Brosimum Alicastrum as a Novel Starch Source for Bioethanol Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
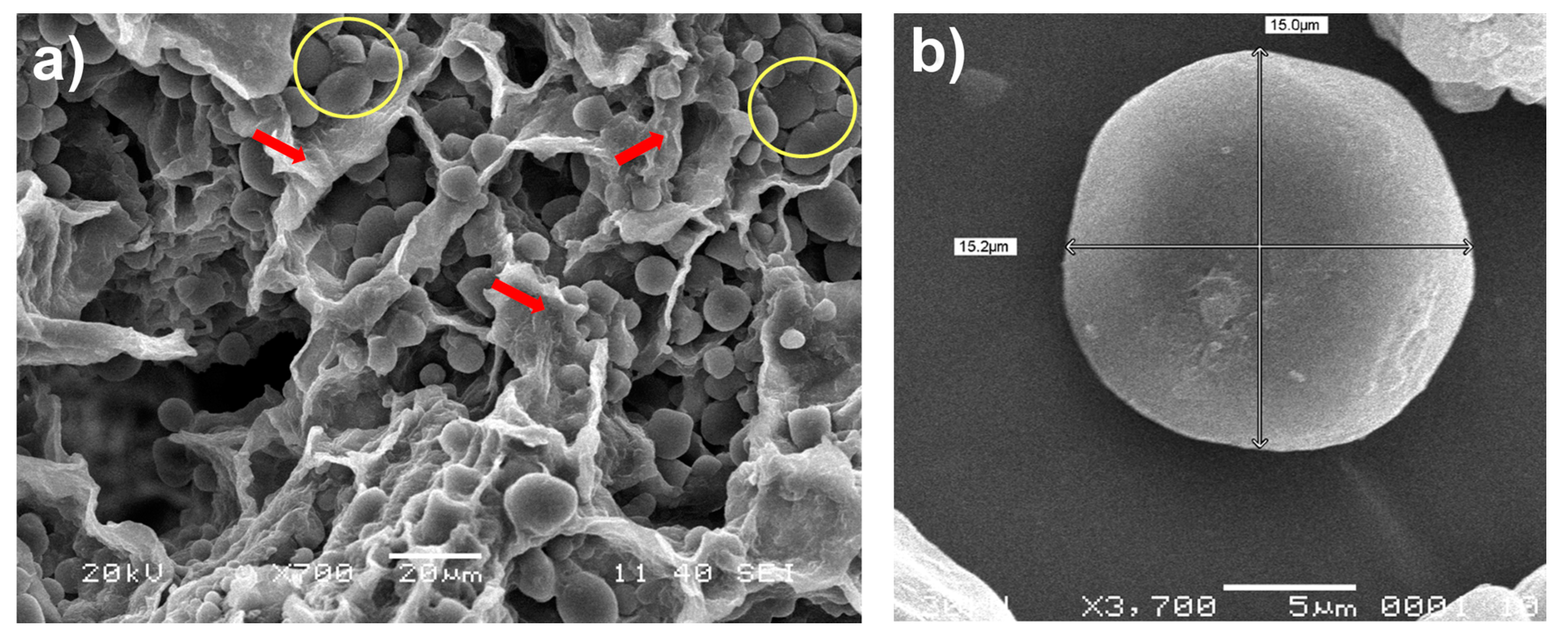
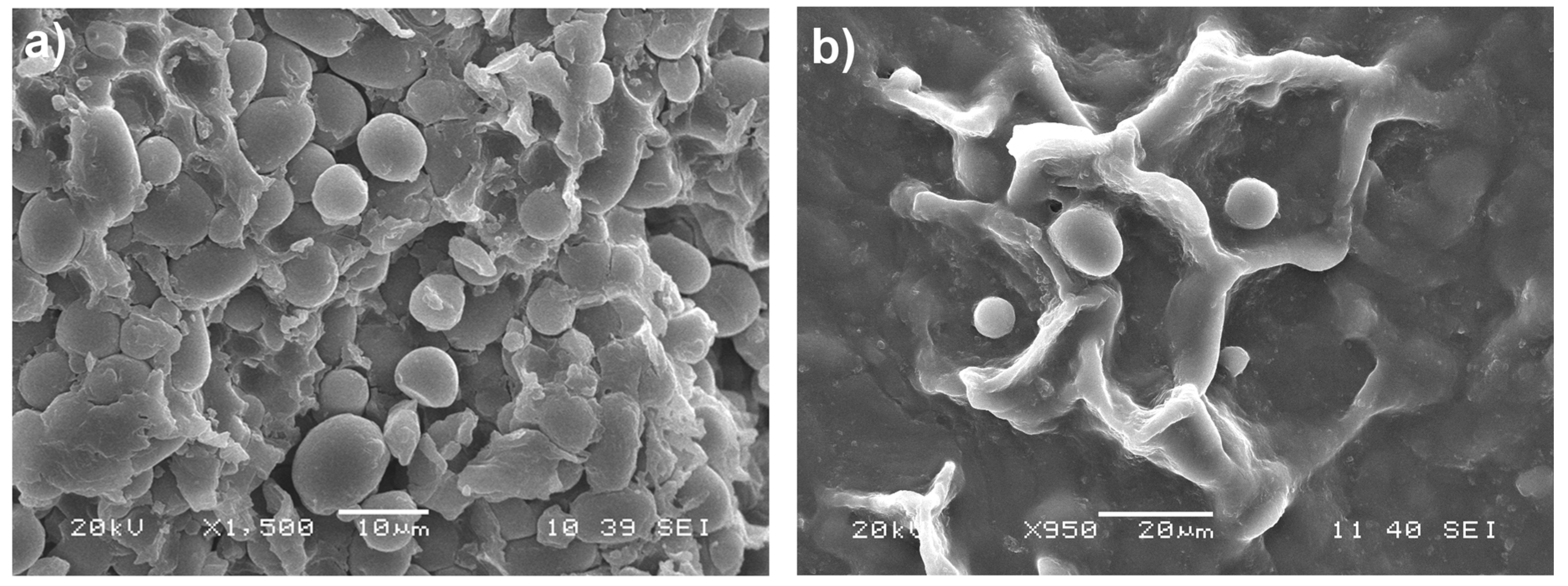
2.2. Scanning Electron Microscopy (SEM)
2.3. Analytical Methods
2.4. Enzymatic Hydrolysis
2.4.1. Liquefaction
2.4.2. Saccharification
2.5. Yeast Strains
2.6. Fermentation Conditions
3. Results and Discussion
3.1. Enzymatic Hydrolysis
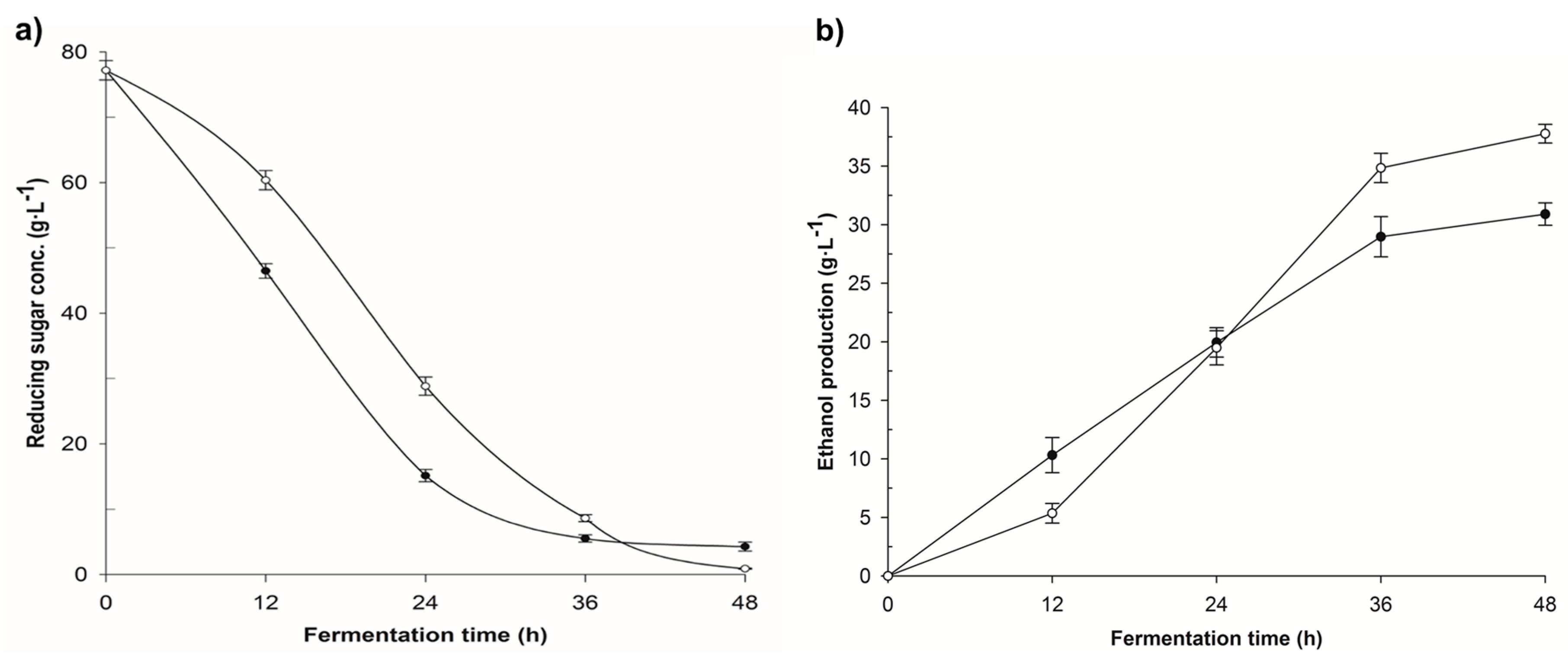
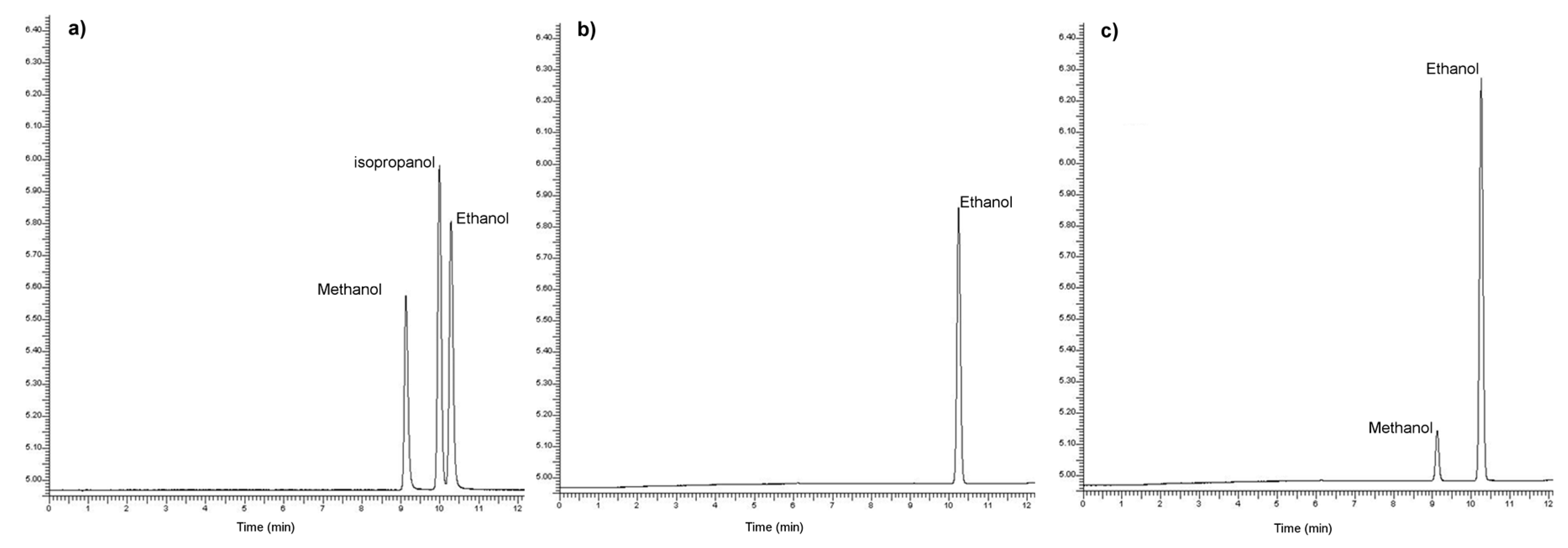
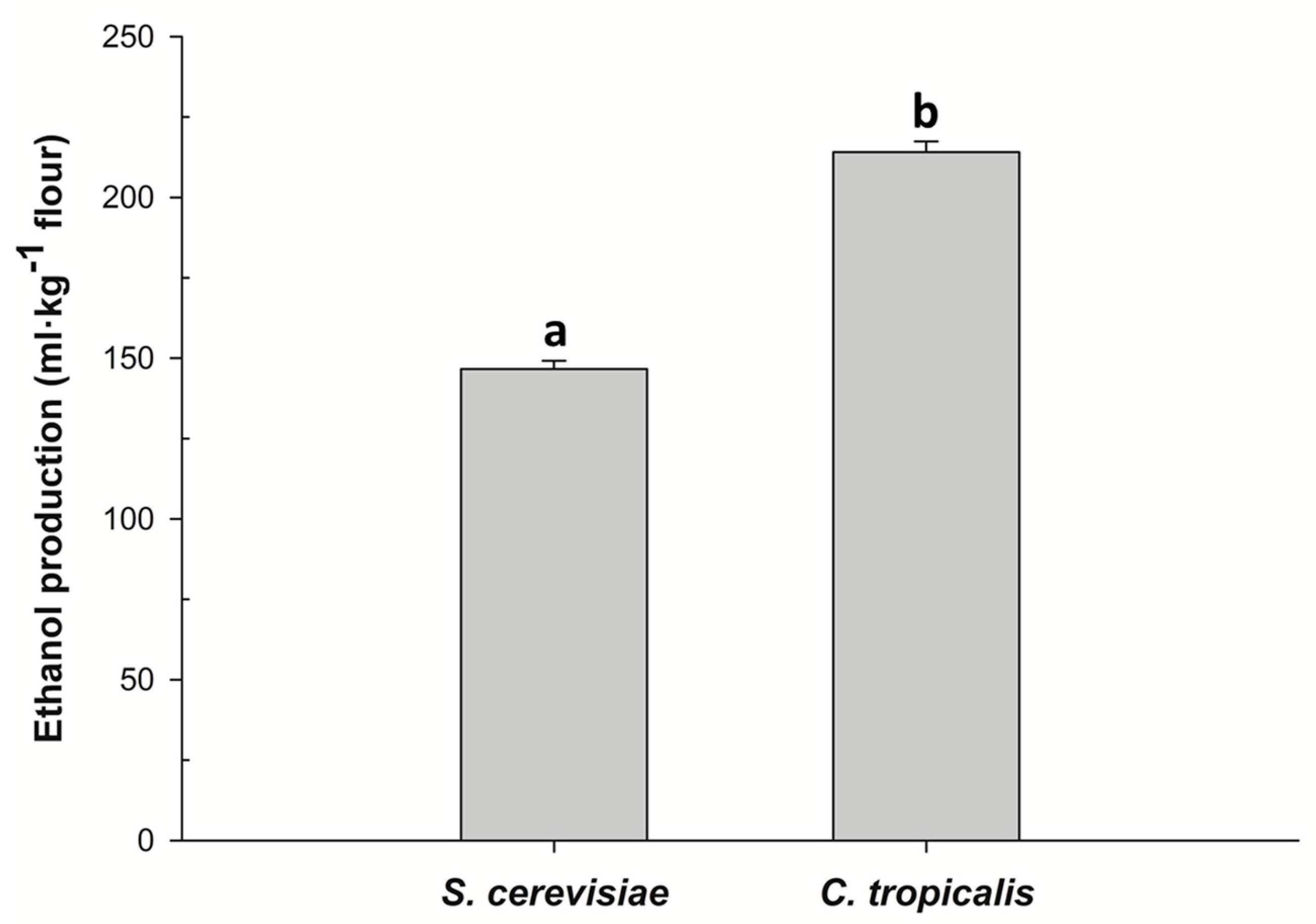
3.2. Fermentation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pervez, S.; Aman, A.; Iqbal, S.; Siddiqui, N.N.; Qader, S.A.U. Saccharification and liquefaction of cassava starch: An alternative source for the production of bioethanol using amylolytic enzymes by double fermentation process. BMC Biotechnol. 2014, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Bhadana, B.; Chauhan, M. Bioethanol production using Saccharomyces cerevisiae with different perspectives: Substrates, growth variables, inhibitor reduction and immobilization. Ferment. Technol. 2016, 5, 131. [Google Scholar] [CrossRef]
- Lamers, P.; Hamelinck, C.; Junginger, M.; Faaij, A. International bioenergy trade. A review of past developments in the liquid biofuel market. Renew. Sustain. Energy Rev. 2011, 15, 2655–2676. [Google Scholar] [CrossRef]
