Thermochemical Storage of Middle Temperature Wasted Heat by Functionalized C/Mg(OH)2 Hybrid Materials
Abstract
:1. Introduction
- cost,
- cycling behavior (reversibility and degradation over large numbers of cycles),
- availability,
- toxicity and safety,
- corrosiveness,
- energy storage density,
- reaction temperature, and
- reaction rate.
2. Materials and Methods
2.1. Carbonaceus Materials
2.2. Carbon-Based Hybrid Materials Preparation and Characterization
2.3. Experimental Simulation of a Heat Storage/Release Cycle
Numerical Evaluation of the Hybrid Materials’ Performances
3. Results
3.1. Samples’ Morphological and Structural Characterizations
3.2. Dehydration and Hydration Reactions
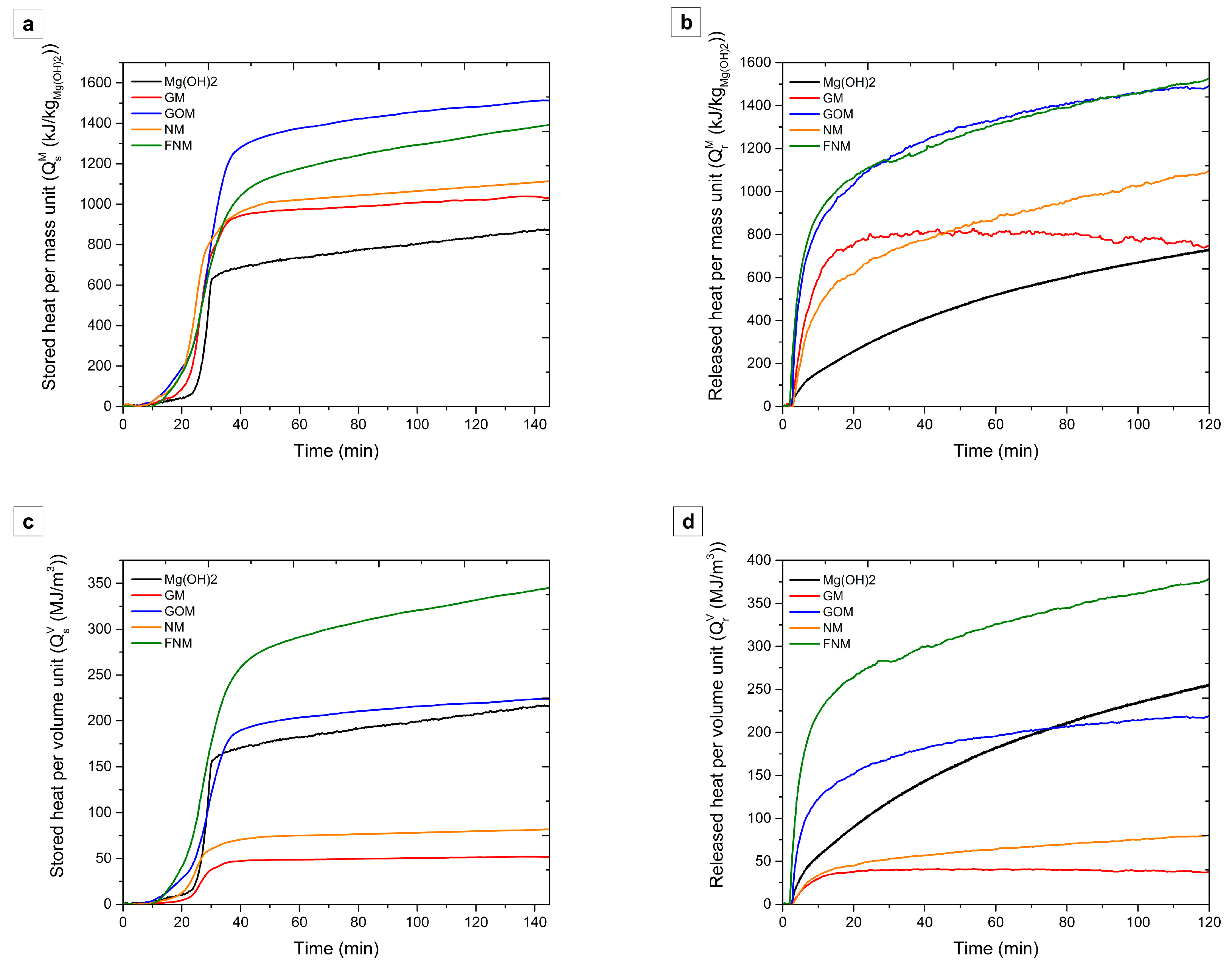
3.3. Heat Storage and Output Capacities
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| EG | Exfoliated graphite |
| CNTs | Carbon nanotubes |
| GO | Graphene oxide |
| F-CNTs | Functionalized carbon nanotubes |
| min | Initial sample mass (g) |
| mist | Instantaneous mass (g) |
| MMg(OH)2 | Molecular weight of Mg(OH)2 (g·mol−1) |
| MMgO | Molecular weight of MgO (g·mol−1) |
| Heat storage and output capacities per mass unit of Mg(OH)2 (kJ·kgMg(OH)2−1) | |
