Identification of Agents That Ameliorate Hyperphosphatemia-Suppressed Myogenin Expression Involved in the Nrf2/p62 Pathway in C2C12 Skeletal Muscle Cells
Abstract
:1. Introduction
2. Results
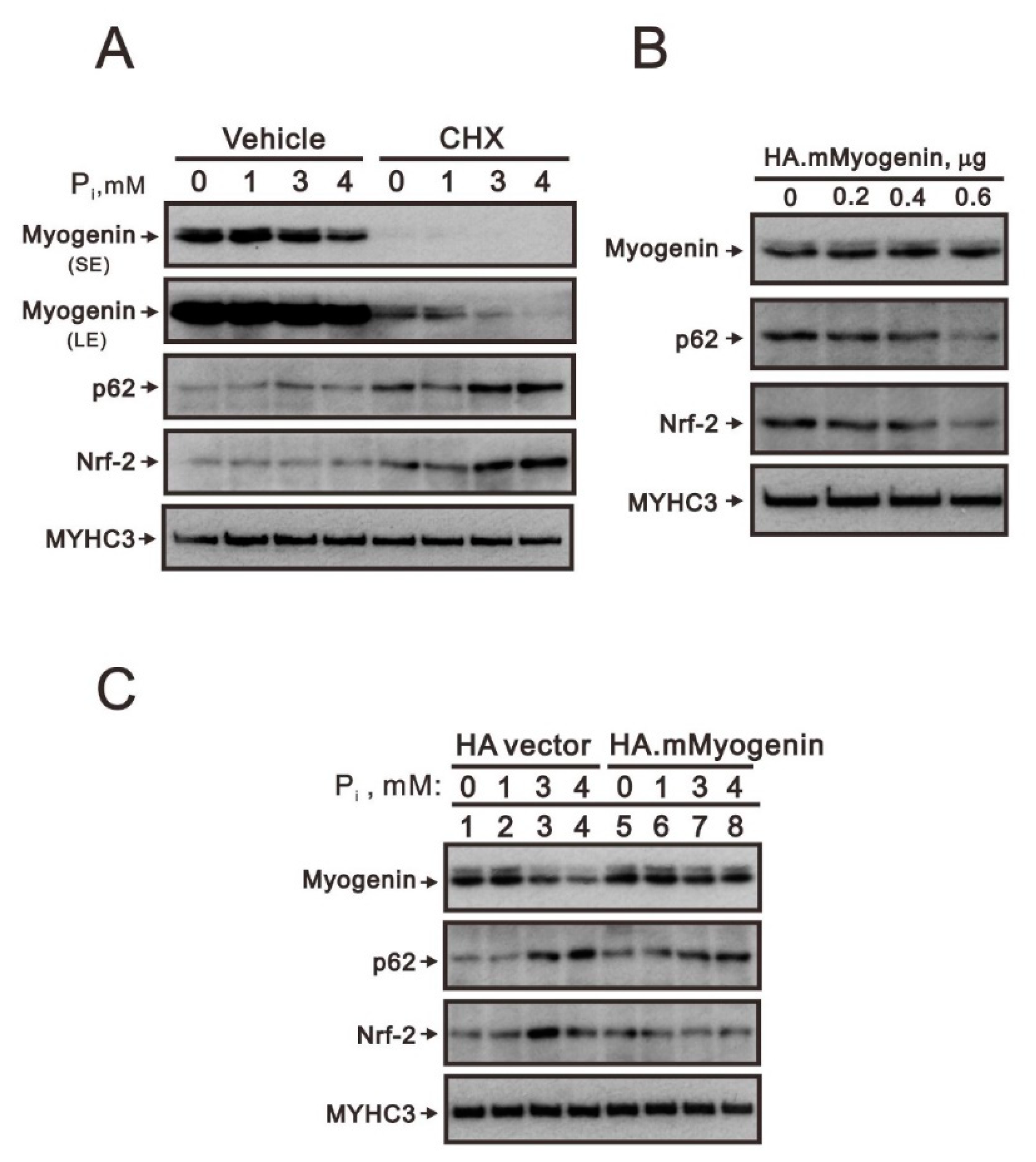
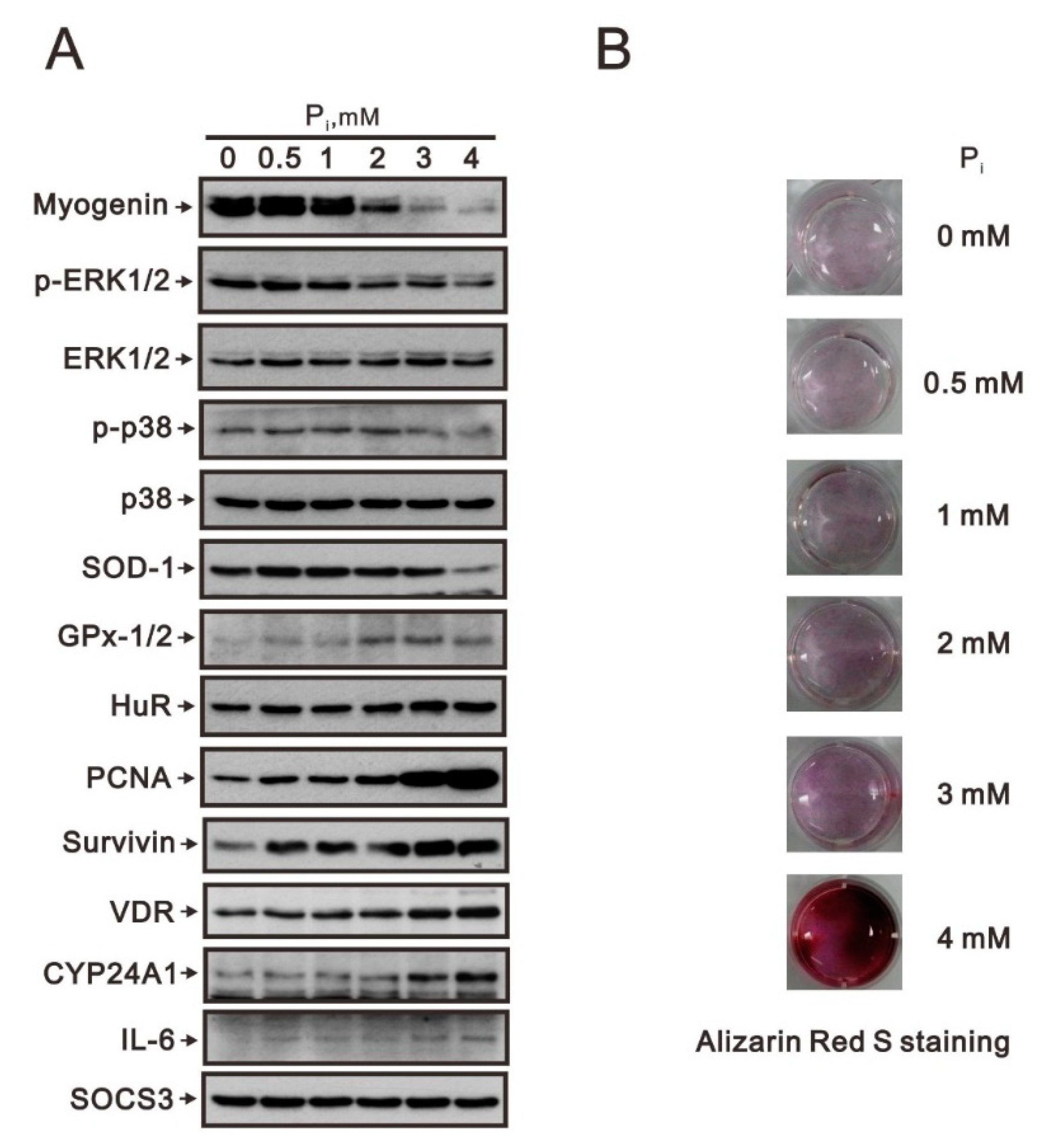
2.1. Crosstalk of Myogenin with the Nrf2/p62 Complex and Other Proteins in High Pi-Treated C2C12 Cells
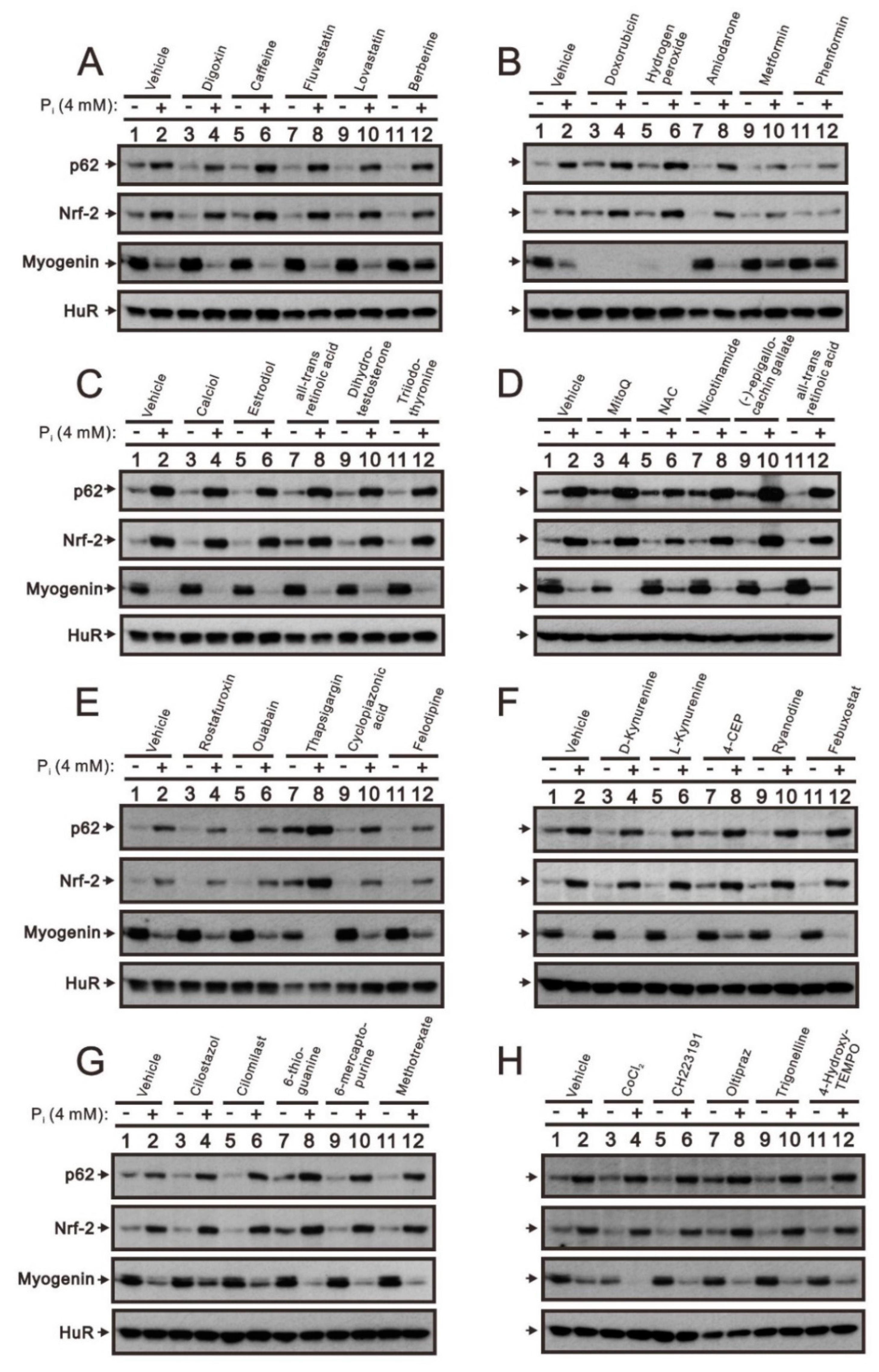
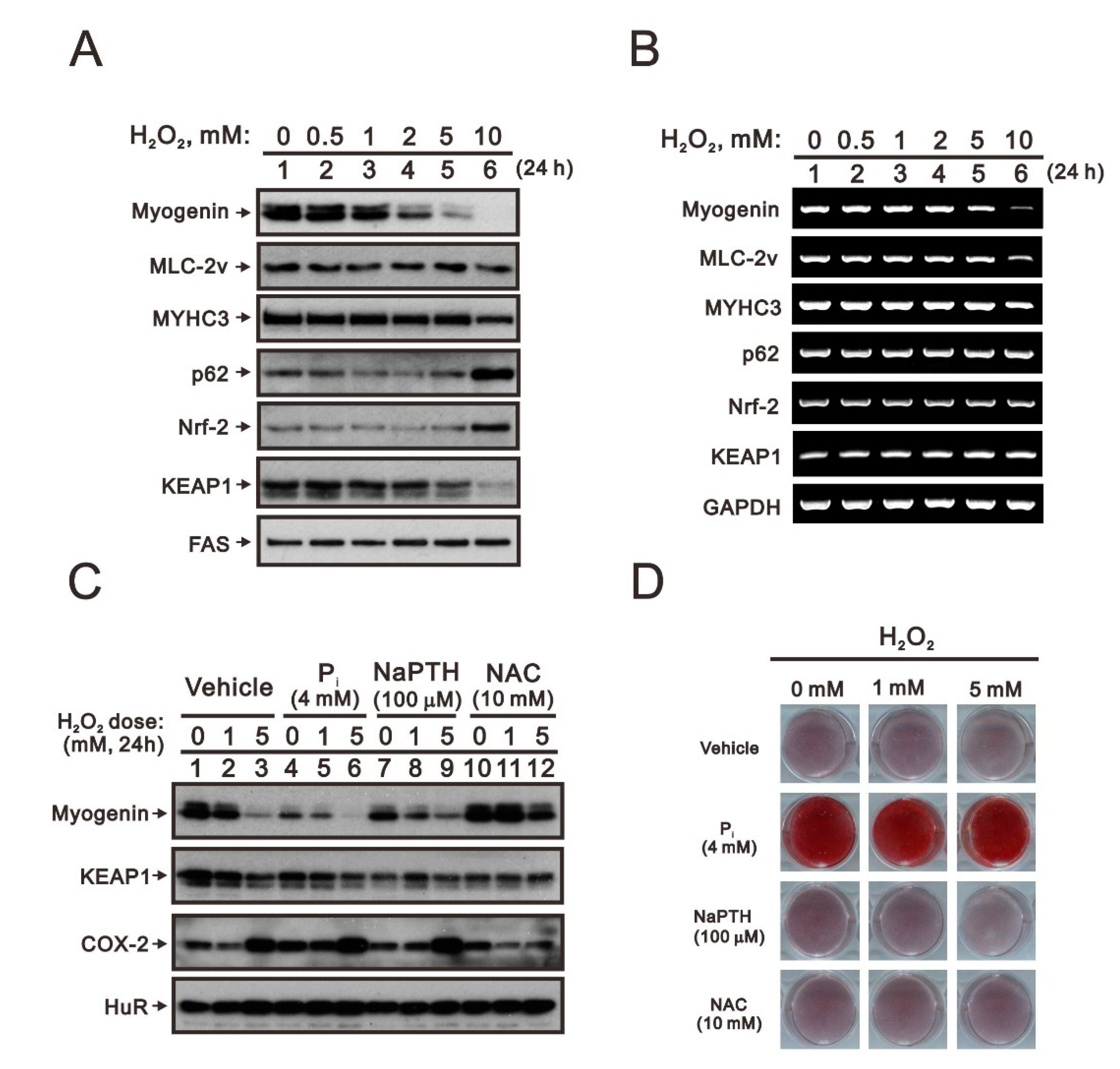
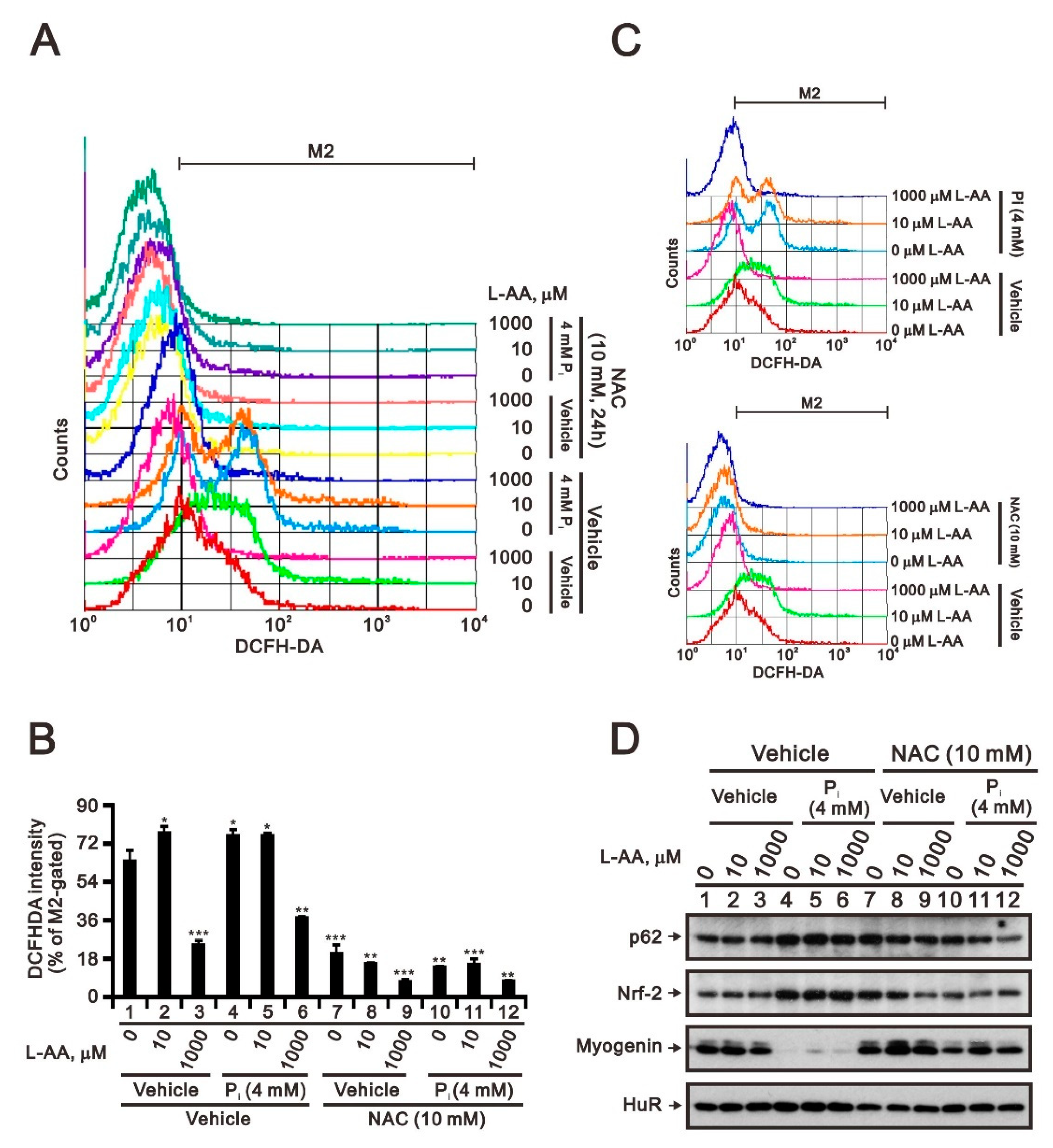
2.2. The Identification of Compounds Involved in the Regulation of Myogenin Expression via a Drug Screening Strategy with the Myogenin/Nrf2/p62 Platform
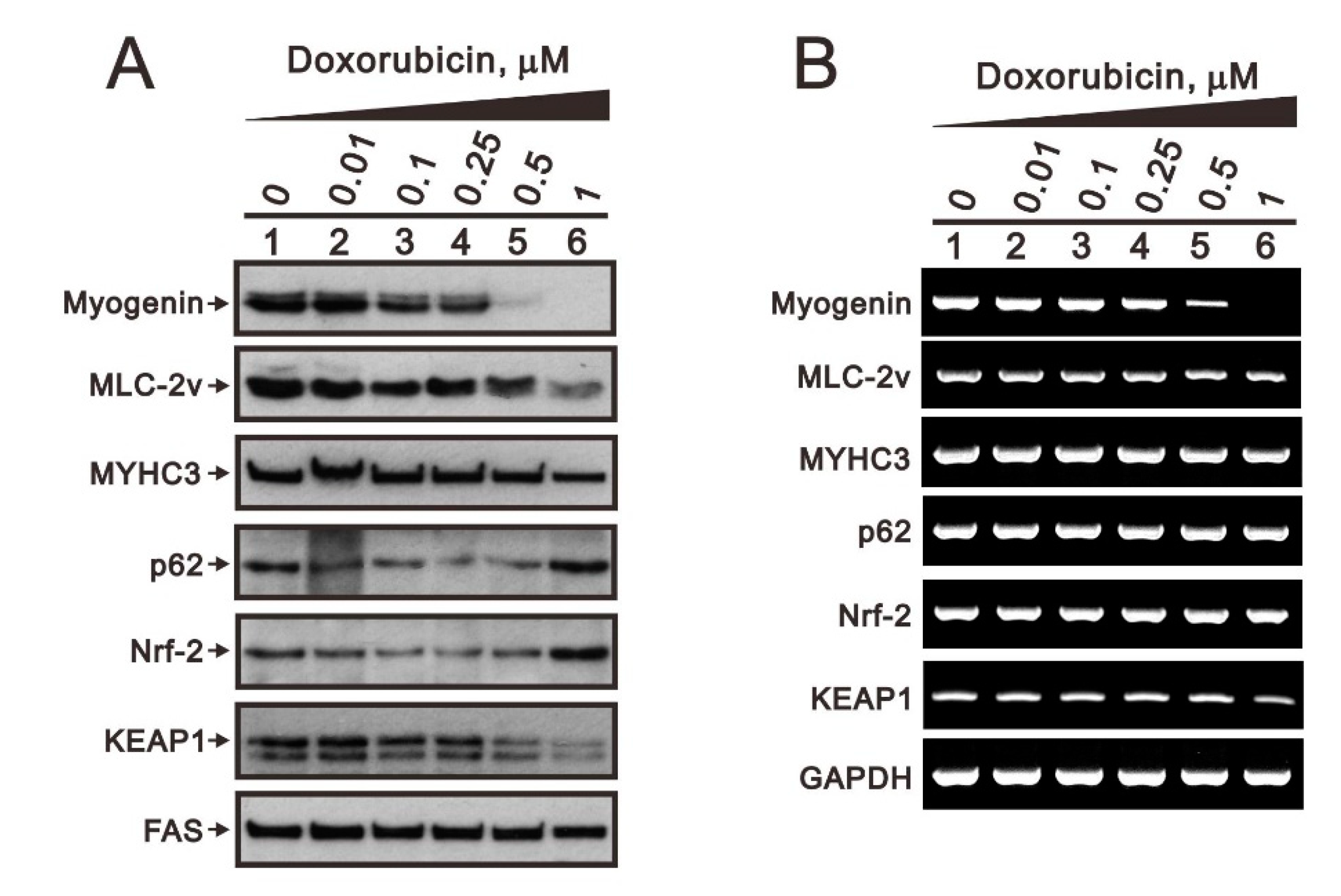
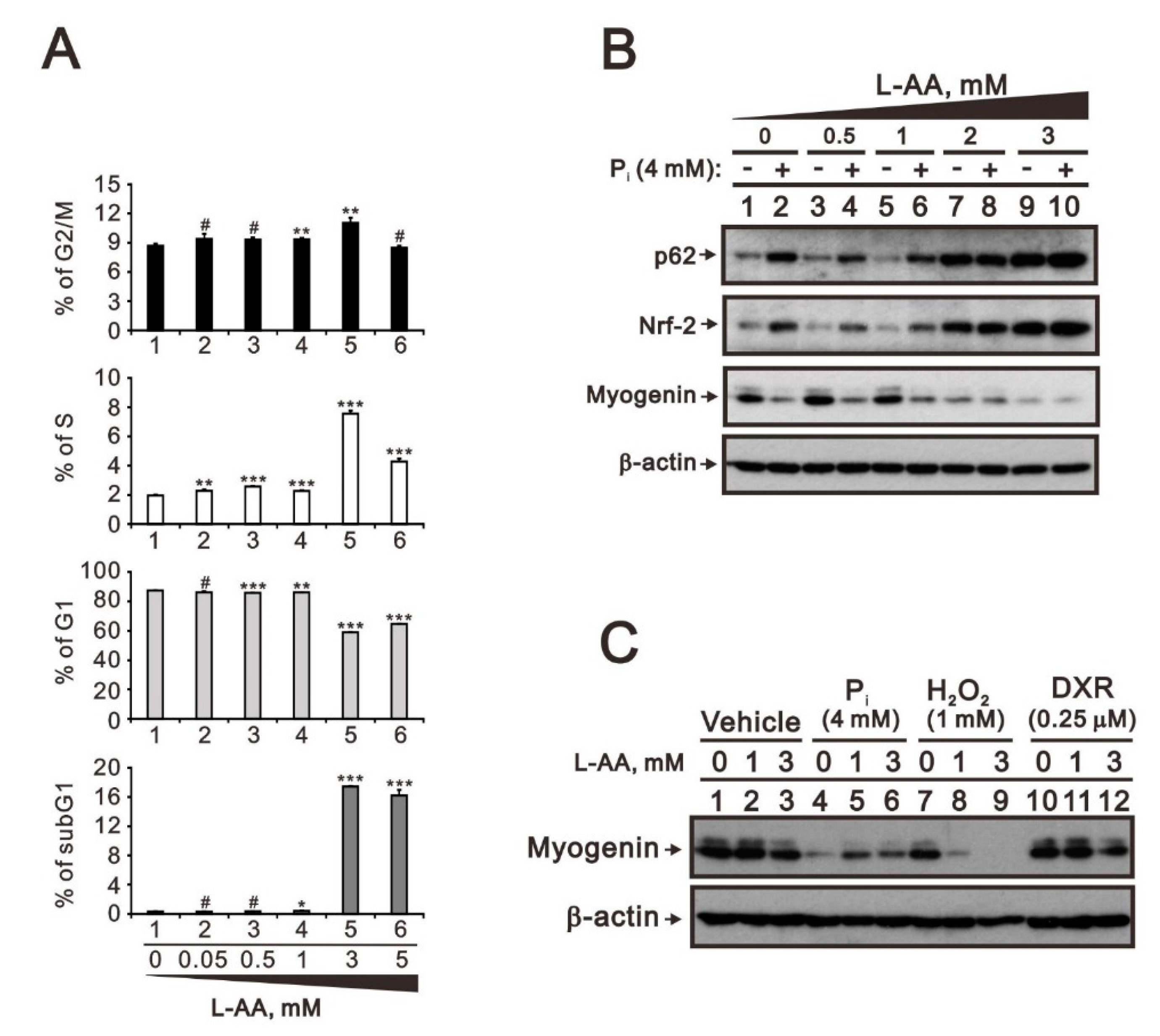
2.3. Comparison of the Effects of Doxorubicin, Hydrogen Peroxide, and L-Ascorbic Acid on the Regulation of Myogenin Expression in C2C12 Cells with High Pi
3. Discussion
4. Materials and Methods
4.1. C2C12 Myotube Culture and Pi Treatment
4.2. Immunoblotting
4.3. Alizarin Red S Staining
4.4. Flow Cytometric Analyses of Cell Cycle Profile and ROS Generation
4.5. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hruska, K.A.; Mathew, S.; Lund, R.; Qiu, P.; Pratt, R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008, 74, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Lederer, E. Regulation of serum phosphate. J. Physiol. 2014, 592, 3985–3995. [Google Scholar] [CrossRef]
- Boengler, K.; Kosiol, M.; Mayr, M.; Schulz, R.; Rohrbach, S. Mitochondria and ageing: Role in heart, skeletal muscle and adipose tissue. J. Cachexia Sarcopenia Muscle 2017, 8, 349–369. [Google Scholar] [CrossRef] [Green Version]