- Renewable Fuels Association. Available online: http://www.ethanolrfa.org/resources/industry/statistics (accessed on 7 July 2017).
- Moshi, A.P.; Crespo, C.F.; Badshah, M.; Hosea, K.M.M.; Mshandete, A.M.; Elisante, E.; Mattiasson, B. Characterization and evaluation of a novel feedstock, Manihot glaziovii, Muell. Arg, for production of bioenergy carriers: Bioethanol and biogas. Bioresour. Technol. 2014, 172, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Silva, P.A.; Toledano-Thompson, T.; Canto-Canché, B.B.; Larqué-Saavedra, A.; Barahona-Pérez, L.F. Hydrolysis of Agave fourcroydes Lemaire (henequen) leaf juice and fermentation with Kluyveromyces marxianus for ethanol production. BMC Biotechnol. 2014, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Hernández-González, O.; Vergara-Yoisura, S.; Larqué-Saavedra, A. Studies on the productivity of Brosimum alicastrum a tropical tree used for animal feed in the Yucatan Peninsula. Bothalia 2014, 44, 70–81. [Google Scholar]
- Pérez-Pacheco, E.; Moo-Huchin, V.M.; Estrada-León, R.J.; Ortiz-Fernández, A.; May-Hernández, L.H.; Ríos-Soberanis, C.R.; Betancur-Ancona, D. Isolation and characterization of starch obtained from Brosimum alicastrum Swarts Seeds. Carbohydr. Polym. 2014, 101, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.D.; Sanchez, F.C.; Ribeiro, D.M.; Freitas, A.S.; da Cruz, P.J. Use of a new Trichoderma harzianum strain isolated from the Amazon rainforest with pretreated sugar cane bagasse for on-site cellulase production. Bioresour. Technol. 2012, 107, 517–521. [Google Scholar]
