Agro-Morphological, Yield and Biochemical Responses of Selected Wheat (Triticum aestivum L.) Genotypes to Salt Stress
Abstract
:1. Introduction
2. Results
2.1. Agro-Morphology and Yield of Wheat
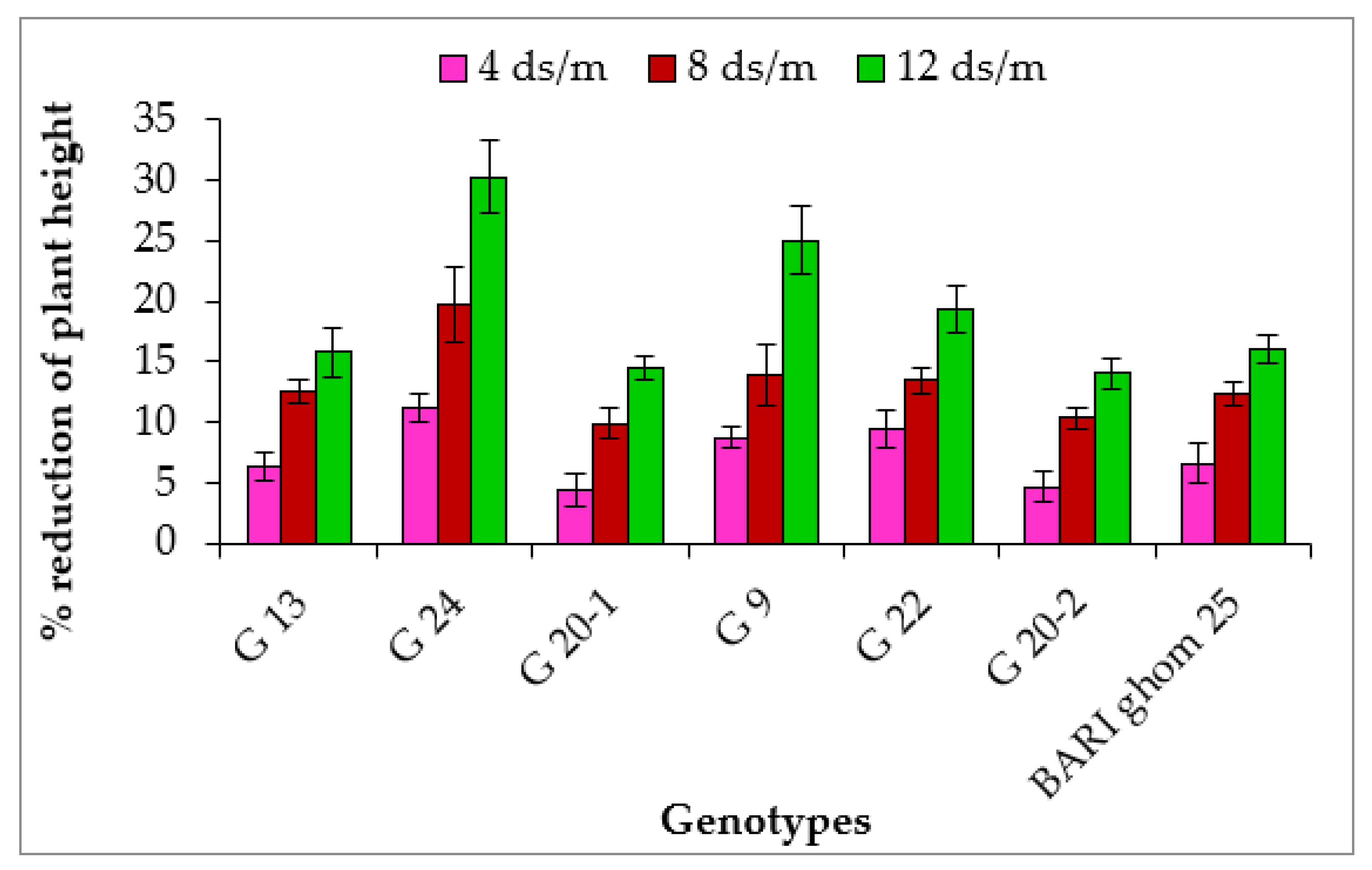
2.1.1. Plant Height
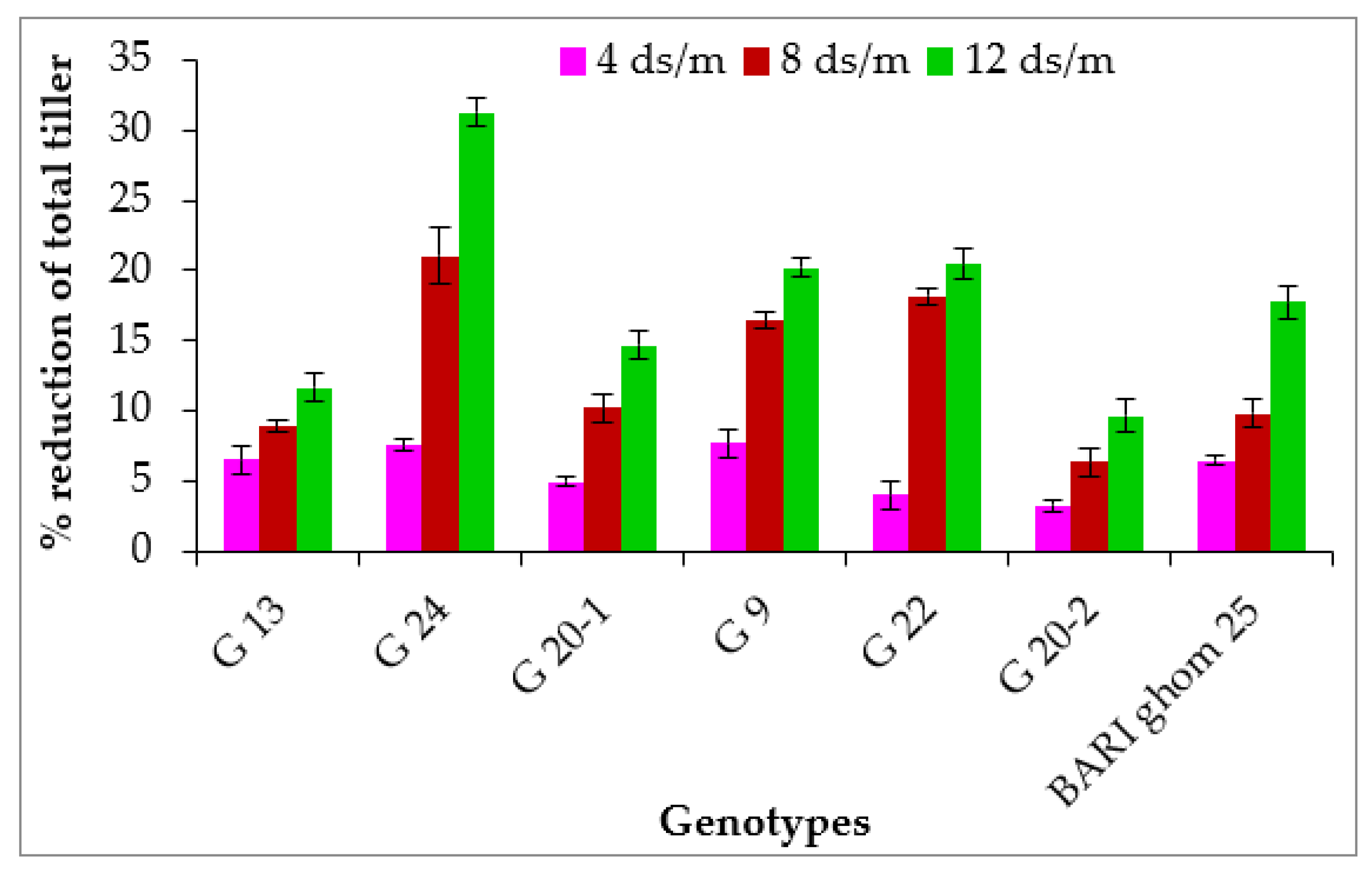
2.1.2. Total Tillers per Plant
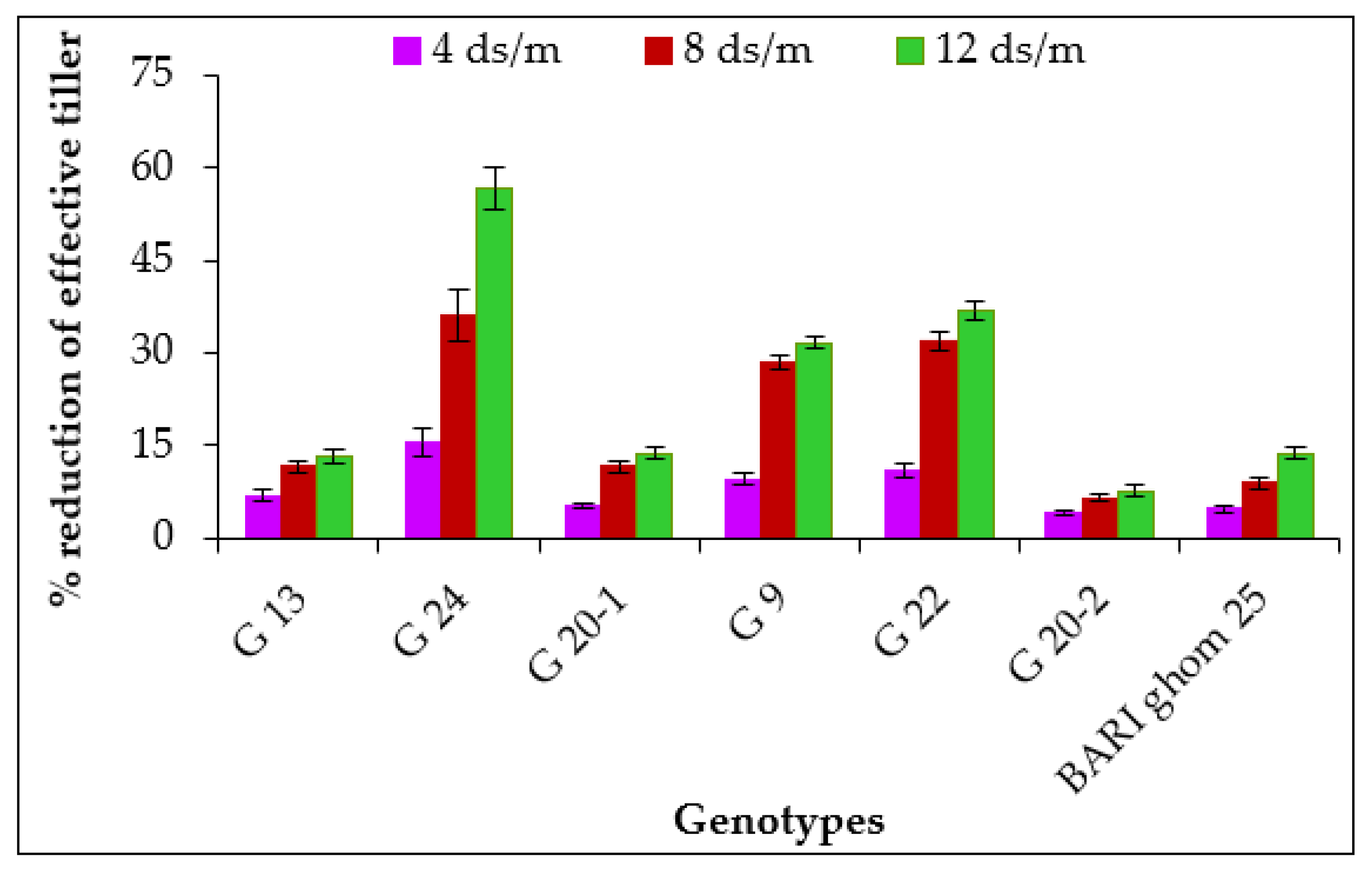
2.1.3. Effective Tillers per Plant
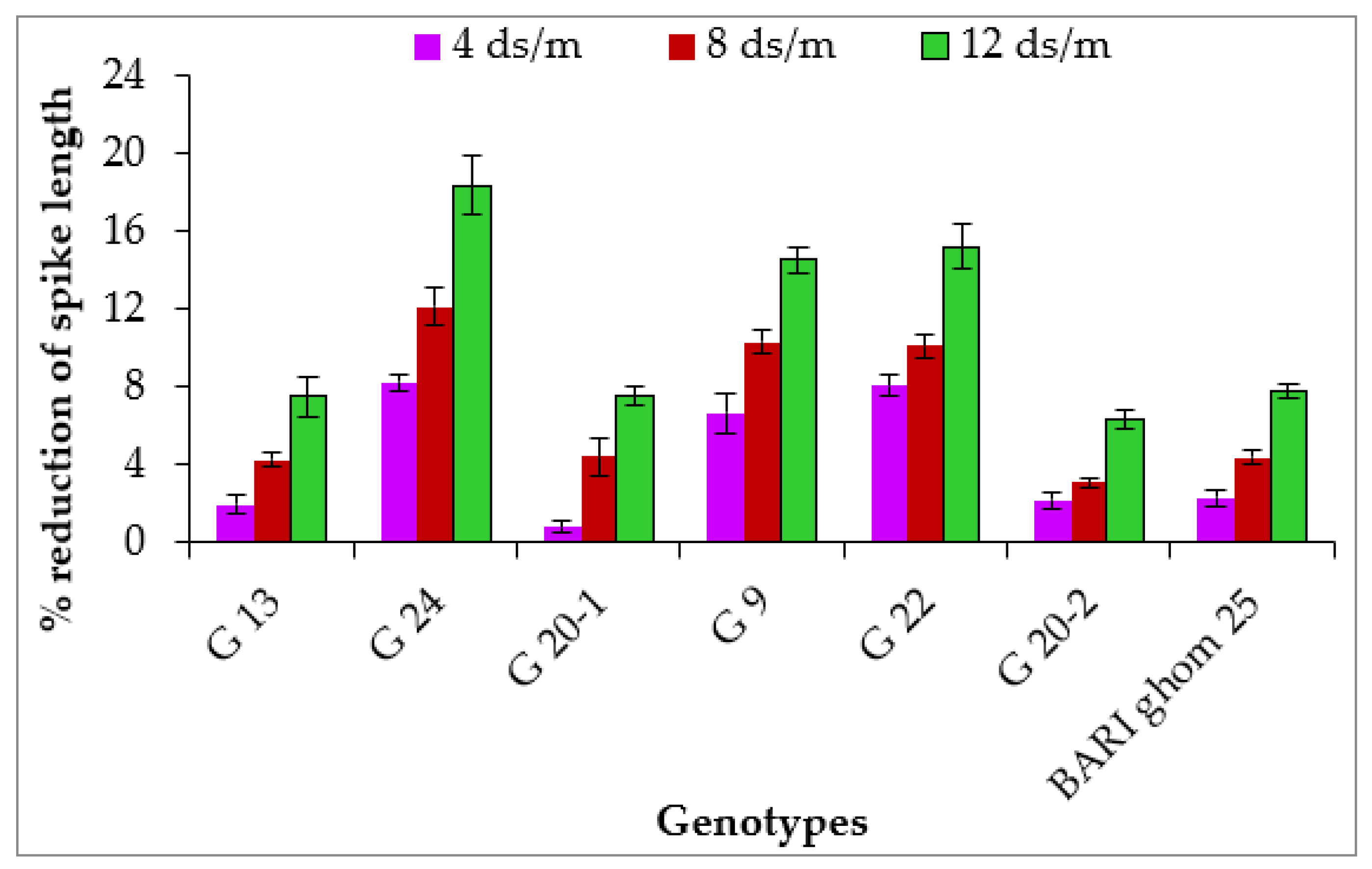
2.1.4. Spike Length
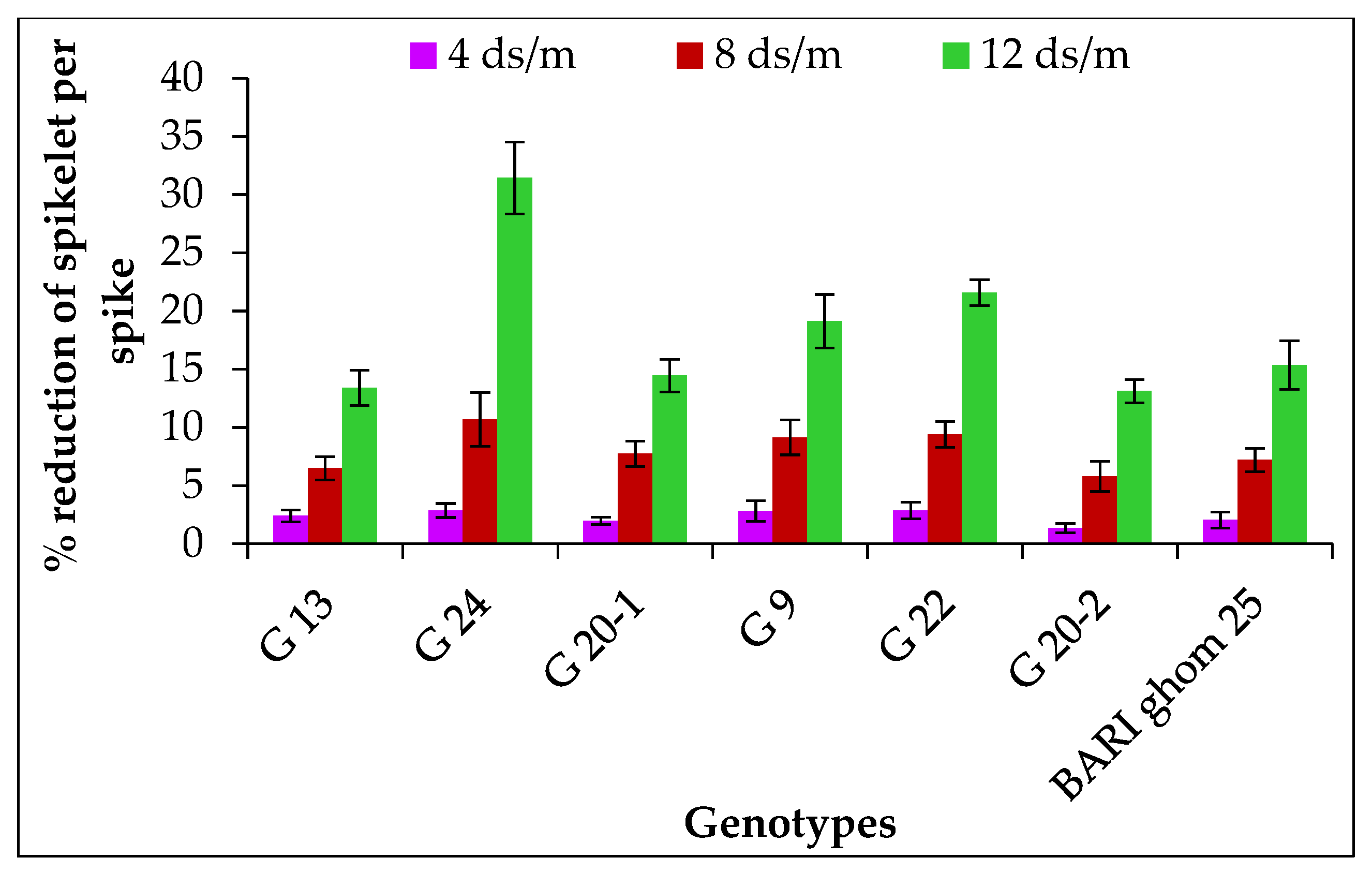
2.1.5. Spikelet Number per Spike
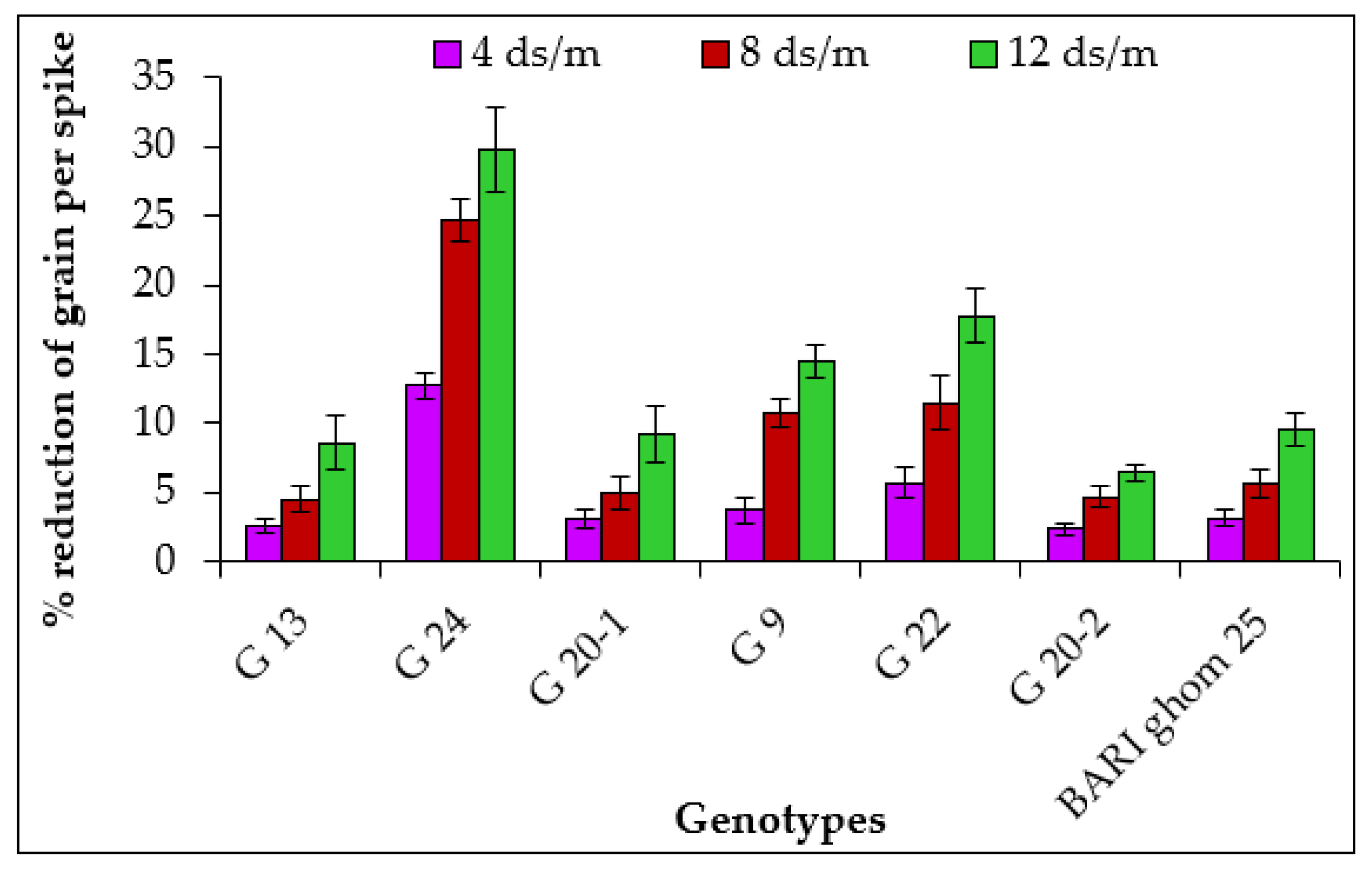
2.1.6. Grains per Spike
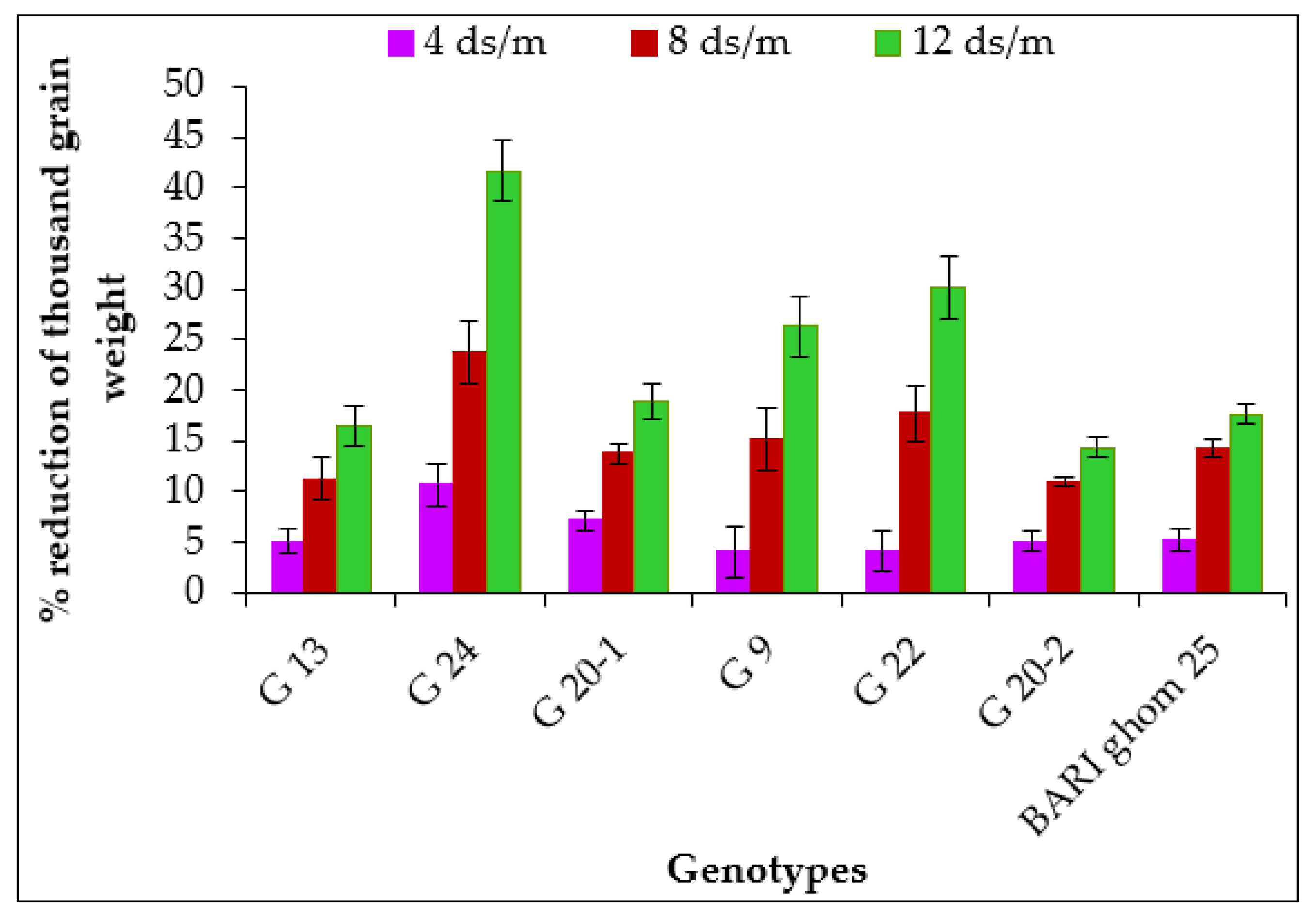
2.1.7. Thousand Grain Weight
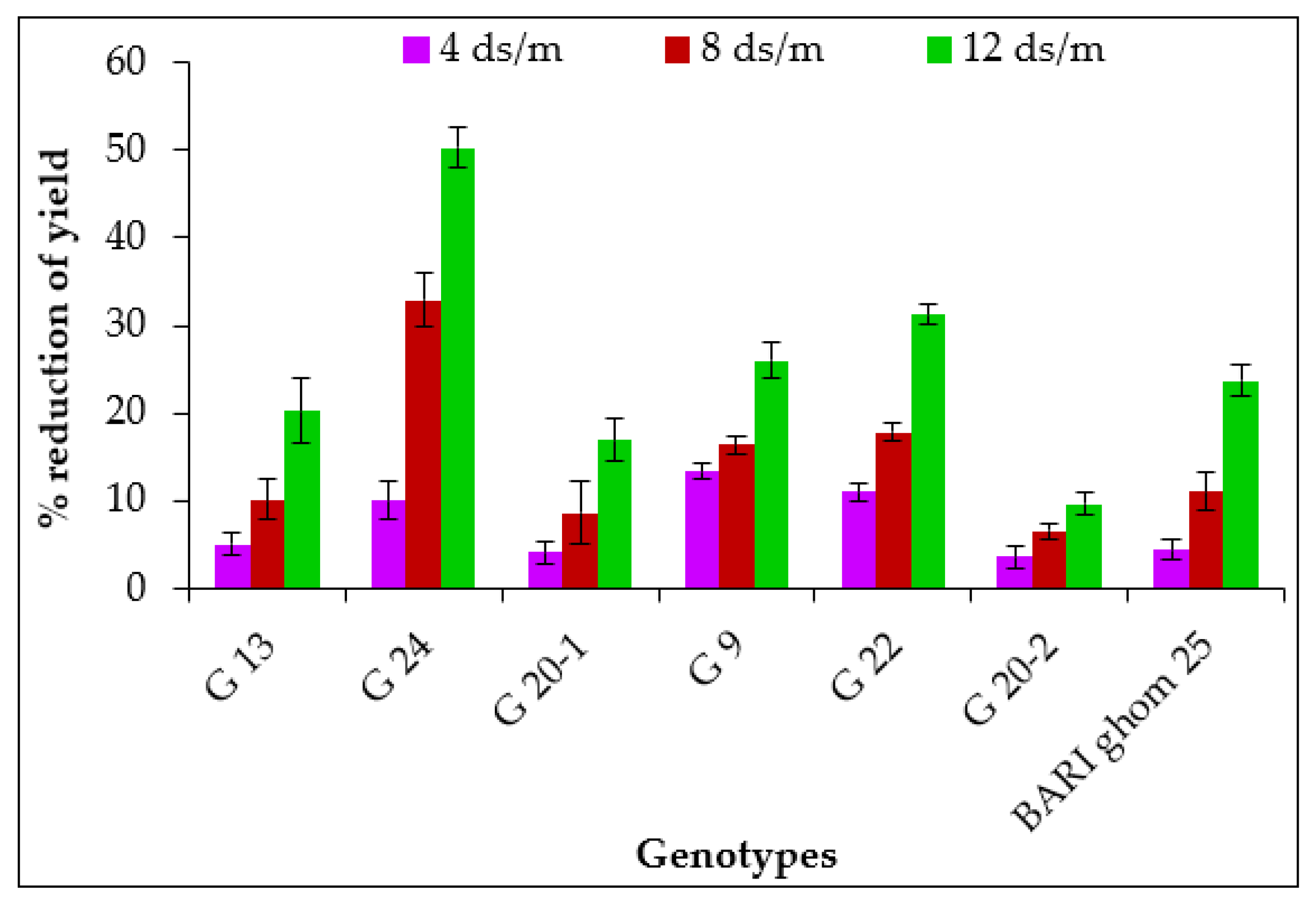
2.1.8. Grain Yield
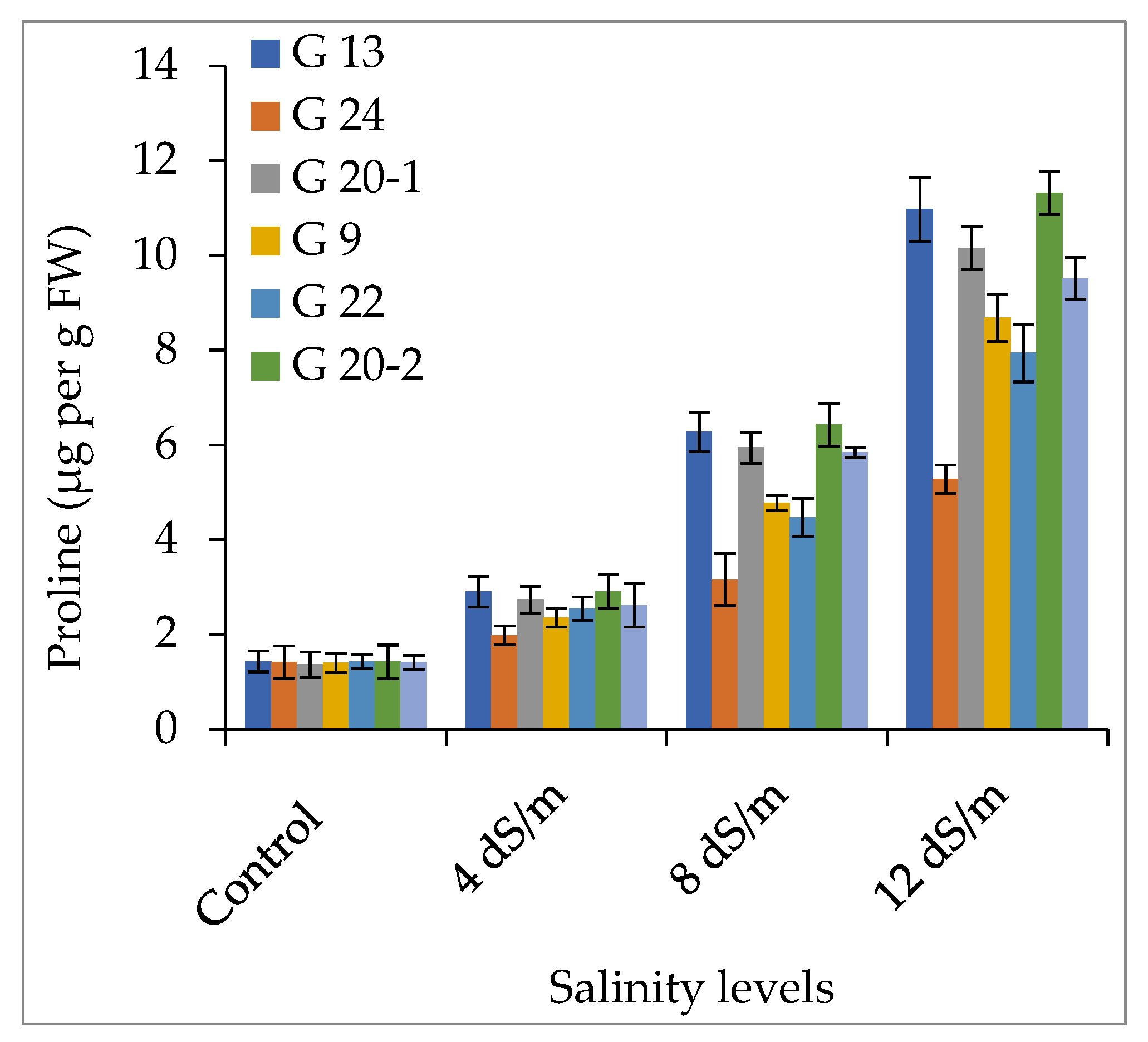
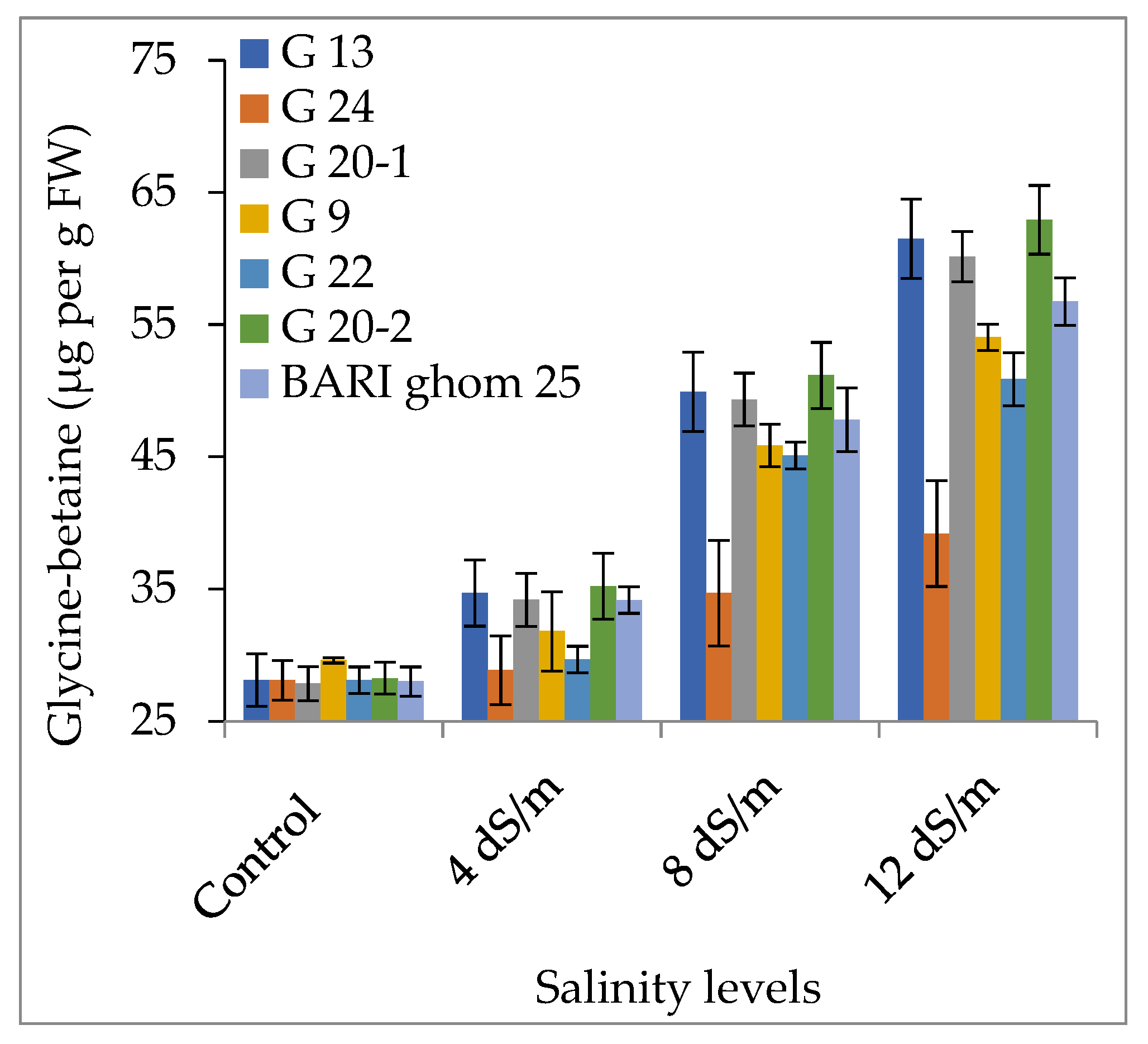
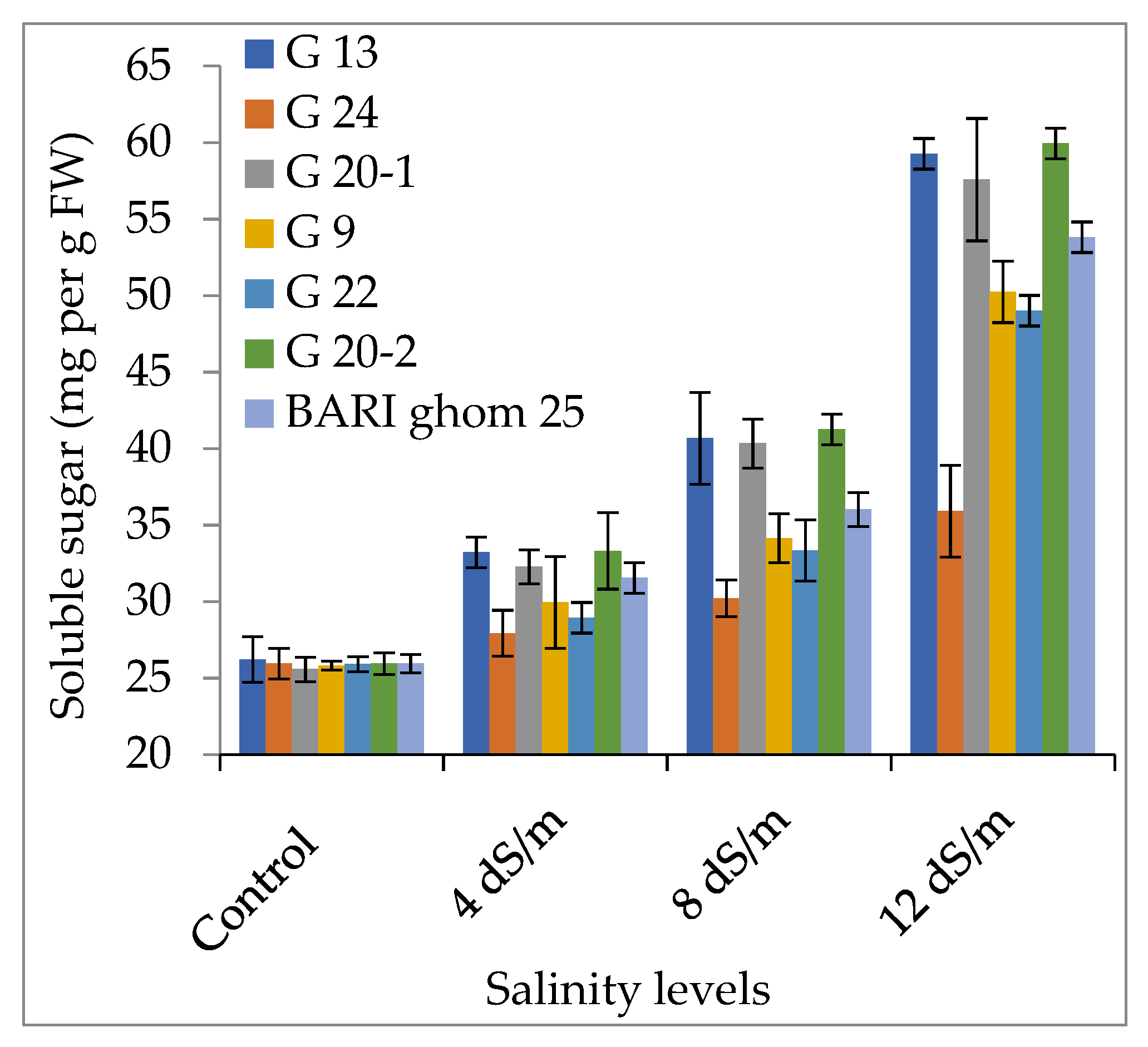
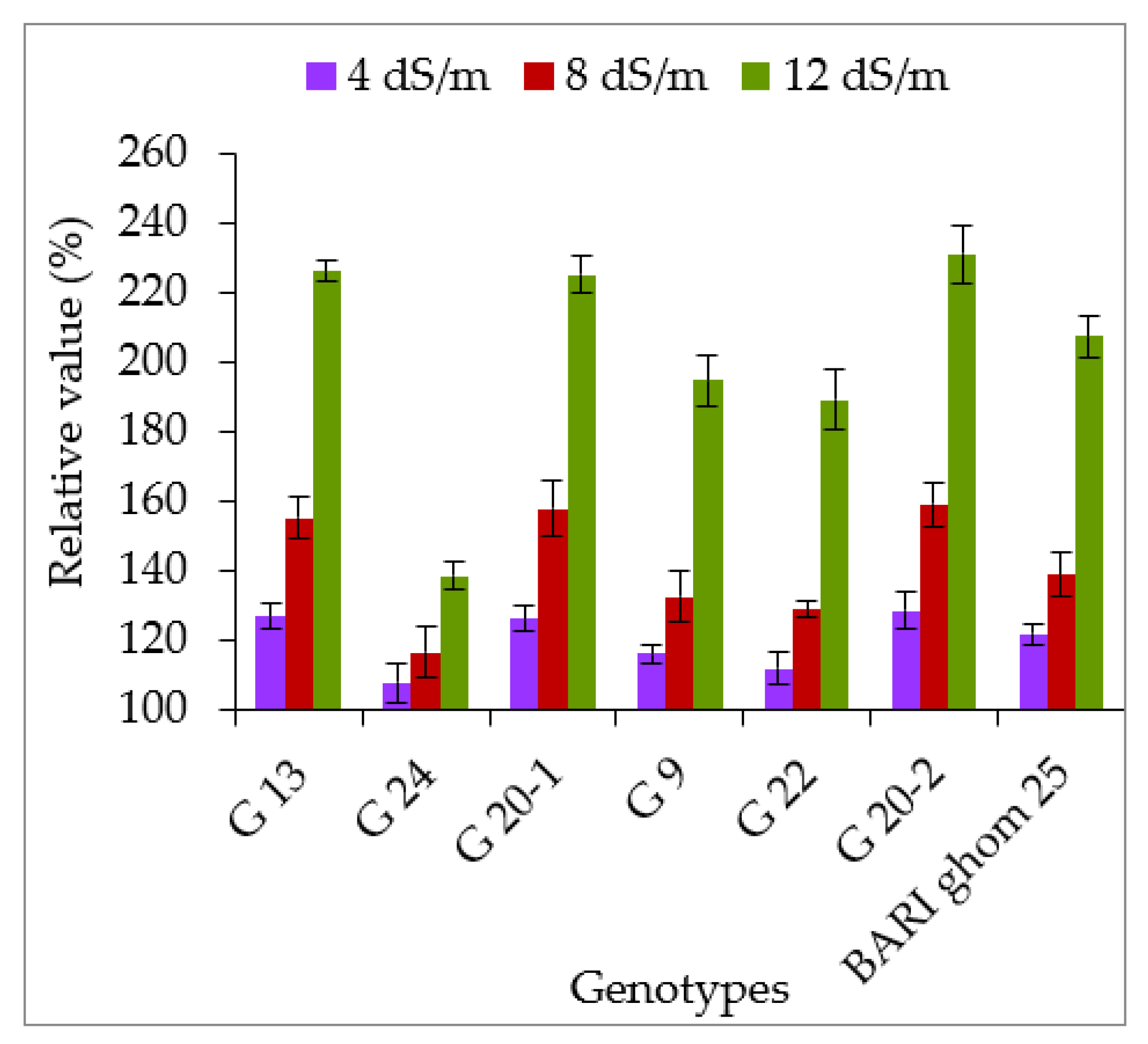
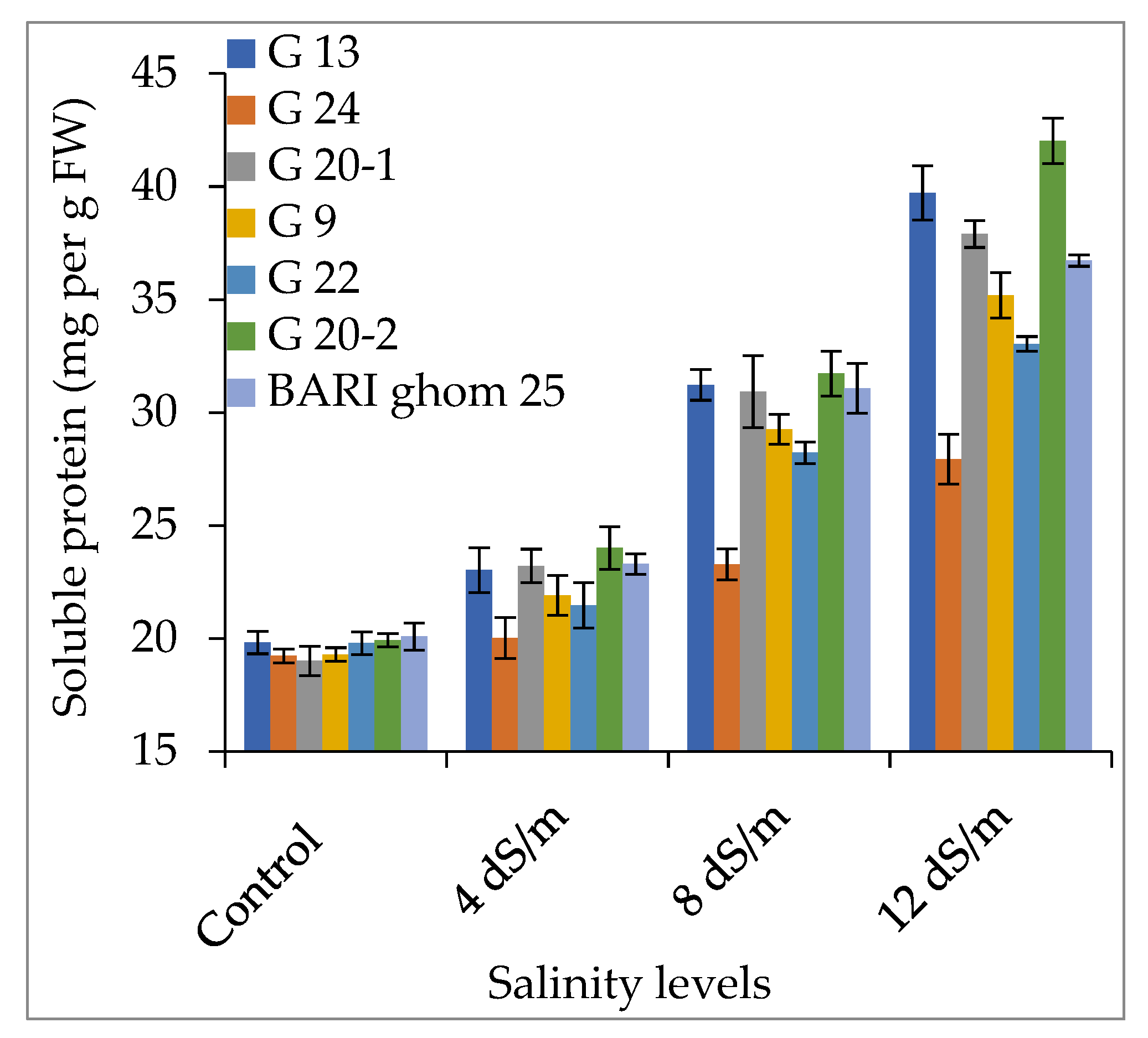
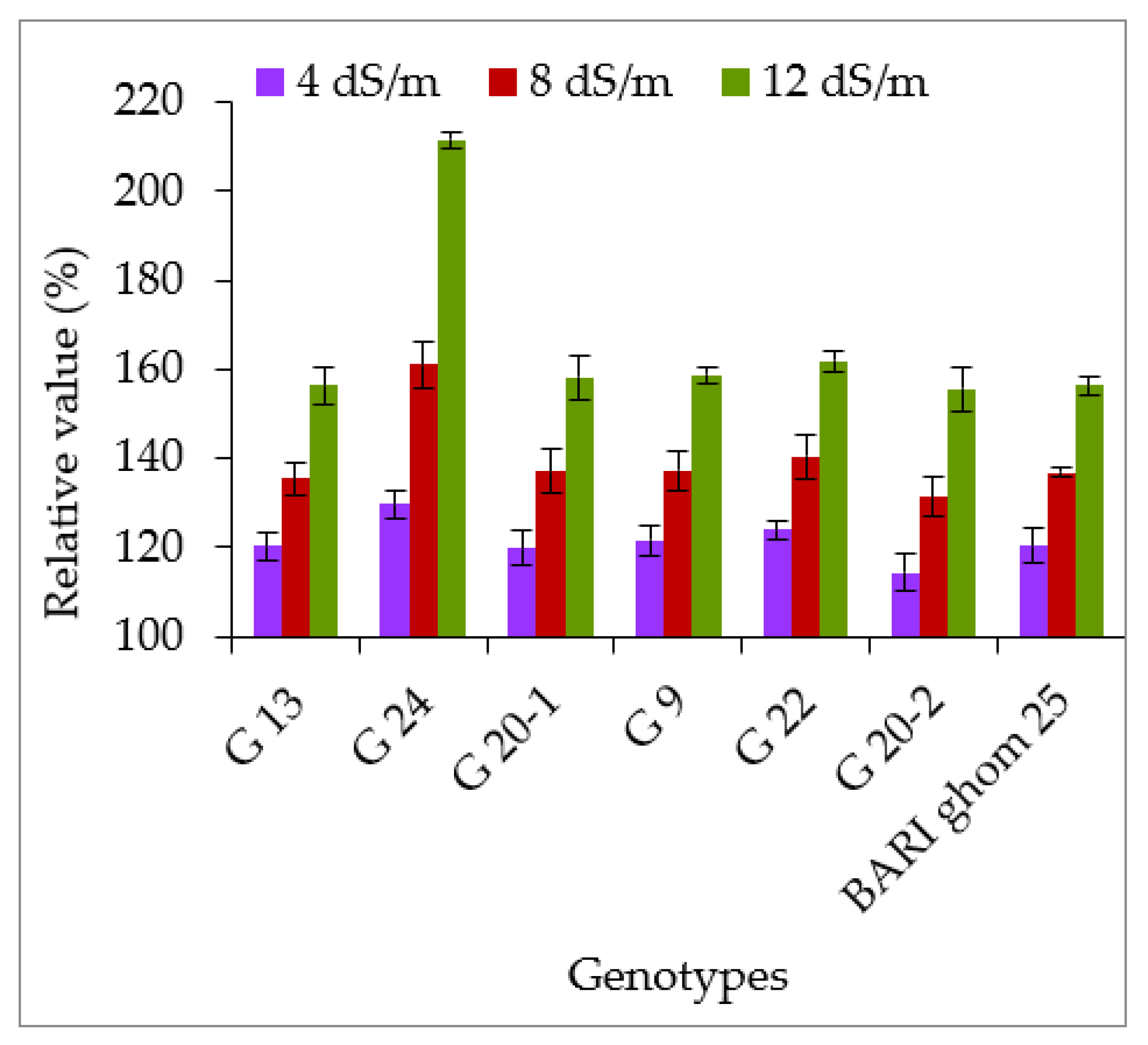
2.2. Compatible Solutes Accumulation
2.2.1. Accumulation of Proline and Glycinebetaine
2.2.2. Soluble Sugar and Soluble Protein Accumulation
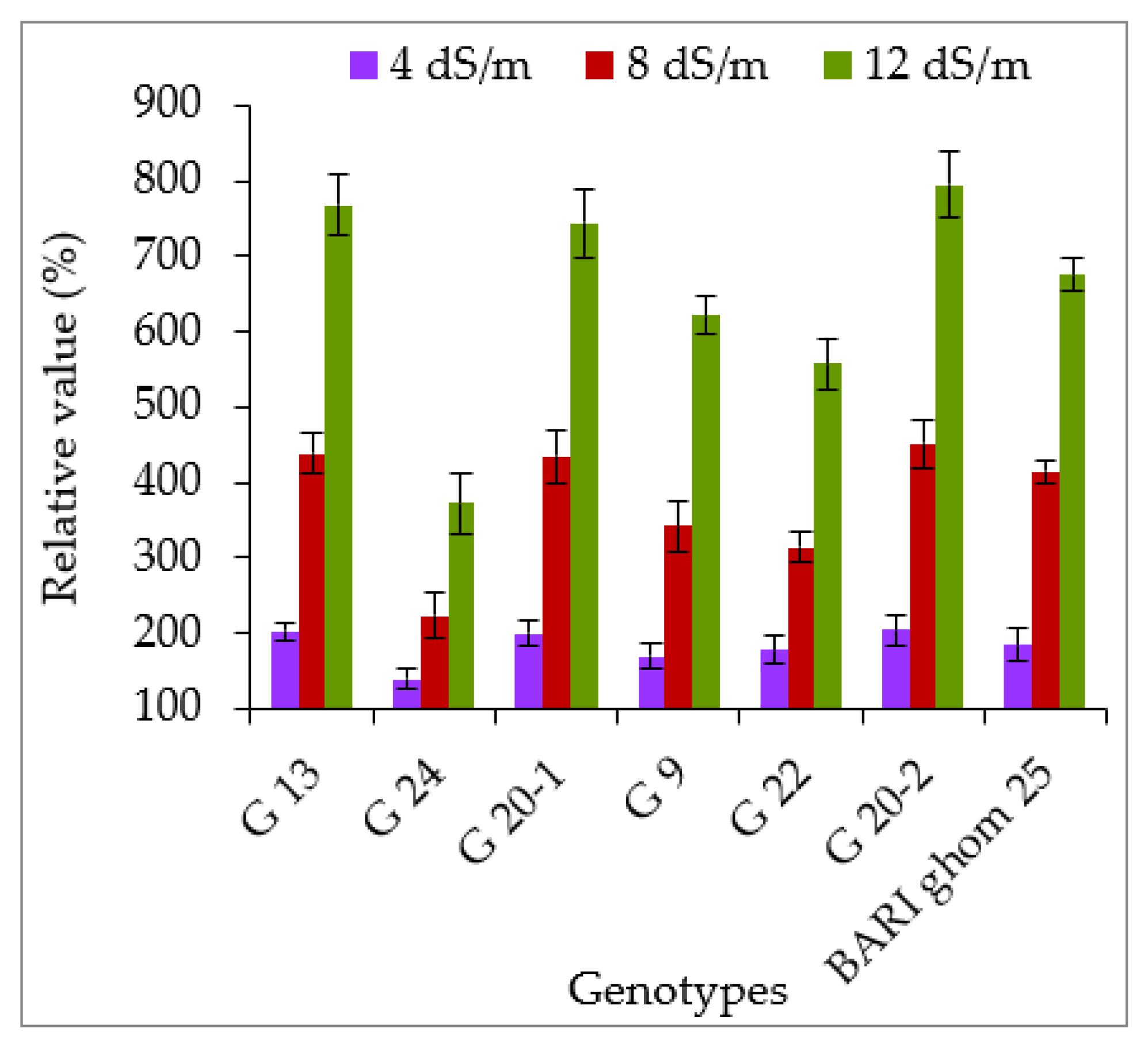
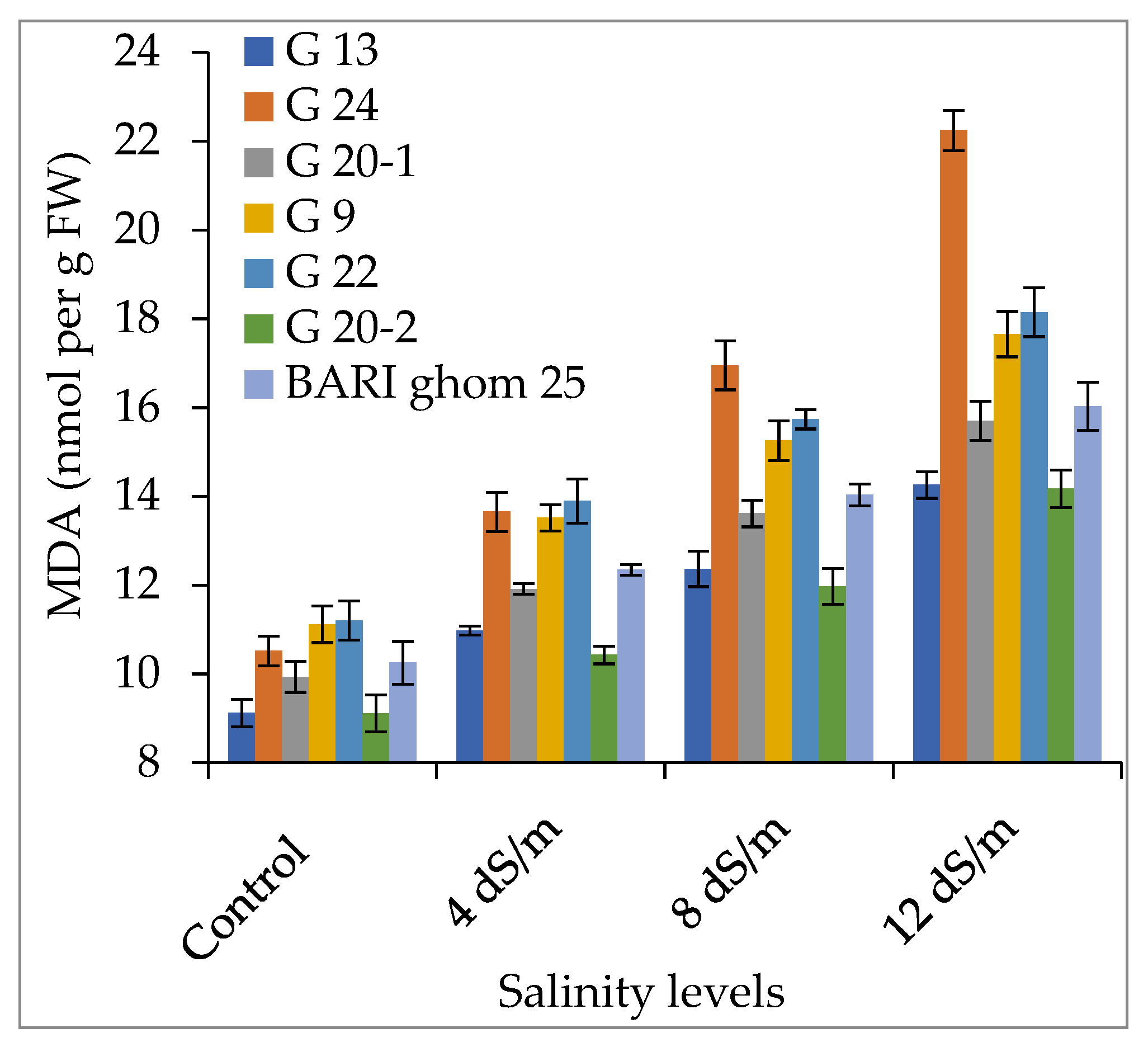
2.3. Accumulation of Hydrogen Peroxide and Malondialdehyde
3. Discussion
3.1. Agro-Morphology and Yield of Wheat
3.2. Accumulation of Compatible Solutes
3.3. Hydrogen Peroxide and Malondialdehyde Accumulation
4. Materials and Methods
4.1. Plant Material and Treatment
4.2. Assessment of Yield Contributing Parameters and Yield
4.3. Proline and Glycine Betaine (GB) Determination
4.4. Soluble Sugar and Soluble Protein Determination
4.5. Determination of Hydrogen Peroxide and Melondealdehyde
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, N.P.; Martin, J.M.; Giroux, M.J. Increased wheat grain hardness conferred by novel puroindoline haplotypes from Aegilopstauschii. Crop Sci. 2010, 50, 1718–1727. [Google Scholar] [CrossRef]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Iqbal, M.; Asif, M.; Hirani, A.H.; Goyal, A. Growing wheat on saline lands: Can a dream come true? Aust. J. Crop Sci. 2013, 7, 515–524. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651. [Google Scholar] [CrossRef] [Green Version]
- Breiman, A.; Graur, D. Wheat evolution. Isr. J. Plant Sci. 1995, 43, 85–98. [Google Scholar] [CrossRef]
- Haque, S.A. Salinity problems and crop production in coastal regions of Bangladesh. Pak. J. Bot. 2006, 38, 1359–1365. [Google Scholar]
- Ibrahim, M.; Zaman, M.A.U.; Mostafizur, A.B.M.; Shahidullah, S.M. Diversity of crops and land use pattern in Barisal region. Bangladesh Rice J. 2017, 21, 57–72. [Google Scholar] [CrossRef]