- Deane, C.S.; Ely, I.A.; Wilkinson, D.J.; Smith, K.; Phillips, B.E.; Atherton, P.J. Dietary protein, exercise, ageing and physical inactivity: Interactive influences on skeletal muscle proteostasis. Proc. Nutr. Soc. 2021, 80, 106–117. [Google Scholar] [CrossRef]
- Chung, L.H.; Liu, S.T.; Huang, S.M.; Salter, D.M.; Lee, H.S.; Hsu, Y.J. High phosphate induces skeletal muscle atrophy and suppresses myogenic differentiation by increasing oxidative stress and activating Nrf2 signaling. Aging 2020, 12, 21446–21468. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Topinkova, E.; Michel, J.P. Understanding sarcopenia as a geriatric syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 1–7. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Scicchitano, B.M.; Pelosi, L.; Sica, G.; Musaro, A. The physiopathologic role of oxidative stress in skeletal muscle. Mech. Ageing Dev. 2018, 170, 37–44. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef]
- Rudnicki, M.A.; Jaenisch, R. The MyoD family of transcription factors and skeletal myogenesis. Bioessays 1995, 17, 203–209. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, J.M.; Garcia-Gonzalez, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef]
- Ohkawa, Y.; Marfella, C.G.; Imbalzano, A.N. Skeletal muscle specification by myogenin and Mef2D via the SWI/SNF ATPase Brg1. EMBO J. 2006, 25, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Mastroyiannopoulos, N.P.; Nicolaou, P.; Anayasa, M.; Uney, J.B.; Phylactou, L.A. Down-regulation of myogenin can reverse terminal muscle cell differentiation. PLoS ONE 2012, 7, e29896. [Google Scholar] [CrossRef] [Green Version]