| Heat storage and output capacities per volume unit (MJ·m−3) | |
| Td | Dehydration temperature (°C) |
| Th | Hydration temperature (°C) |
| t | Reaction time (min) |
| Tpeak | Temperatures of the derivative reacted fraction maximum peak (°C) |
| Greek Symbols | |
| ρ | Density (kg·m−3) |
| β | Reacted fraction (%) |
| dβ/dt | The reaction conversion rate |
| β fd | Reacted fraction at the end of the dehydration treatment (%) |
| β id | Reacted fraction at the beginning of the dehydration treatment (%) |
| βh | Final reacted fraction of MgO at the point of water supply termination (%) |
| Δmreal | Instantaneous real mass change (%) |
| Δmth | Theoretical mass change due to the dehydration of Mg(OH)2 normalized to the total amount present in the sample (%) |
| Δβd | Dehydration conversion (%) |
| Δβh | Hydration conversion (%) |
| ΔH0 | Standard reaction enthalpy (kJ/mol) |
Appendix A
Appendix A.1
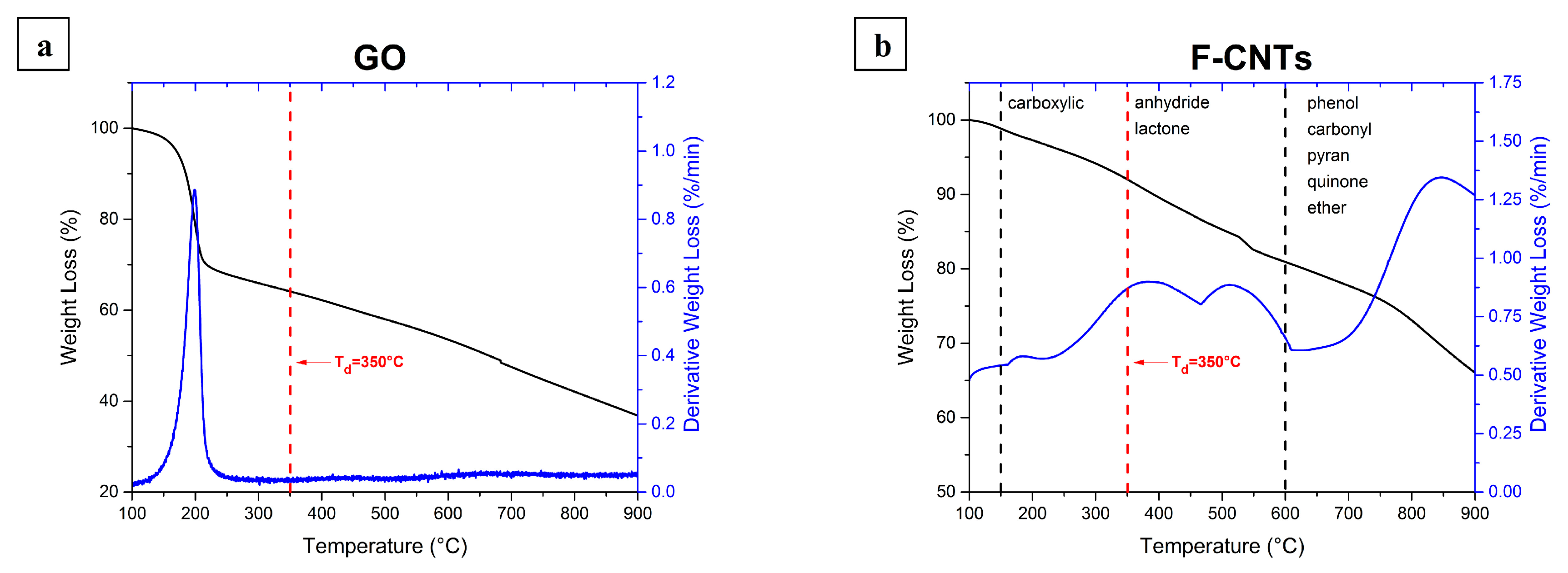
Appendix A.2

Appendix A.3
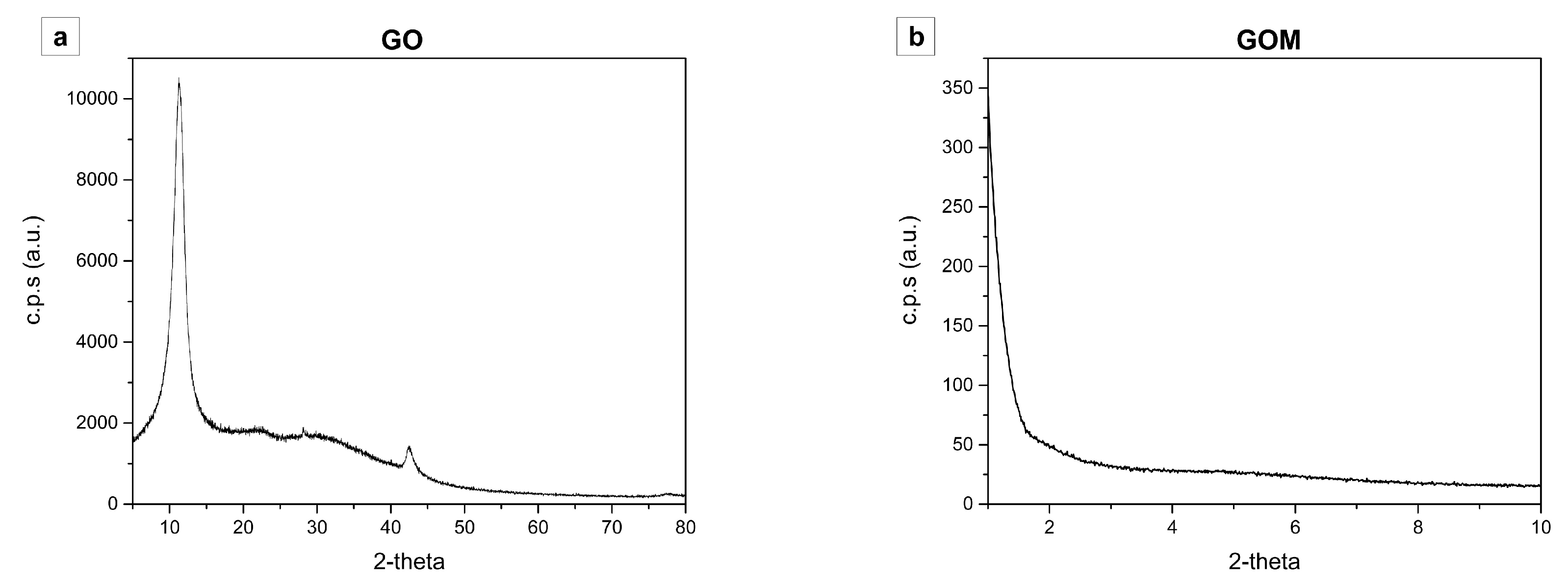
References
- BCS, Incorporated. Waste Heat Recovery: Technology Opportunities in the US Industry; U.S. Department of Energy: Washington, DC, USA, 2008; pp. 1–112.