- Orencio-Trejo, M.; Torres-Granados, J.; Rangel-Lara, A.; Beltrán-Guerrero, E.; García-Aguilar, S.; Moss-Acosta, C.; Valenzuela-Soto, H.; De la Torre-Zavala, S.; Gastelum-Arellanez, A.; Martinez, A.; et al. Cellulase and Xylanase Production by the Mexican Strain Talaromyces stollii LV186 and Its Application in the Saccharification of Pretreated Corn and Sorghum Stover. Bioenergy Res. 2016, 9, 1034–1045. [Google Scholar]
- Huchin-Poot, E.G. Aislamiento de la microbiota del fruto de ramon Brosimum alicastrum Swartz para su uso en la producción de bioetanol. Master’s Thesis, Centro de Investigación Científica de Yucatán A.C., Mérida, Mexico, 2015. [Google Scholar]
- Holm, J.; Björck, I.; Drews, A.; Asp, N.G. A rapid method for the analysis of starch. Starch Stärke 1986, 38, 224–226. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Analytical Chemists: Washington, DC, USA, 1997. [Google Scholar]
- Miller, G.L. Use of Dinitrisalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1859, 31, 426–428. [Google Scholar] [CrossRef]
- Barquera, B.Z. Obtención de bioethanol a partir de la semilla del ramón (Brosimum alicastrum Sw.). Master’s Thesis, Centro de Investigación Científica de Yucatán A.C., Mérida, Mexico, 2013. [Google Scholar]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Santana, A.L.; Meireless, M.A.A. New starches are the trend for industry applications: A review. Food Public Health 2014, 4, 229–241. [Google Scholar] [CrossRef]