- Buckingham, M.; Tajbakhsh, S. Expression of myogenic factors in the mouse: Myf-5, the first member of the MyoD gene family to be transcribed during skeletal myogenesis. C. R. Acad. Sci. III 1993, 316, 1032–1046. [Google Scholar]
- Valdez, M.R.; Richardson, J.A.; Klein, W.H.; Olson, E.N. Failure of Myf5 to support myogenic differentiation without myogenin, MyoD, and MRF4. Dev. Biol. 2000, 219, 287–298. [Google Scholar] [CrossRef]
- Denes, L.T.; Riley, L.A.; Mijares, J.R.; Arboleda, J.D.; McKee, K.; Esser, K.A.; Wang, E.T. Culturing C2C12 myotubes on micromolded gelatin hydrogels accelerates myotube maturation. Skelet Muscle 2019, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Katagiri, T.; Yamaguchi, A.; Komaki, M.; Abe, E.; Takahashi, N.; Ikeda, T.; Rosen, V.; Wozney, J.M.; Fujisawa-Sehara, A.; Suda, T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 1994, 127, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.Y.; Kim, T.S.; Ahn, J.H.; Yoon, J.U.; Kim, H.J.; Kim, E.J. Remifentanil promotes osteoblastogenesis by upregulating Runx2/osterix expression in preosteoblastic C2C12 cells. J. Dent. Anesth. Pain Med. 2019, 19, 91–99. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K. Non-invasive biomarkers of oxidative stress: Reproducibility and methodological issues. Redox Rep. 2004, 9, 125–143. [Google Scholar] [CrossRef]
- Kazmierczak-Baranska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Wu, T.M.; Liu, S.T.; Chen, S.Y.; Chen, G.S.; Wu, C.C.; Huang, S.M. Mechanisms and Applications of the Anti-cancer Effect of Pharmacological Ascorbic Acid in Cervical Cancer Cells. Front. Oncol. 2020, 10, 1483. [Google Scholar] [CrossRef]