- Abdallah, M.S.; Abdelgawad, Z.A.; El-Bassiouny, H.M.S. Alleviation of the adverse effects of salinity stress using trehalose in two rice varieties. S. Afr. J. Bot. 2016, 103, 275–282. [Google Scholar] [CrossRef]
- Akbarimoghaddam, H.; Galavi, M.; Ghanbari, A.; Panjehkeh, N. Salinity effects on seed germination and seedling growth of bread wheat cultivars. Trakia J. Sci. 2011, 9, 43–50. [Google Scholar]
- Mbarki, S.; Sytar, O.; Cerda, A.; Zivcak, M.; Rastogi, A.; He, X.; Zoghlami, A.; Abdelly, C.; Brestic, M. Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants. In Salinity Responses and Tolerance in Plants; Springer: New York, NY, USA, 2018; Volume 1, pp. 85–136. [Google Scholar]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Hussan, M.U.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar]
- Muhammad, Z.; Hussain, F. Effect of NaCl salinity on the germination and seedling growth of seven wheat genotypes. Pak. J. Bot. 2012, 44, 1845–1850. [Google Scholar]
- Munns, R. Physiological processes limiting plant growth in saline soils: Some dogmas and hypotheses. Plant Cell Environ. 1993, 16, 15–24. [Google Scholar] [CrossRef]
- Ashraf, M.F.M.R.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef]
- Nawaz, K.; Hussain, K.; Majeed, A.; Khan, F.; Afghan, S.; Ali, K. Fatality of salt stress to plants: Morphological, physiological and biochemical aspects. Afr. J. Biotechnol. 2010, 9, 34. [Google Scholar]
- Rahnama, A.; James, R.A.; Poustini, K.; Munns, R. Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct. Plant Biol. 2010, 37, 255–263. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J.K. Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ. 2002, 25, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.K.; Goyal, M.R.; Singh, A. Physiological and biochemical changes in plants under soil salinity stress: A review. In Engineering Practices Management Soil Salinity; Apple Academic Press: Palm Bay, FL, USA, 2018; pp. 159–200. [Google Scholar]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [Green Version]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Navarro, A.; Bañon, S.; Olmos, E.; Sánchez-Blanco, M.D.J. Effects of sodium chloride on water potential components, hydraulic conductivity, gas exchange and leaf ultrastructure of Arbutus unedo plants. Plant Sci. 2007, 172, 473–480. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptations to environmental stresses. Plant Cell. 1995, 7, 1099. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.A.; Banu, M.N.A.; Okuma, E.; Amako, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline and glycinebetaine increase NaCl-induced ascorbate–glutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco Bright Yellow-2 suspension-cultured cells. J. Plant Physiol. 2007, 164, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Jaleel, C.A.; Sharma, S. Antioxidant defense system, lipid peroxidation, proline-metabolizing enzymes, and biochemical activities in two Morusalba genotypes subjected to NaCl stress. Russ. J. Plant Physiol. 2010, 57, 509–517. [Google Scholar] [CrossRef]
- Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of endogenous abscisic acid in methyl jasmonate-induced stomatal closure in Arabidopsis. Plant Physiol. 2011, 156, 430–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nounjan, N.; Nghia, P.T.; Theerakulpisut, P. Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J. Plant Physiol. 2012, 169, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.A.; Aziz, T.; Farooq, M.; Sarwar, G. Silicon-induced changes in growth, ionic composition, water relations, chlorophyll contents and membrane permeability in two salt-stressed wheat genotypes. Arch. Agron. Soil Sci. 2012, 58, 247–256. [Google Scholar] [CrossRef]
- Khan, M.A.; Ungar, I.A.; Showalter, A.M. Effects of sodium chloride treatments on growth and ion accumulation of the halophyte Haloxylonrecurvum. Commun. Soil Sci. Plant Anal. 2000, 31, 2763–2774. [Google Scholar] [CrossRef]
- Wang, Y.; Nii, N. Changes in chlorophyll, ribulosebisphosphate carboxylase-oxygenase, glycine betaine content, photosynthesis and transpiration in Amaranthus tricolor leaves during salt stress. J. Hortic. Sci. Biotechnol. 2000, 75, 623–627. [Google Scholar] [CrossRef]
- Kerepesi, I.; Galiba, G. Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci. 2000, 40, 482–487. [Google Scholar] [CrossRef]
- Saxena, S.C.; Kaur, H.; Verma, P.; Petla, B.P.; Andugula, V.R.; Majee, M. Osmoprotectants: Potential for crop improvement under adverse conditions. In Plant Acclimation to Environmental Stress; Springer: New York, NY, USA, 2013; pp. 197–232. [Google Scholar]
- Ben Ahmed, C.; Ben Rouina, B.; Sensoy, S.; Boukhriss, M.; Ben Abdullah, F. Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J. Agric. Food Chem. 2010, 58, 4216–4222. [Google Scholar] [CrossRef]
- Shamsi, K.; Kobraee, S. Biochemical Responses of Three Iranian Bread Wheat Cultivars (Triticum aestivum L.) to Salinity Stress. Glob. J. Pure Appl. Sci. 2014, 2, 42–45. [Google Scholar]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, C.; Jiang, D.; Liu, F.; Dai, T.; Jing, Q.; Cao, W. Effects of salt and waterlogging stresses and their combination on leaf photosynthesis, chloroplast ATP synthesis, and antioxidant capacity in wheat. Plant Sci. 2009, 176, 575–582. [Google Scholar] [CrossRef]