- Sabihuddin, S.; Kiprakis, A.E.; Mueller, M. A numerical and graphical review of energy storage technologies. Energies 2015, 8, 172–216. [Google Scholar] [CrossRef]
- Hauer, A. Thermal Energy Storage. Technology Brief; IEA-ETSAP and IRENA© Technology Brief E17; International Renewable Energy Agency (IRENA): Abu Dhabi, UAE; Energy Technology Systems Analysis Programme (ETSAP): Paris, France, 2013; pp. 1–24. [Google Scholar]
- Tomlinson, J.J. Thermal Energy Storage Technical Progress Report; Oak Ridge National Lab.: Oak Ridge, TN, USA, 1992; pp. 1–27. [Google Scholar]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sustain. Energy Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef]
- Abedin, A.; Rosen, M. A Critical Review of Thermochemical Energy Storage Systems. Open Renew. Energy J. 2011, 4, 42–46. [Google Scholar] [CrossRef]
- Cot-Gores, J.; Castell, A.; Cabeza, L.F. Thermochemical energy storage and conversion: A-state-of-the-art review of the experimental research under practical conditions. Renew. Sustain. Energy Rev. 2012, 16, 5207–5224. [Google Scholar] [CrossRef]
- Kato, Y.; Harada, N.; Yoshizawa, Y. Kinetic feasibility of a chemical heat pump for heat utilization of high-temperature processes. Appl. Therm. Eng. 1999, 19, 239–254. [Google Scholar] [CrossRef]