- Volkova, M.; Russell, R., 3rd. Anthracycline cardiotoxicity: Prevalence, pathogenesis and treatment. Curr. Cardiol. Rev. 2011, 7, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Wenningmann, N.; Knapp, M.; Ande, A.; Vaidya, T.R.; Ait-Oudhia, S. Insights into Doxorubicin-induced Cardiotoxicity: Molecular Mechanisms, Preventive Strategies, and Early Monitoring. Mol. Pharmacol. 2019, 96, 219–232. [Google Scholar] [CrossRef]
- Valiyil, R.; Christopher-Stine, L. Drug-related myopathies of which the clinician should be aware. Curr. Rheumatol. Rep. 2010, 12, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Walton, R.G.; Dungan, C.M.; Long, D.E.; Tuggle, S.C.; Kosmac, K.; Peck, B.D.; Bush, H.M.; Villasante Tezanos, A.G.; McGwin, G.; Windham, S.T.; et al. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: A randomized, double-blind, placebo-controlled, multicenter trial: The MASTERS trial. Aging Cell 2019, 18, e13039. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.J.; Moon, J.W.; Lee, J.O.; Kim, J.H.; Jung, E.J.; Kim, S.J.; Oh, J.Y.; Wu, S.W.; Lee, P.R.; Park, S.H.; et al. Metformin induces muscle atrophy by transcriptional regulation of myostatin via HDAC6 and FoxO3a. J. Cachexia Sarcopenia Muscle 2022, 13, 605–620. [Google Scholar] [CrossRef]
- Miller, R.A.; Birnbaum, M.J. An energetic tale of AMPK-independent effects of metformin. J. Clin. Investig. 2010, 120, 2267–2270. [Google Scholar] [CrossRef]
- Espinoza, S.E.; Musi, N.; Wang, C.P.; Michalek, J.; Orsak, B.; Romo, T.; Powers, B.; Conde, A.; Moris, M.; Bair-Kelps, D.; et al. Rationale and Study Design of a Randomized Clinical Trial of Metformin to Prevent Frailty in Older Adults With Prediabetes. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Halfon, M.; Phan, O.; Teta, D. Vitamin D: A review on its effects on muscle strength, the risk of fall, and frailty. BioMed Res. Int. 2015, 2015, 953241. [Google Scholar] [CrossRef] [Green Version]