- Ali, Q.; Javed, M.T.; Noman, A.; Haider, M.Z.; Waseem, M.; Iqbal, N.; Waseem, M.; Shah, M.S.; Shahzad, F.; Perveen, R. Assessment of drought tolerance in mung bean cultivars/lines as depicted by the activities of germination enzymes, seedling’s antioxidative potential and nutrient acquisition. Arch. Agron. Soil Sci. 2018, 64, 84–102. [Google Scholar] [CrossRef]
- Ashik, T.; Islam, M.M.; Rana, M.S.; Jahan, K.; Urmi, T.A.; Jahan, N.A.; Rahman, M.M. Evaluation of Salinity Tolerant Wheat (Triticum aestivum L.) Genotypes through Multivariate Analysis of Agronomic Traits. Agric. Sci. Dig. 2021. [Google Scholar] [CrossRef]
- Ouhaddach, M.; ElYacoubi, H.; Douaik, A.; Rochdi, A. Morpho-physiological and biochemical responses to salt stress in wheat (Triticum aestivum L.) at the heading stage. J. Mater. Environ. Sci. 2018, 9, 1899–1907. [Google Scholar]
- Ibrahimova, U.; Suleymanova, Z.; Brestic, M.; Mammadov, A.; Ali, O.M.; Abdel Latef, A.A.H.; Hossain, A. Assessing the Adaptive Mechanisms of Two Bread Wheat (Triticum aestivum L.) Genotypes to Salinity Stress. Agronomy 2021, 11, 1979. [Google Scholar] [CrossRef]
- Asgari, H.R.; Cornelis, W.; Damme, P.V. Effect of salinity on wheat (Triticum aestivum L.) grain yield, yield components and ion uptake. Desert 2012, 16, 169–175. [Google Scholar]
- Asgari, H.R.; Cornelis, W.; Damme, P.V. Salt stress effect on wheat (Triticum aestivum L.) growth and leaf ion concentrations. Int. J. Plant Prod. 2012, 195–208. [Google Scholar]
- Abbas, G.; Saqib, M.; Rafique, Q.; Rahman, A.U.; Akhtar, J.; Haq, M.A.; Nasim, M. Effect of salinity on grain yield and grain quality of wheat (Triticum aestivum L.). Pak. J. Bot. 2013, 50, 185–189. [Google Scholar]
- Sadak, M.S. Physiological role of trehalose on enhancing salinity tolerance of wheat plant. Bull. Natl. Res. Cent. 2019, 43, 1–10. [Google Scholar]
- Kalhoro, N.A.; Rajpar, I.; Kalhoro, S.A.; Ali, A.; Raza, S.; Ahmed, M.; Kalhoro, F.A.; Ramzan, M.; Wahid, F. Effect of salts stress on the growth and yield of wheat (Triticum aestivum L.). Am. J. Plant Sci. 2016, 7, 2257–2271. [Google Scholar] [CrossRef]
- Chamekh, Z.; Ayed, S.; Sahli, A.; Ayadi, S.; Hammemi, Z.; Jallouli, S.; Trifa, Y.; Amara, H.; Abidi, W.; Zeballos, J.L.; et al. Eff ect of salt stress on the flag leaf area and yield components in twenty five durum wheat genotypes (Triticum turgidum ssp. durum). J. New Sci. 2014, 6, 15–23. [Google Scholar]
- Islam, M.M.; Faruqe, M.H.; Rana, M.S.; Akter, M.; Karim, M.A. Screening of rice (Oryza sativa L.) genotypes at reproductive stage for their tolerance to salinity. Agriculturists 2018, 16, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Aref, F.; Rad, H.E. Physiological characterization of rice under salinity stress during vegetative and reproductive stages. Indian J. Sci. Technol. 2012, 5, 2578–2586. [Google Scholar]