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemical pathways: A review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Global potential bioethanol production from wasted crops and crop residues. Bioenergy 2004, 24, 361–375. [Google Scholar] [CrossRef]
- Wang, T.; Wang, M.; Hu, S.; Xiao, Y.; Tong, H.; Pan, Q.; Xue, J.; Yan, J.; Li, J.; Yang, X. Genetic basis of maize kernel starch content revealed by high-density single nucleotide polymorphism markers in a recombinant inbred line population. BMC Plant Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Somavat, P.; Li, Q.; Gonzalez de Mejia, E.; Liu, W.; Singh, V. Coproduct yield comparisons of purple, blue and yellow dent corn for various milling processes. Ind. Crop. Prod. 2016, 87, 266–272. [Google Scholar] [CrossRef]
- Khan, A.H.; Minhas, N.M.; Asad, M.J.; Iqbal, A.; Ilyas, M.; Mahmood, R.J. Estimation of protein, carbohydrate, starch and oil contents of indigenous maize (Zea mayz L.) germoplasm. Eur. Acad. Res. 2014, 2, 5230–5240. [Google Scholar]
- Altan, A. Effects of pretreatments and moisture on microestructure and physical properties of microwave expanded hull-less barley. Food Res. Int. 2014, 56, 126–135. [Google Scholar] [CrossRef]