- Vaes, A.M.M.; Brouwer-Brolsma, E.M.; Toussaint, N.; de Regt, M.; Tieland, M.; van Loon, L.J.C.; de Groot, L. The association between 25-hydroxyvitamin D concentration, physical performance and frailty status in older adults. Eur. J. Nutr. 2019, 58, 1173–1181. [Google Scholar] [CrossRef] [Green Version]
- Rejnmark, L. Effects of vitamin d on muscle function and performance: A review of evidence from randomized controlled trials. Ther. Adv. Chronic Dis. 2011, 2, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Schneider, J.W.; Gu, W.; Zhu, L.; Mahdavi, V.; Nadal-Ginard, B. Reversal of terminal differentiation mediated by p107 in Rb-/- muscle cells. Science 1994, 264, 1467–1471. [Google Scholar] [CrossRef]
- Tada, H.; Nemoto, E.; Foster, B.L.; Somerman, M.J.; Shimauchi, H. Phosphate increases bone morphogenetic protein-2 expression through cAMP-dependent protein kinase and ERK1/2 pathways in human dental pulp cells. Bone 2011, 48, 1409–1416. [Google Scholar] [CrossRef]
- Gallea, S.; Lallemand, F.; Atfi, A.; Rawadi, G.; Ramez, V.; Spinella-Jaegle, S.; Kawai, S.; Faucheu, C.; Huet, L.; Baron, R.; et al. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone 2001, 28, 491–498. [Google Scholar] [CrossRef]