- Barker, R. The reactivity of calcium oxide towards carbon dioxide and its use for energy storage. J. Appl. Chem. Biotechnol. 2007, 24, 221–227. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, C.Y. Thermodynamic and kinetic study of the dehydration process of CaO/Ca(OH)2 thermochemical heat storage system with Li doping. Chem. Eng. Sci. 2015, 138, 86–92. [Google Scholar] [CrossRef]
- Felderhoff, M.; Bogdanović, B. High temperature metal hydrides as heat storage materials for solar and related applications. Int. J. Mol. Sci. 2009, 10, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Agrafiotis, C.; Roeb, M.; Schmücker, M.; Sattler, C. Exploitation of thermochemical cycles based on solid oxide redox systems for thermochemical storage of solar heat. Part 1: Testing of cobalt oxide-based powders. Sol. Energy 2014, 102, 189–211. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Sastre, D.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Revisiting the BaO2/BaO redox cycle for solar thermochemical energy storage. Phys. Chem. Chem. Phys. 2016, 18, 8039–8048. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Takahashi, F.; Watanabe, A.; Yoshizawa, Y. Thermal analysis of a magnesium oxide/water chemical heat pump for cogeneration. Appl. Therm. Eng. 2001, 21, 1067–1081. [Google Scholar] [CrossRef]