- Darko, E.; Gierczik, K.; Hudaak, O.; Forgoa, P.; Paal, M.; Tuerkoesi, E. Differing metabolic responses to salt strein wheat-barley addition lines containing different 7H chromosomal fragments. PLoS ONE 2017, 12, e0174170. [Google Scholar] [CrossRef] [Green Version]
- Ali, Q.; Daud, M.K.; Haider, M.Z.; Ali, S.; Rizwan, M.; Aslam, N.; Noman, A.; Iqbal, N.; Shahzad, F.; Deeba, F.; et al. Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant Physiol. Biochem. 2017, 119, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Aslam, M.T.; Alhammad, B.A.; Hassan, M.U.; Maqbool, R.; Chattha, M.U.; Khan, I.; Gitari, H.I.; Uslu, O.S.; Rana, R.; et al. Salinity stress in wheat: Effects, mechanisms and management strategies. Phyton 2022, 91, 667. [Google Scholar]
- Munns, R.; Schachtman, D.P.; Condon, A.G. The significance of a two-phase growth response to salinity in wheat and barley. Funct. Plant Biol. 1995, 22, 561–569. [Google Scholar] [CrossRef]
- Heshmat, S.A.; Zakaria, A.B.; Omar, A.E.S.; Hanan, E.G. Efficacy of seawater salinity on osmotic adjustment and solutes allocation in wheat (Triticum aestivum) flag leaf during grain filling. Int. J. Plant Physiol. Biochem. 2012, 4, 33–45. [Google Scholar]
- Shamsi, K.; Kobraee, S. Biochemical and physiological responses of three wheat cultivars (Triticum aestivum L.) to salinity stress. Ann. Biol. Res. 2013, 4, 180–185. [Google Scholar]
- Sun, K.; Hunt, K.; Hauser, B.A. Ovule abortion in Arabidopsis triggered by stress. Plant Physiol. 2004, 135, 2358–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating prolinemetabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Banu, M.N.A.; Hoque, M.A.; Watanabe-Sugimoto, M.; Matsuoka, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol. 2009, 166, 146–156. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L.) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017, 115, 449–460. [Google Scholar] [CrossRef]
- Kibria, M.G.; Hossain, M.; Murata, Y.; Hoque, M.A. Antioxidant defense mechanisms of salinity tolerance in rice genotypes. Rice Sci. 2017, 24, 155–162. [Google Scholar] [CrossRef]
- Giri, J. Glycinebetaine and abiotic stress tolerance in plants. Plant Signal. Behav. 2011, 6, 1746–1751. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Reddy, P.S.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kishor, P.K. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor L. Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Bhaskara, G.B.; Yang, T.H.; Verslues, P.E. Dynamic proline metabolism: Importance and regulation in water limited environments. Front. Plant Sci. 2015, 6, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parida, A.; Das, A.B.; Das, P. NaCl stress causes changes in photosynthetic pigments, proteins, and other metabolic components in the leaves of a true mangrove, Bruguiera parviflora, in hydroponic cultures. J. Plant Biol. 2002, 45, 28–36. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Goudarzi, M.; Pakniyat, H. Evaluation of wheat cultivars under salinity stress based on some agronomic and physiological traits. J. Agric. Soc. Sci. 2008, 4, 35–38. [Google Scholar]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Flowers, T.J.; Yeo, A.R. Breeding for salinity resistance in crop plants: Where next? Funct. Plant Biol. 1995, 22, 875–884. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Alam, M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed Res. Int. 2014, 2014, 757219. [Google Scholar] [CrossRef] [Green Version]
- Manai, J.; Gouia, H.; Corpas, F.J. Redox and nitric oxide homeostasis are affected in tomato (Solanum lycopersicum) roots under salinity-induced oxidative stress. J. Plant Physiol. 2014, 171, 1028–1035. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.V.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxifi cation system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Chunthaburee, S.; Dongsansuk, A.; Sanitchon, J.; Pattanagul, W.; Theerakulpisut, P. Physiological and biochemical parameters for evaluation and clustering of rice cultivars differing in salt tolerance at seedling stage. Saudi J. Biol. Sci. 2016, 23, 467–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Chen, Q.I.N.; Liu, Q.; Zhang, W.; Ding, R. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef]
- Brankova, L.; Ivanov, S.; Alexieva, V.; Karanov, E. Salt-induced alteration in the levels of some oxidative parameters and unspecific defence compounds in leaves of two plant species (cotton and bean) with different sensitivity to salinity. Comptes Rendus-Acad. Bulg. Sci. 2005, 58, 1307. [Google Scholar]
- Ashraf, M.A.; Ashraf, M.U.H.A.M.M.A.D.; Ali, Q. Response of two genetically diverse wheat cultivars to salt stress at different growth stages: Leaf lipid peroxidation and phenolic contents. Pak. J. Bot. 2010, 42, 559–565. [Google Scholar]
- Rasool, S.; Ahmad, A.; Siddiqi, T.O.; Ahmad, P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol. Plant. 2013, 35, 1039–1050. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Abd Allah, E.F.; Hashem, A.; Sarwat, M.; Anjum, N.A.; Gucel, S. Calcium and potassium supplementation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed chickpea (Cicerarietinum L.). Front. Plant Sci. 2016, 7, 513. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil. 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Yoshida, S.; Coronel, V. Nitrogen nutrition, leaf resistance, and leaf photosynthetic rate of the rice plant. Soil Sci. Plant Nutr. 1976, 22, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Lowry, O.H. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Madhava Rao, K.V.; Sresty, T.V. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef]