- El Halabi, I.; Bejjany, R.; Nasr, R.; Mukherji, D.; Temraz, S.; Nassar, F.J.; El Darsa, H.; Shamseddine, A. Ascorbic Acid in Colon Cancer: From the Basic to the Clinical Applications. Int. J. Mol. Sci. 2018, 19, 2752. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Venturelli, S.; Sinnberg, T.W.; Niessner, H.; Busch, C. Molecular mechanisms of pharmacological doses of ascorbate on cancer cells. Wien. Med. Wochenschr. 2015, 165, 251–257. [Google Scholar] [CrossRef]
- Suh, J.; Zhu, B.Z.; Frei, B. Ascorbate does not act as a pro-oxidant towards lipids and proteins in human plasma exposed to redox-active transition metal ions and hydrogen peroxide. Free Radic. Biol. Med. 2003, 34, 1306–1314. [Google Scholar]
- Lane, D.J.; Richardson, D.R. The active role of vitamin C in mammalian iron metabolism: Much more than just enhanced iron absorption! Free Radic. Biol. Med. 2014, 75, 69–83. [Google Scholar] [CrossRef]
- Lane, D.J.; Chikhani, S.; Richardson, V.; Richardson, D.R. Transferrin iron uptake is stimulated by ascorbate via an intracellular reductive mechanism. Biochim. Biophys. Acta 2013, 1833, 1527–1541. [Google Scholar] [CrossRef] [Green Version]
- Bakalova, R.; Zhelev, Z.; Miller, T.; Aoki, I.; Higashi, T. New potential biomarker for stratification of patients for pharmacological vitamin C in adjuvant settings of cancer therapy. Redox Biol. 2020, 28, 101357. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef]
- Sinnberg, T.; Noor, S.; Venturelli, S.; Berger, A.; Schuler, P.; Garbe, C.; Busch, C. The ROS-induced cytotoxicity of ascorbate is attenuated by hypoxia and HIF-1alpha in the NCI60 cancer cell lines. J. Cell Mol. Med. 2014, 18, 530–541. [Google Scholar] [CrossRef]