- Shkatulov, A.; Ryu, J.; Kato, Y.; Aristov, Y. Composite material Mg(OH)2/vermiculite: A promising new candidate for storage of middle temperature heat. Energy 2012, 44, 1028–1034. [Google Scholar] [CrossRef]
- Zamengo, M.; Ryu, J.; Kato, Y. Magnesium hydroxide–Expanded graphite composite pellets for a packed bed reactor chemical heat pump. Appl. Therm. Eng. 2013, 61, 853–858. [Google Scholar] [CrossRef]
- Mastronardo, E.; Bonaccorsi, L.; Kato, Y.; Piperopoulos, E.; Milone, C. Efficiency improvement of heat storage materials for MgO/H2O/Mg(OH)2 chemical heat pumps. Appl. Energy 2016, 162, 31–39. [Google Scholar] [CrossRef]
- Mastronardo, E.; Bonaccorsi, L.; Kato, Y.; Piperopoulos, E.; Lanza, M.; Milone, C. Thermochemical performance of carbon nanotubes based hybrid materials for MgO/H2O/Mg(OH)2 chemical heat pumps. Appl. Energy 2016, 181, 232–243. [Google Scholar] [CrossRef]
- Milone, C.; Piperopoulos, E.; Ansari, S.; Faggio, G.; Santangelo, S. Highly Versatile and Efficient Process for CNT Oxidation in Vapor Phase by Means of Mg(NO3)2–HNO3–H2O Ternary Mixture. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 1–5. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Messina, G.; Modafferi, V.; Santangelo, S.; Tripodi, P.; Donato, M.G.; Lanza, M.; Galvagno, S.; Milone, C.; Piperopoulos, E.; Pistone, A. Large-scale production of high-quality multi-walled carbon nanotubes: Role of precursor gas and of Fe-catalyst support. Diam. Relat. Mater. 2008, 17, 1482–1488. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Schott, J. Experimental study of brucite dissolution and precipitation in aqueous solutions: Surface speciation and chemical affinity control. Geochim. Cosmochim. Acta 2004, 68, 31–45. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, L.; Lu, Z.; Gu, L.; Cao, S.; Cao, X. Selectively expanding graphene oxide paper for creating multifunctional carbon materials. J. Phys. Chem. C 2012, 116, 23075–23082. [Google Scholar] [CrossRef]
- Liu, C.; He, C.; Xie, T.; Yang, J. Reduction of Graphite Oxide Using Ammonia Solution and Detection Cr(VI) with Graphene-Modified Electrode. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 125–130. [Google Scholar] [CrossRef]