| Genotypes | Plant Height (cm) | Total Tillers Plant−1 | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | 4 dS m−1 | 8 dS m−1 | 12 dS m−1 | Control | 4 dS m−1 | 8 dS m−1 | 12 dS m−1 | |
| G 13 | 83.3 c | 77.9 c | 72.8 c | 70.1 b | 5.9 a | 5.5 ab | 5.4 abc | 5.2 ab |
| G 24 | 84.6 bc | 75.2 c | 67.9 d | 59.0 d | 5.7 a | 5.3 b | 4.5 d | 3.9 d |
| G 20-1 | 89.3 ab | 85.3 a | 80.4 a | 76.3 a | 6.2 a | 5.9 ab | 5.6 ab | 5.3 ab |
| G 9 | 89.0 ab | 81.2 b | 76.6 bc | 66.7 c | 5.9 a | 5.5 ab | 4.9 bcd | 4.8 bc |
| G 22 | 90.9 a | 82.3 ab | 78.7 ab | 73.3 a | 5.7 a | 5.5 ab | 4.7 cd | 4.6 c |
| G 20-2 | 86.0 abc | 82.0 b | 77.1 ab | 73.9 a | 6.3 a | 6.1 a | 5.9 a | 5.7 a |
| BARI ghom 25 | 89.3 ab | 83.4 ab | 78.3 ab | 74.9 a | 6.2 a | 5.8 ab | 5.6 ab | 5.1 bc |
| CV (%) | 3.45 | 2.24 | 2.78 | 2.47 | 5.61 | 5.96 | 6.78 | 6.16 |
| Genotypes | Effective Tiller No. Plant−1 | Spike Length (cm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | 4 dS m−1 | 8 dS m−1 | 12 dS m−1 | Control | 4 dS m−1 | 8 dS m−1 | 12 dS m−1 | |
| G 13 | 5.29 a | 4.93 abc | 4.68 a | 4.59 a | 10.28 bcd | 10.08 bc | 9.84 abc | 9.51 ab |
| G 24 | 5.15 a | 4.35 c | 3.30 b | 2.23 c | 11.83 a | 10.86 a | 10.40 a | 9.66 ab |
| G 20-1 | 5.45 a | 5.17 ab | 4.82 a | 4.70 a | 10.60 bc | 10.51 ab | 10.13 ab | 9.80 a |
| G 9 | 5.11 a | 4.62 bc | 3.65 b | 3.49 b | 10.69 b | 9.98 bc | 9.59 bc | 9.14 b |
| G 22 | 5.06 a | 4.51 c | 3.45 b | 3.20 b | 10.95 b | 10.07 bc | 9.85 abc | 9.29 ab |
| G 20-2 | 5.53 a | 5.31 a | 5.17 a | 5.11 a | 9.83 d | 9.62 c | 9.53 bc | 9.21 ab |
| BARI ghom 25 | 5.49 a | 5.23 a | 5.00 a | 4.73 a | 9.96 cd | 9.73 c | 9.52 c | 9.18 b |
| CV (%) | 6.86 | 6.19 | 6.34 | 6.96 | 3.48 | 3.04 | 3.29 | 3.30 |
| Genotypes | Spikelets Number Spike−1 | Grains Spike−1 | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | 4 dS m−1 | 8 dS m−1 | 12 dS m−1 | Control | 4 dS m−1 | 8 dS m−1 | 12 dS m−1 | |
| G 13 | 19.26 ab | 18.80 a | 18.01 a | 16.66 a | 57.53 ab | 56.00 ab | 54.56 ab | 51.85 ab |
| G 24 | 17.50 d | 17.00 c | 15.63 e | 12.00 d | 53.95 bc | 46.91 c | 40.00 e | 36.90 e |
| G 20-1 | 17.36 d | 17.02 c | 16.02 de | 14.85 c | 54.84 abc | 53.10 b | 51.73 bc | 49.04 bc |
| G 9 | 19.43 a | 18.88 a | 17.65 ab | 15.71 b | 54.40 abc | 52.32 b | 48.10 cd | 45.72 c |