- Shenoy, N.; Bhagat, T.D.; Cheville, J.; Lohse, C.; Bhattacharyya, S.; Tischer, A.; Machha, V.; Gordon-Mitchell, S.; Choudhary, G.; Wong, L.F.; et al. Ascorbic acid-induced TET activation mitigates adverse hydroxymethylcytosine loss in renal cell carcinoma. J. Clin. Investig. 2019, 130, 1612–1625. [Google Scholar] [CrossRef] [Green Version]
- Kuiper, C.; Vissers, M.C. Ascorbate as a co-factor for fe- and 2-oxoglutarate dependent dioxygenases: Physiological activity in tumor growth and progression. Front. Oncol. 2014, 4, 359. [Google Scholar] [CrossRef]
| Santa Cruz Biotechnoglog (Santa Cruz, CA, USA) | ||
|---|---|---|
| MYHC3 | sc-376157 | 1:2000 |
| SOD-1 | sc-101523 | 1:1000 |
| GPx-1/2 | sc-133160 | 1:1000 |
| beta-actin | sc-47778 | 1:10,000 |
| HuR | sc-5261 | 1:2000 |
| Nrf-2 | sc-365949 | 1:1000 |
| p62 | sc-28359 | 1:1000 |
| PCNA | sc-25280 | 1:2000 |
| VDR | sc-13133 | 1:1000 |
| CYP24A1 | sc-365700 | 1:1000 |
| SOCS3 | sc-51699 | 1:1000 |
| FAS | sc-55580 | 1:1000 |
| COX-2 | sc-1745 | 1:1000 |
| Abcam (Trumpington, Cambridg, UK) | ||
| Myogenin | ab124800 | 1:2000 |
| Survivin | ab76424 | 1:1000 |
| Cell signaling Biotechnology (Topsfield, MA, USA) | ||
| p-ERK1/2 | #4370 | 1:1000 |
| ERK1/2 | #4695 | 1:1000 |
| p-p38 | #9212 | 1:1000 |
| p38 | #9211 | 1:1000 |
| IL-6 | #12153 | 1:1000 |
| MLC2v | #8505 | 1:1000 |
| Proteintech Group (Rosemont, IL, USA) | ||
| Keap1 | 60027-I-Ig | 1:1000 |
| Gene Name | Sequence (5′→3′) |
|---|---|
| Myogenin | Forward: 5′-CTACCTTCCTGTCCACCTTC-3′ |
| Reverse: 5′-CTCCAGTGCATTGCCCCACT-3′ | |
| MYHC3 | Forward: 5′-GCCTCATCCACACCAAGAAGA-3′ |
| Reverse: 5′-TCCACCAGATCCTGCAATCTC-3′ | |
| MLC-2v | Forward: 5′-CTCCAACGTGTTCTCCATG-3′ |
| Reverse: 5′-AGTCCTTCTCTTCTCCGTGGG-3′ | |
| p62 | Forward: 5′-GTGATGAGGAGCTGACAATGG-3′ |
| Reverse: 5′-TGGAGCAGAAGCTGACTCAG-3′ | |