- Compton, O.C.; Dikin, D.A.; Putz, K.W.; Brinson, L.C.; Nguyen, S.T. Electrically conductive “alkylated” graphene paper via chemical reduction of amine-functionalized graphene oxide paper. Adv. Mater. 2010, 22, 892–896. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Luzan, S.M.; Szabó, T.S.; Talyzin, A.V. Effect of synthesis method on solvation and exfoliation of graphite oxide. Carbon 2013, 52, 171–180. [Google Scholar] [CrossRef]
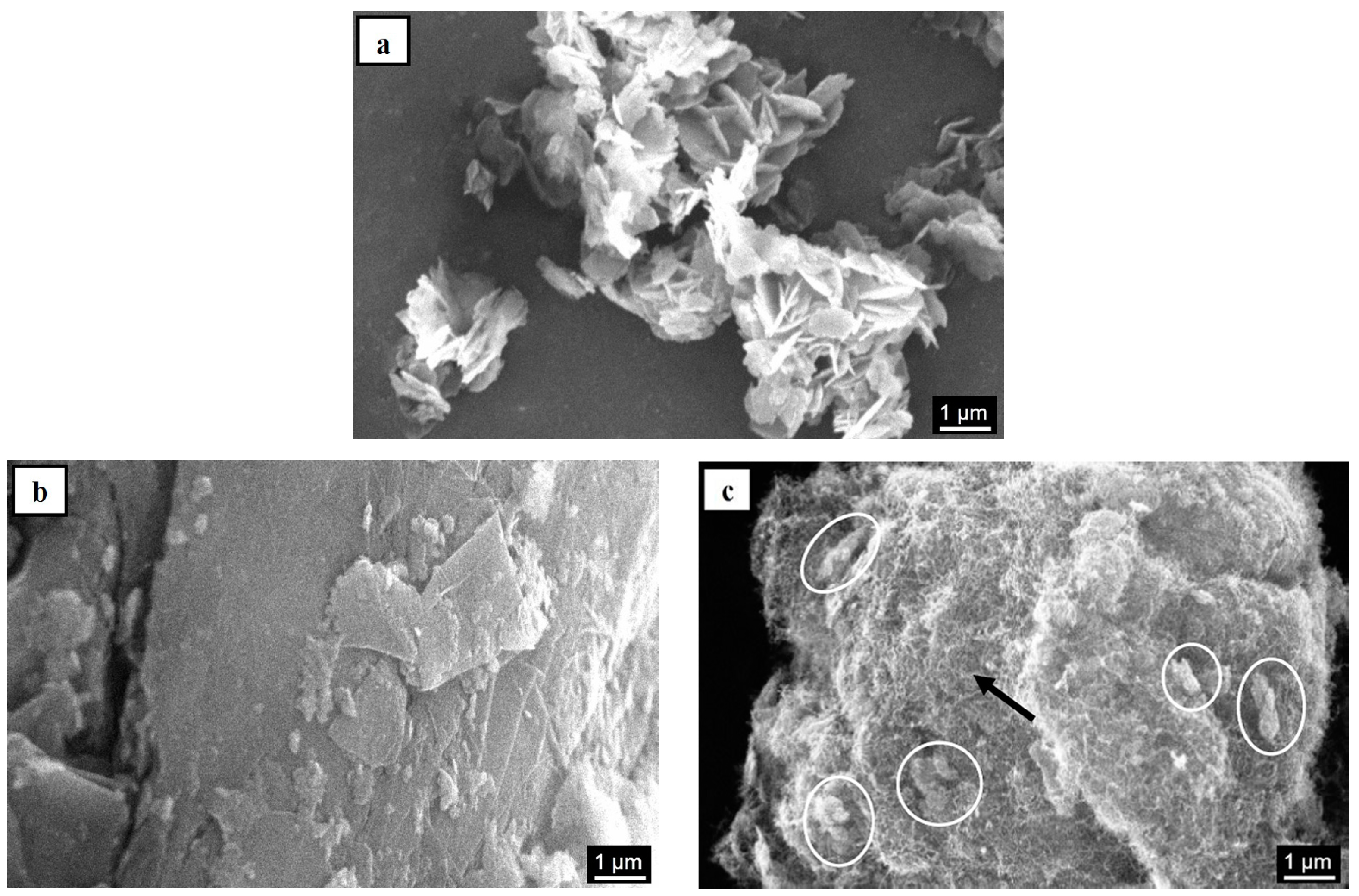
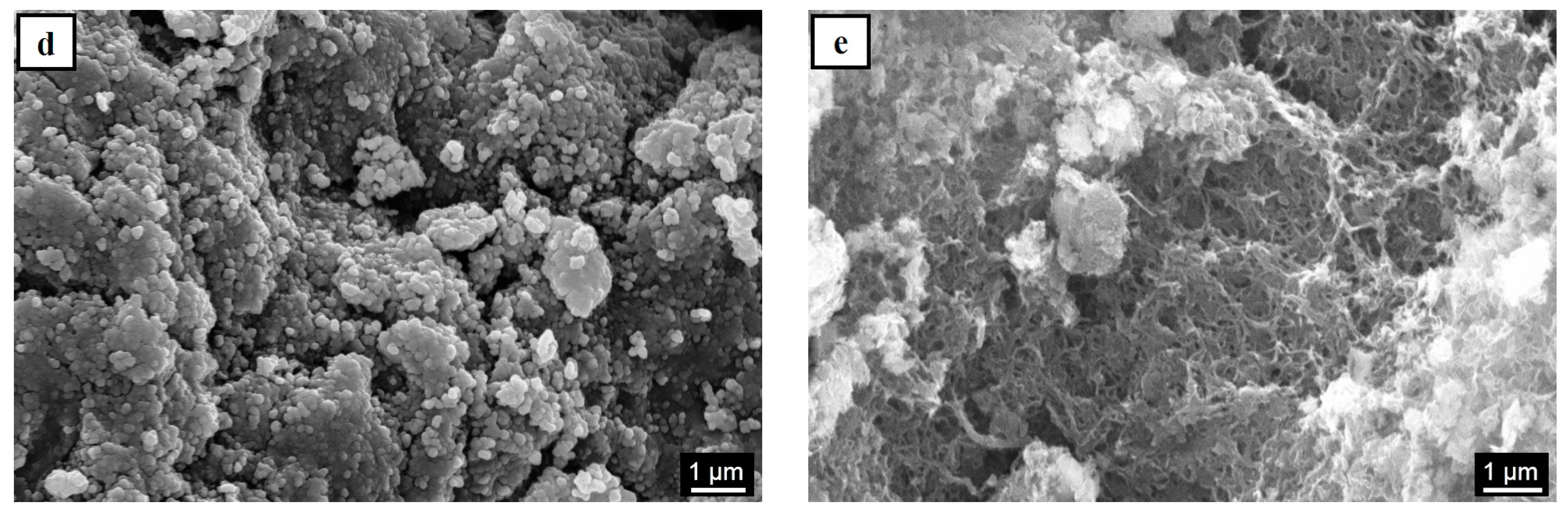
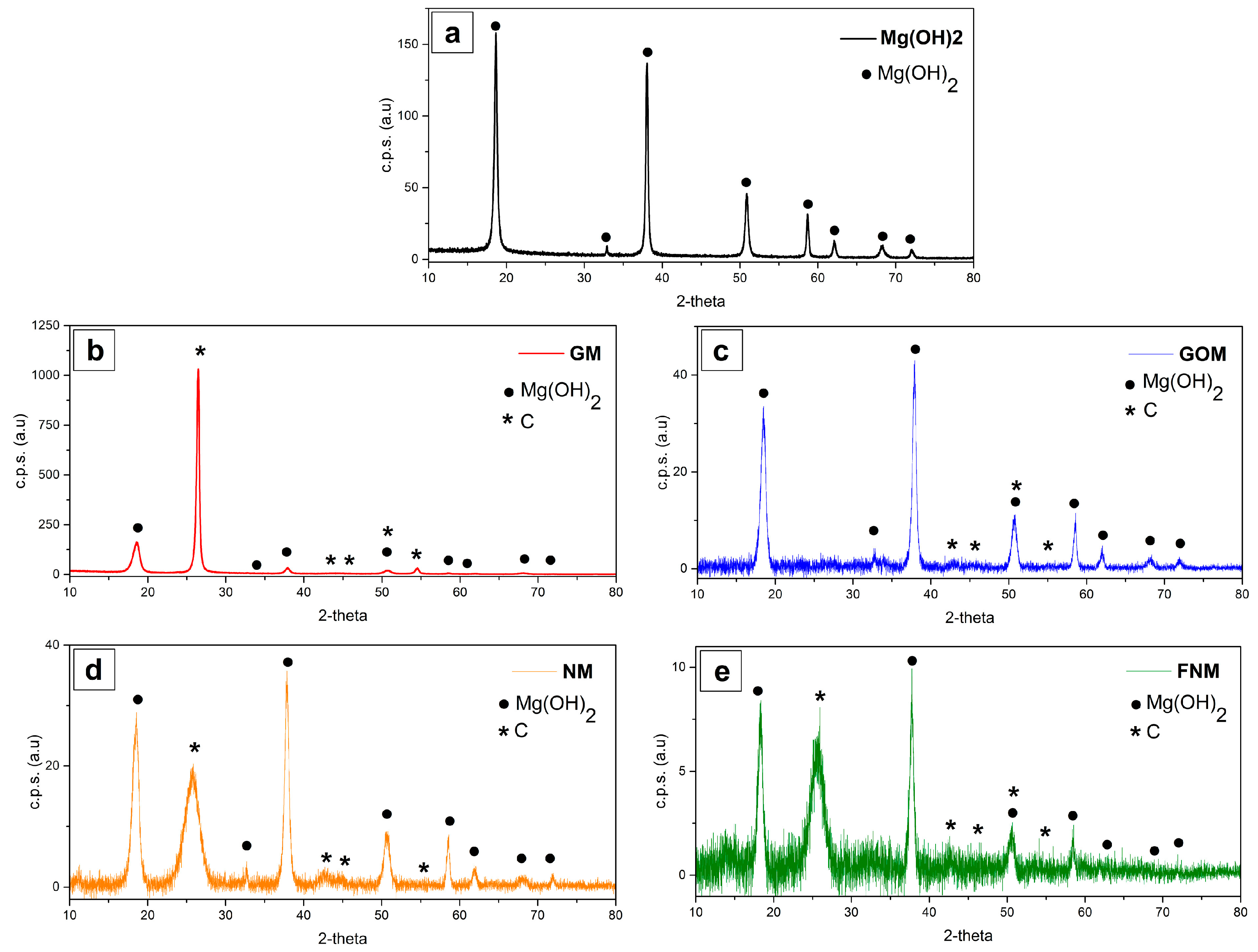
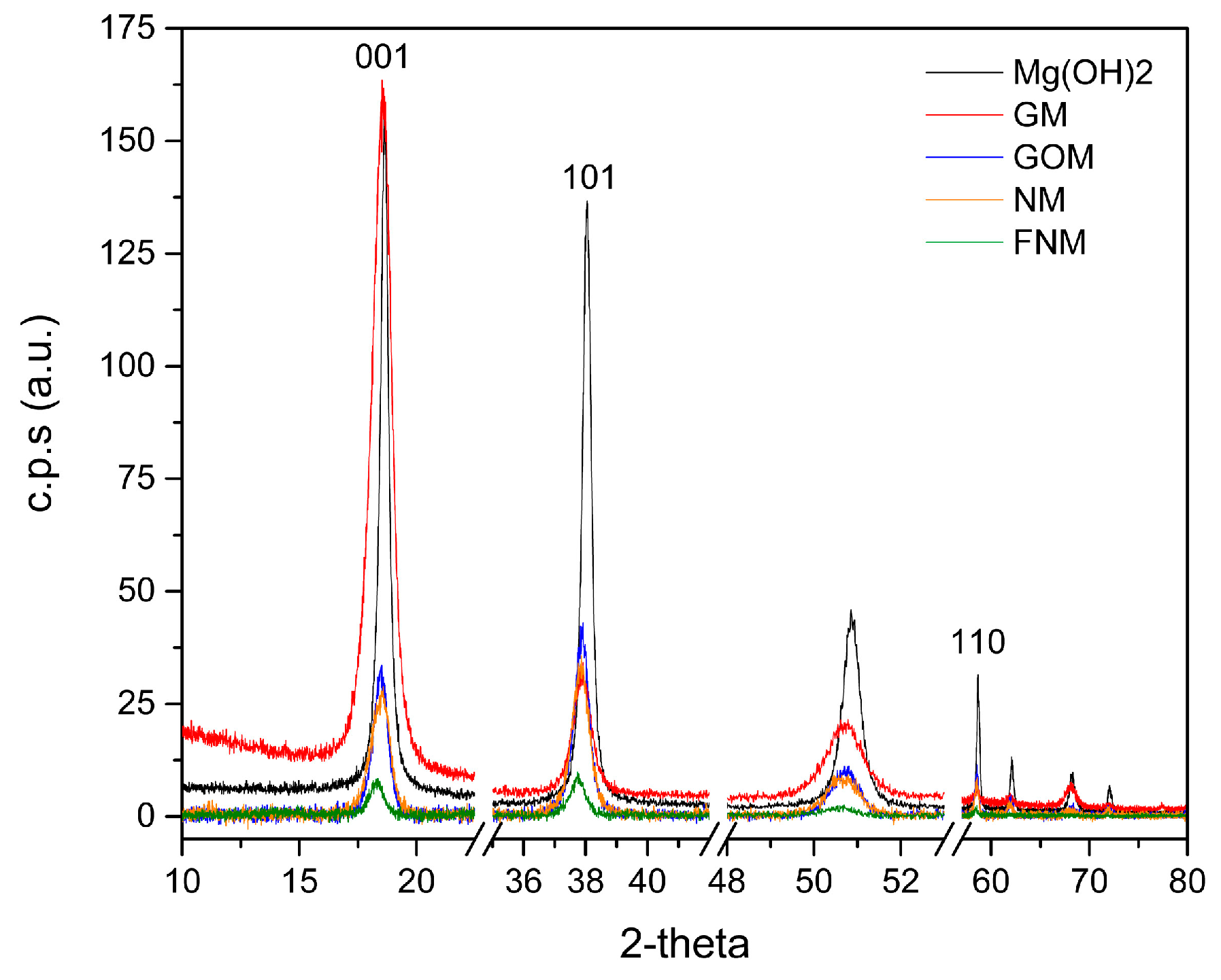
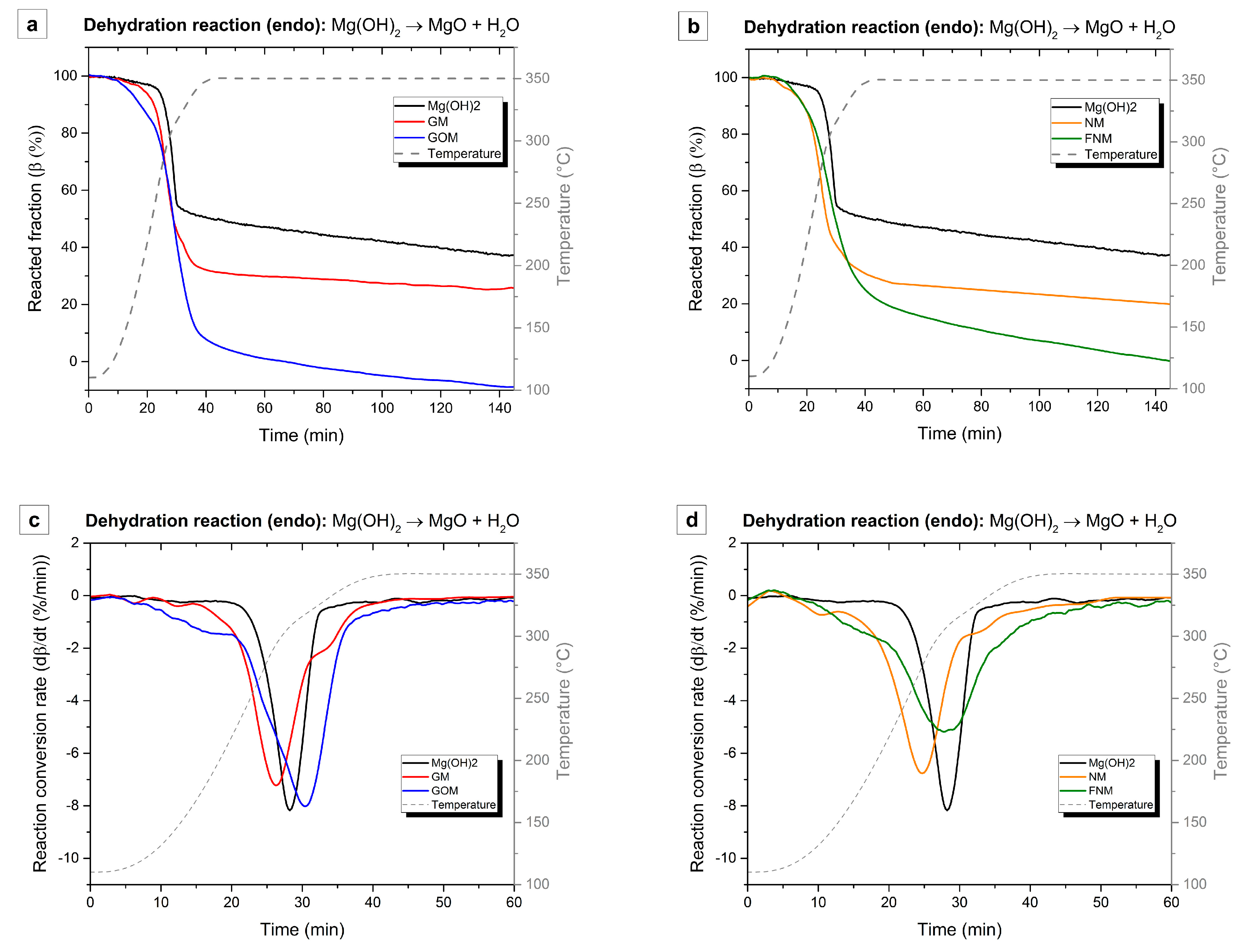
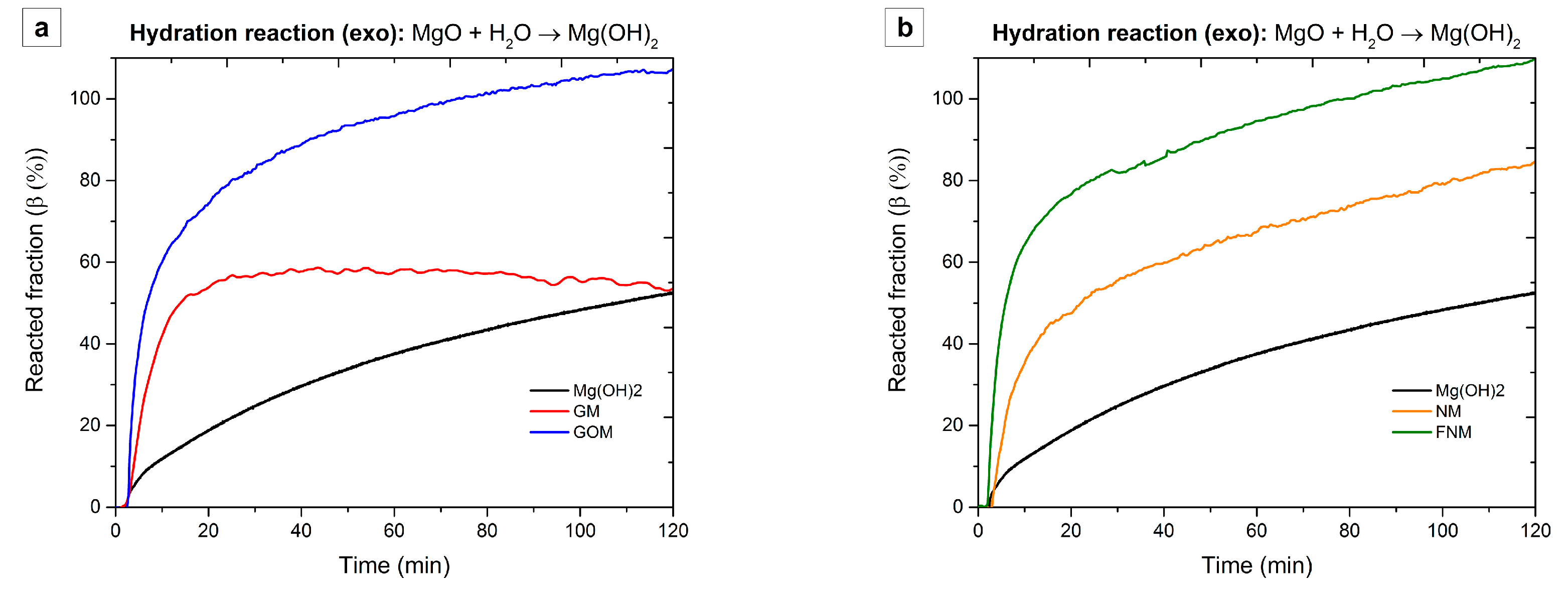
| Sample Code | Carbon Type | Mg(OH)2 Load (WMg(OH)2 (wt %)) | Density (ρ (kg/m3)) |
|---|---|---|---|
| Mg(OH)2 | - | 100 | 350 |
| GM | EG | 33.4 | 150 |
| GOM | GO | 34.7 | 423 |
| NM | CNTs | 35.5 | 207 |
| FNM | F-CNTs | 31.0 | 765 |
| Sample Code | I001/I101 | I001/I110 |
|---|---|---|
| Mg(OH)2 | 1.1 | 5.7 |
| GM | 5.3 | 23.6 |
| GOM | 0.8 | 3.3 |
| NM | 0.8 | 3.3 |
| FNM | 0.9 | 4.4 |
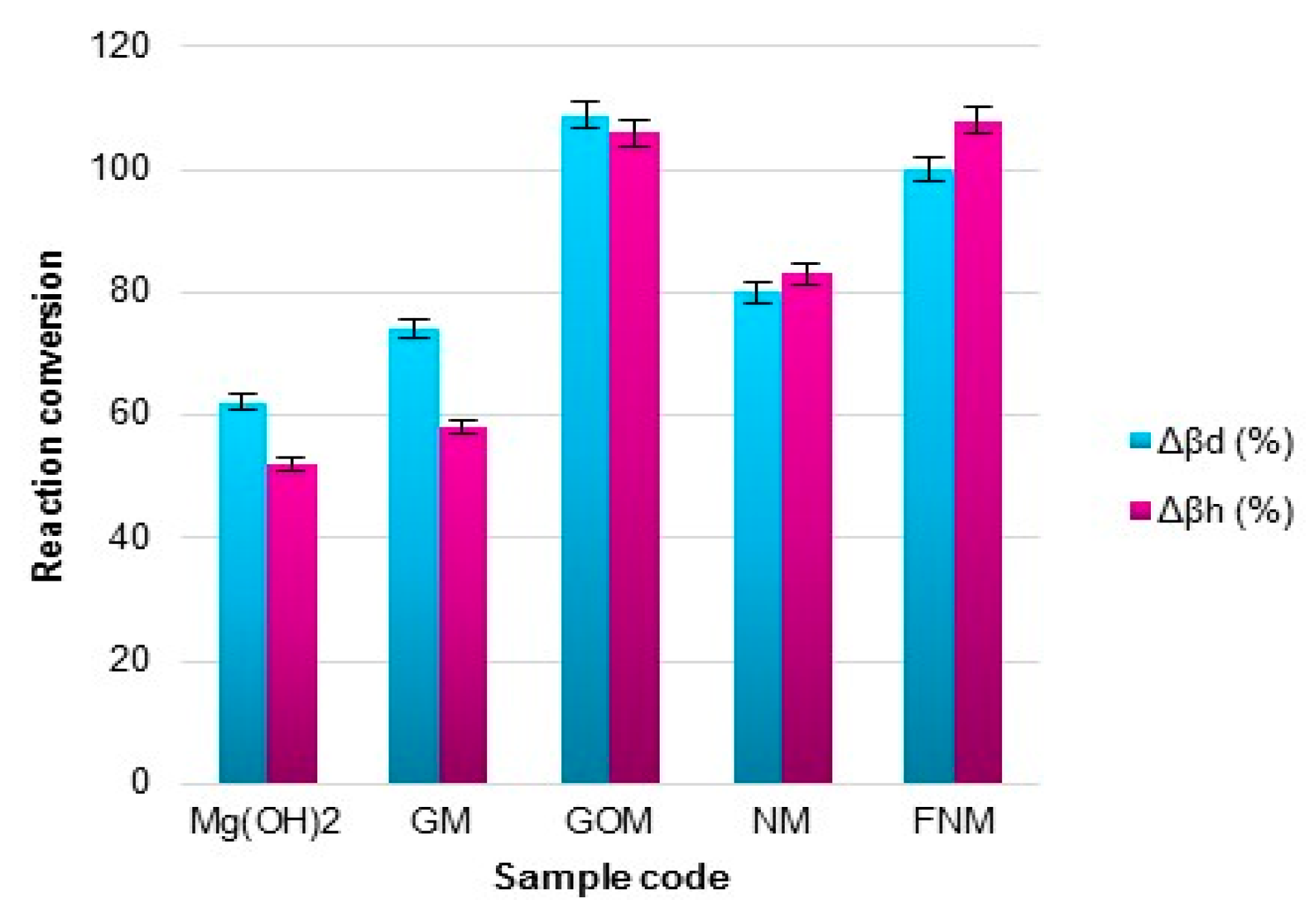
| Sample Code | Tpeak (°C) | dβ/dt (%/min) | Δβd (%) | Δβh (%) |
|---|---|---|---|---|
| Mg(OH)2 | 307 | 8.2 ± 0.2 | 62.1 ± 1.2 | 52.0 ± 1.0 |
| GM | 292 | 7.2 ± 0.1 | 74.2 ± 1.5 | 58.1 ± 1.2 |