- Dhital, S.; Warren, F.J.; Butterworth, P.J.; Ellis, P.R.; Gidley, M.J. Mechanism of starch digestion by amylase-estructural basis for kinetic properties. Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef]
- Martín, J.C.; López, E. Modificación física del almidón de yuca y evaluación de la susceptibilidad a la hidrólisis enzimática por una alfa amilasa. Rev. Colomb. Quim. 2009, 38, 395–408. [Google Scholar]
- Hernández-Medina, M.; Torruco-Uco, J.G.; Chel-Guerrero, L.; Betancur-Ancona, D. Caracterización fisicoquímica de almidones de tubérculos cultivados en Yucatán, México. Cienc. Tecnol. Aliment. 2008, 28, 718–726. [Google Scholar] [CrossRef]
- Medina, J.A.; Salas, J.C. Caracterización morfológica del gránulo de almidón nativo: Apariencia, forma, tamaño y su distribución. Rev. Ing. 2008, 27, 56–62. [Google Scholar]
- Chen, X.; He, X.; Huang, Q. Effects of hidrothermal pretreatment on subsequent octenysuccinic anhydride (OSA) modification of cornstarch. Carbohydr. Polym. 2014, 101, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Sakinah, A.M.; Ismail, A.F.; Hassan, O.; Zularisam, A.W.; Illias, R.M. Influence of starch pretreatment on yield of cyclodextrins and performance of ultrafiltration membranes. Desalination 2009, 239, 317–333. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Chiming, M.T. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Baks, T.; Bruins, M.E.; Matser, A.M.; Janssen, A.E.M.; Boom, R.M. Effect of gelatinization and hydrolysis conditions on the selectivity of starch hydrolysis with amylase from Bacillus lincheniformis. J. Agric. Food Chem. 2008, 56, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Pepe, L.S.; Moraes, J.; Albano, K.M.; Telis, V.R.N.; Franco, C.M.L. Effect of heat moisture treatment on the structural, physicochemical, and rheological characteristics of arrowroot starch. Food Sci. Technol. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Copeland, L. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: A review. Food Funct. 2013, 4, 1564–1580. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A Comprehensive Review. Comp. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Nakamura, L.K. Influence of the acceptor during transglucosylation by transglucosylamylase of Candida tropicalis. Can. J. Biochem. 1970, 48, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Jamai, L.; Ettayebi, K.; El Yamani, J.; Ettayebi, M. Production of ethanol from starch by free and immobilized Candida tropicalis in the presence of α-amylase. Bioresour. Technol. 2007, 98, 2765–2770. [Google Scholar] [CrossRef] [PubMed]
- Gumienna, M.; Szwengiel, A.; Lasik, M.; Szambelan, K.; Majchrzycki, D.; Adamczyk, J.; Nowak, J.; Czarnecki, Z. Effect of corn grain variety on the bioethanol production efficiency. Fuel 2016, 164, 386–392. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olguin-Maciel, E.; Larqué-Saavedra, A.; Pérez-Brito, D.; Barahona-Pérez, L.F.; Alzate-Gaviria, L.; Toledano-Thompson, T.; Lappe-Oliveras, P.E.; Huchin-Poot, E.G.; Tapia-Tussell, R. Brosimum Alicastrum as a Novel Starch Source for Bioethanol Production. Energies 2017, 10, 1574. https://doi.org/10.3390/en10101574
Olguin-Maciel E, Larqué-Saavedra A, Pérez-Brito D, Barahona-Pérez LF, Alzate-Gaviria L, Toledano-Thompson T, Lappe-Oliveras PE, Huchin-Poot EG, Tapia-Tussell R. Brosimum Alicastrum as a Novel Starch Source for Bioethanol Production. Energies. 2017; 10(10):1574. https://doi.org/10.3390/en10101574
Chicago/Turabian StyleOlguin-Maciel, Edgar, Alfonso Larqué-Saavedra, Daisy Pérez-Brito, Luis F. Barahona-Pérez, Liliana Alzate-Gaviria, Tanit Toledano-Thompson, Patricia E. Lappe-Oliveras, Emy G. Huchin-Poot, and Raúl Tapia-Tussell. 2017. "Brosimum Alicastrum as a Novel Starch Source for Bioethanol Production" Energies 10, no. 10: 1574. https://doi.org/10.3390/en10101574
APA StyleOlguin-Maciel, E., Larqué-Saavedra, A., Pérez-Brito, D., Barahona-Pérez, L. F., Alzate-Gaviria, L., Toledano-Thompson, T., Lappe-Oliveras, P. E., Huchin-Poot, E. G., & Tapia-Tussell, R. (2017). Brosimum Alicastrum as a Novel Starch Source for Bioethanol Production. Energies, 10(10), 1574. https://doi.org/10.3390/en10101574