| Nrf-2 | Forward: 5′-GAGAATTCCTCCCAATTCAGC-3′ |
| Reverse: 5′-ACCATGAAGGAAATGTGGACC-3′ | |
| KEAP1 | Forward: 5′-CACACTAGAGGATCACACCAAG-3′ |
| Reverse: 5′-GCTGGTGCAGTTCAGTGCAG-3′ | |
| GAPDH | Forward: 5′-CTTCATTGACCTCAACTAC-3′ |
| Reverse: 5′-GCCATCCACAGTCTTCTG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh Li, S.-M.; Liu, S.-T.; Chang, Y.-L.; Chen, G.-S.; Huang, S.-M. Identification of Agents That Ameliorate Hyperphosphatemia-Suppressed Myogenin Expression Involved in the Nrf2/p62 Pathway in C2C12 Skeletal Muscle Cells. Int. J. Mol. Sci. 2022, 23, 15324. https://doi.org/10.3390/ijms232315324
Hsieh Li S-M, Liu S-T, Chang Y-L, Chen G-S, Huang S-M. Identification of Agents That Ameliorate Hyperphosphatemia-Suppressed Myogenin Expression Involved in the Nrf2/p62 Pathway in C2C12 Skeletal Muscle Cells. International Journal of Molecular Sciences. 2022; 23(23):15324. https://doi.org/10.3390/ijms232315324
Chicago/Turabian StyleHsieh Li, Shu-Man, Shu-Ting Liu, Yung-Lung Chang, Gunng-Shinng Chen, and Shih-Ming Huang. 2022. "Identification of Agents That Ameliorate Hyperphosphatemia-Suppressed Myogenin Expression Involved in the Nrf2/p62 Pathway in C2C12 Skeletal Muscle Cells" International Journal of Molecular Sciences 23, no. 23: 15324. https://doi.org/10.3390/ijms232315324
APA StyleHsieh Li, S.-M., Liu, S.-T., Chang, Y.-L., Chen, G.-S., & Huang, S.-M. (2022). Identification of Agents That Ameliorate Hyperphosphatemia-Suppressed Myogenin Expression Involved in the Nrf2/p62 Pathway in C2C12 Skeletal Muscle Cells. International Journal of Molecular Sciences, 23(23), 15324. https://doi.org/10.3390/ijms232315324