| GOM | 318 | 8.0 ± 0.2 | 108.1 ± 2.2 | 106.0 ± 2.1 |
| NM | 282 | 6.7 ± 0.1 | 80.0 ± 1.6 | 83.2 ± 1.7 |
| FNM | 307 | 5.2 ± 0.1 | 100.3 ± 2.0 | 108.1 ± 2.2 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastronardo, E.; Kato, Y.; Bonaccorsi, L.; Piperopoulos, E.; Milone, C. Thermochemical Storage of Middle Temperature Wasted Heat by Functionalized C/Mg(OH)2 Hybrid Materials. Energies 2017, 10, 70. https://doi.org/10.3390/en10010070
Mastronardo E, Kato Y, Bonaccorsi L, Piperopoulos E, Milone C. Thermochemical Storage of Middle Temperature Wasted Heat by Functionalized C/Mg(OH)2 Hybrid Materials. Energies. 2017; 10(1):70. https://doi.org/10.3390/en10010070
Chicago/Turabian StyleMastronardo, Emanuela, Yukitaka Kato, Lucio Bonaccorsi, Elpida Piperopoulos, and Candida Milone. 2017. "Thermochemical Storage of Middle Temperature Wasted Heat by Functionalized C/Mg(OH)2 Hybrid Materials" Energies 10, no. 1: 70. https://doi.org/10.3390/en10010070
APA StyleMastronardo, E., Kato, Y., Bonaccorsi, L., Piperopoulos, E., & Milone, C. (2017). Thermochemical Storage of Middle Temperature Wasted Heat by Functionalized C/Mg(OH)2 Hybrid Materials. Energies, 10(1), 70. https://doi.org/10.3390/en10010070