| G 22 | 18.38 bcd | 17.85 bc | 16.65 cd | 14.41 c | 50.86 c | 47.88 c | 44.56 d | 40.98 d |
| G 20-2 | 18.00 cd | 17.76 bc | 16.96 bc | 15.64 b | 59.53 a | 58.10 a | 56.36 a | 55.00 a |
| BARI ghom 25 | 18.70 abc | 18.32 ab | 17.36 abc | 15.83 b | 54.66 abc | 52.88 b | 51.20 bc | 48.75 bc |
| CV (%) | 2.88 | 2.63 | 2.47 | 2.29 | 5.24 | 4.40 | 4.44 | 4.75 |
| Genotypes | Thousand Grain Weight (g) | Grain Yield (g plant−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | 4 dS m−1 | 8 dS m−1 | 12 dS m−1 | Control | 4 dS m−1 | 8 dS m−1 | 12 dS m−1 | |
| G 13 | 47.85 ab | 45.41 ab | 42.45 ab | 40.00 a | 9.79 a | 9.29 a | 8.80 ab | 7.81 b |
| G 24 | 44.75 b | 39.96 c | 34.14 c | 26.09 c | 9.76 a | 8.78 b | 6.55 d | 4.86 e |
| G 20-1 | 51.25 a | 47.58 a | 44.18 a | 41.54 a | 9.18 bc | 8.80 b | 8.39 ab | 7.62 b |
| G 9 | 46.43 b | 44.55 ab | 39.38 b | 34.19 b | 8.91 cd | 7.72 c | 7.45 c | 6.59 c |
| G 22 | 48.45 ab | 46.40 ab | 39.87 b | 33.89 b | 8.58 d | 7.64 c | 7.05 cd | 5.90 d |
| G 20-2 | 45.34 b | 43.05 bc | 40.37 b | 38.84 a | 9.54 ab | 9.19 ab | 8.91 a | 8.62 a |
| BARI ghom 25 | 47.03 b | 44.60 ab | 40.32 b | 38.72 a | 9.29 abc | 8.87 ab | 8.26 b | 7.08 c |
| CV (%) | 4.80 | 4.63 | 4.71 | 5.37 | 3.29 | 3.13 | 3.86 | 4.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sen, A.; Islam, M.M.; Zaman, E.; Ghosh, U.K.; Momtaz, M.B.; Islam, M.A.; Urmi, T.A.; Mamun, M.A.A.; Rahman, M.M.; Kamal, M.Z.U.; et al. Agro-Morphological, Yield and Biochemical Responses of Selected Wheat (Triticum aestivum L.) Genotypes to Salt Stress. Agronomy 2022, 12, 3027. https://doi.org/10.3390/agronomy12123027
Sen A, Islam MM, Zaman E, Ghosh UK, Momtaz MB, Islam MA, Urmi TA, Mamun MAA, Rahman MM, Kamal MZU, et al. Agro-Morphological, Yield and Biochemical Responses of Selected Wheat (Triticum aestivum L.) Genotypes to Salt Stress. Agronomy. 2022; 12(12):3027. https://doi.org/10.3390/agronomy12123027
Chicago/Turabian StyleSen, Arpita, Md. Moshiul Islam, Erin Zaman, Uttam Kumar Ghosh, Milia Bente Momtaz, Md. Ariful Islam, Tahmina Akter Urmi, Md. Abdullah Al Mamun, Md. Mamunur Rahman, Mohammed Zia Uddin Kamal, and et al. 2022. "Agro-Morphological, Yield and Biochemical Responses of Selected Wheat (Triticum aestivum L.) Genotypes to Salt Stress" Agronomy 12, no. 12: 3027. https://doi.org/10.3390/agronomy12123027
APA StyleSen, A., Islam, M. M., Zaman, E., Ghosh, U. K., Momtaz, M. B., Islam, M. A., Urmi, T. A., Mamun, M. A. A., Rahman, M. M., Kamal, M. Z. U., Rahman, G. K. M. M., Haque, M. M., & Murata, Y. (2022). Agro-Morphological, Yield and Biochemical Responses of Selected Wheat (Triticum aestivum L.) Genotypes to Salt Stress. Agronomy, 12(12), 3027. https://doi.org/10.3390/agronomy12123027







