94S–ZnxCd1-xS(0 ≤ x ≤ 1) | 2.57–3.88 | λ > 420 | [69] |
| CdS–ZnS | N/A | λ > 420 | [70] |
| CdSe/CdS–MoS3 | 1.75–2.44 | 450 | [71,72] |
| MoS2/CuInS2 | N/A | λ > 420 | [73] |
| Cu2O/CuO | 1.54–2.01 | λ > 400 | [74] |
| Ni3N/CdS | 2.54 | λ > 420 | [75] |
| BaZrO3/BaTaO2N | 1.8 | λ > 420 | [76] |
| Ir/CoOx/Ta3N5–Rh,Ru/SrTiO3 | ~2.1 | λ > 420 | [77] |
| Pt/BaZrO3–BaTaO2N | 1.8–1.9 | λ > 420 | [78] |
| TaOxN: Tantalum oxynitride | |||
| Ru,Rh/SrTiO3–BiVO4 | N/A | λ > 420 | [79] |
| WO3/BiVO4 | ~2.4 | λ > 420 | [80] |
| CdS-ZnO/RGO | N/A | λ > 400 | [81] |
| RGO: reduced graphene oxide | |||
| CdS-TaON/RGO | 2.4–2.5 | λ > 420 | [82] |
| Sulfide based semiconductors | 2.0–2.3 | λ > 420 | [22] |
| (µmoles H2/h·g) | Material | Sacrificial Agent | Irradiation Type | Reference |
|---|---|---|---|---|
| >4.3 × 104 | metal/niobates | methanol | UV-A | [126] |
| 1.1 × 104 | Sr/tantalates | methanol | UV-A | [127] |
| 6.3 × 104 | Ni/CdSnanorods | ethanol | visible light | [128] |
| 5.6 × 104 | CdS/RGO | lactic acid | visible light | [129] |
| 4.0 × 104 | Pt/CdSe-CdS | isopropanol | visible light | [130] |
| >3.7 × 104 | N/Zn,Ga-mixed oxide-Rh/Cr2O3 | methanol | visible light | [131] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarizia, L.; Russo, D.; Di Somma, I.; Andreozzi, R.; Marotta, R. Hydrogen Generation through Solar Photocatalytic Processes: A Review of the Configuration and the Properties of Effective Metal-Based Semiconductor Nanomaterials. Energies 2017, 10, 1624. https://doi.org/10.3390/en10101624
Clarizia L, Russo D, Di Somma I, Andreozzi R, Marotta R. Hydrogen Generation through Solar Photocatalytic Processes: A Review of the Configuration and the Properties of Effective Metal-Based Semiconductor Nanomaterials. Energies. 2017; 10(10):1624. https://doi.org/10.3390/en10101624
Chicago/Turabian StyleClarizia, Laura, Danilo Russo, Ilaria Di Somma, Roberto Andreozzi, and Raffaele Marotta. 2017. "Hydrogen Generation through Solar Photocatalytic Processes: A Review of the Configuration and the Properties of Effective Metal-Based Semiconductor Nanomaterials" Energies 10, no. 10: 1624. https://doi.org/10.3390/en10101624
APA StyleClarizia, L., Russo, D., Di Somma, I., Andreozzi, R., & Marotta, R. (2017). Hydrogen Generation through Solar Photocatalytic Processes: A Review of the Configuration and the Properties of Effective Metal-Based Semiconductor Nanomaterials. Energies, 10(10), 1624. https://doi.org/10.3390/en10101624








