Hypoxia-Induced HIF-1α Expression Promotes Neurogenic Bladder Fibrosis via EMT and Pyroptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and NB Model
2.2. Cystometry
2.3. Doppler Ultrasonography
2.4. BUN/Scr Analysis
2.5. Histological and Immunohistochemical Staining
2.6. RNA Sequencing and Functional Enrichment Analysis
2.7. TUNEL Analysis
2.8. Cell Culture and Hypoxia Treatment
2.9. Western Blotting
2.10. Cellular and Tissue Immunofluorescence
2.11. Scanning Electron Microscopy (SEM)
2.12. Lactate Dehydrogenase (LDH) Release Assays
2.13. Calcein-AM/Propidium Iodine (PI) Staining
2.14. Enzyme-Linked Immunosorbent Assay for IL-1β and IL-18
2.15. siRNA Transfection
2.16. Statistical Analysis
3. Results
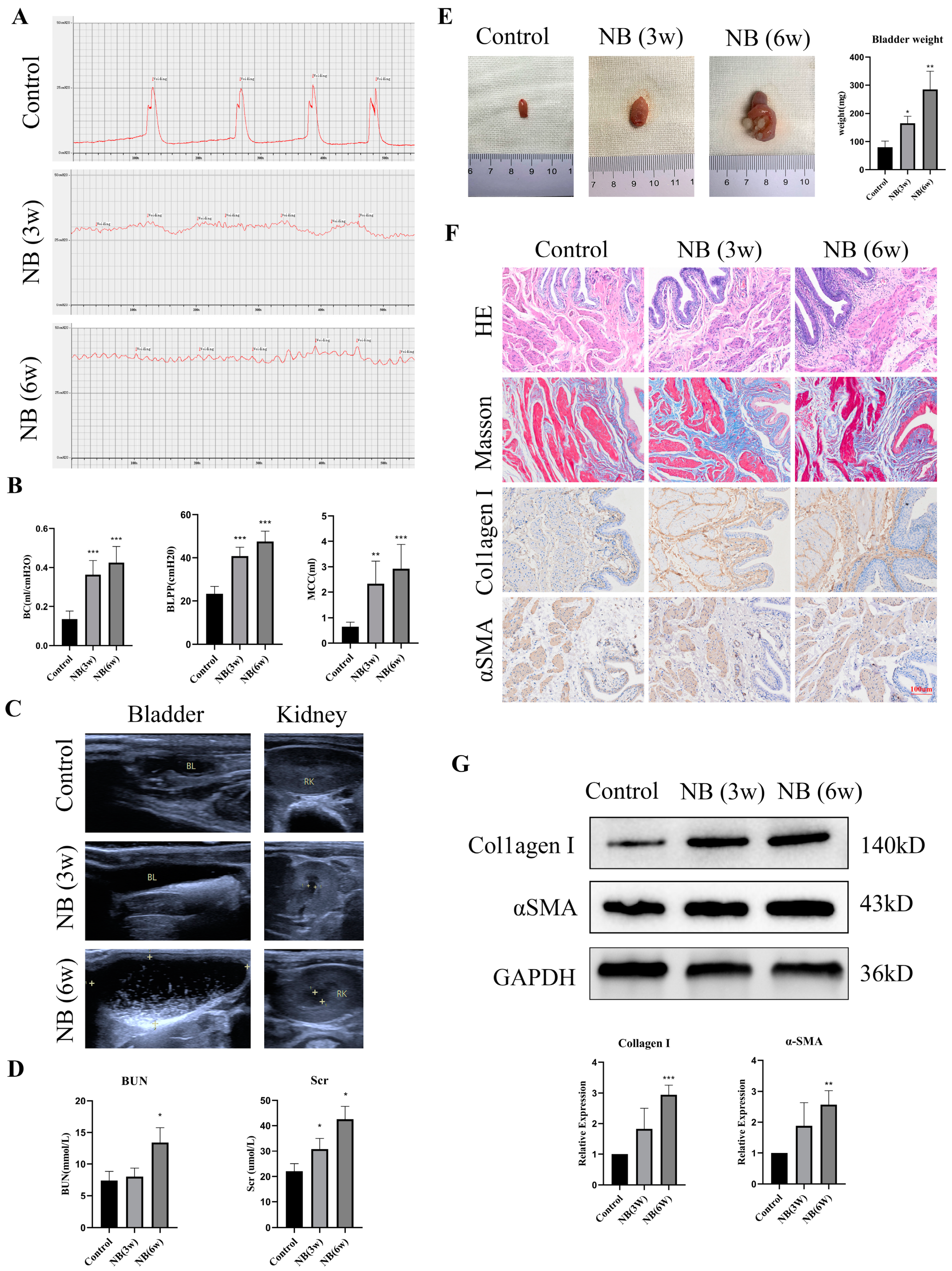
3.1. The Rats Showed Bladder Dysfunction, Upper Urinary Tract Damage and Bladder Fibrosis after SCI
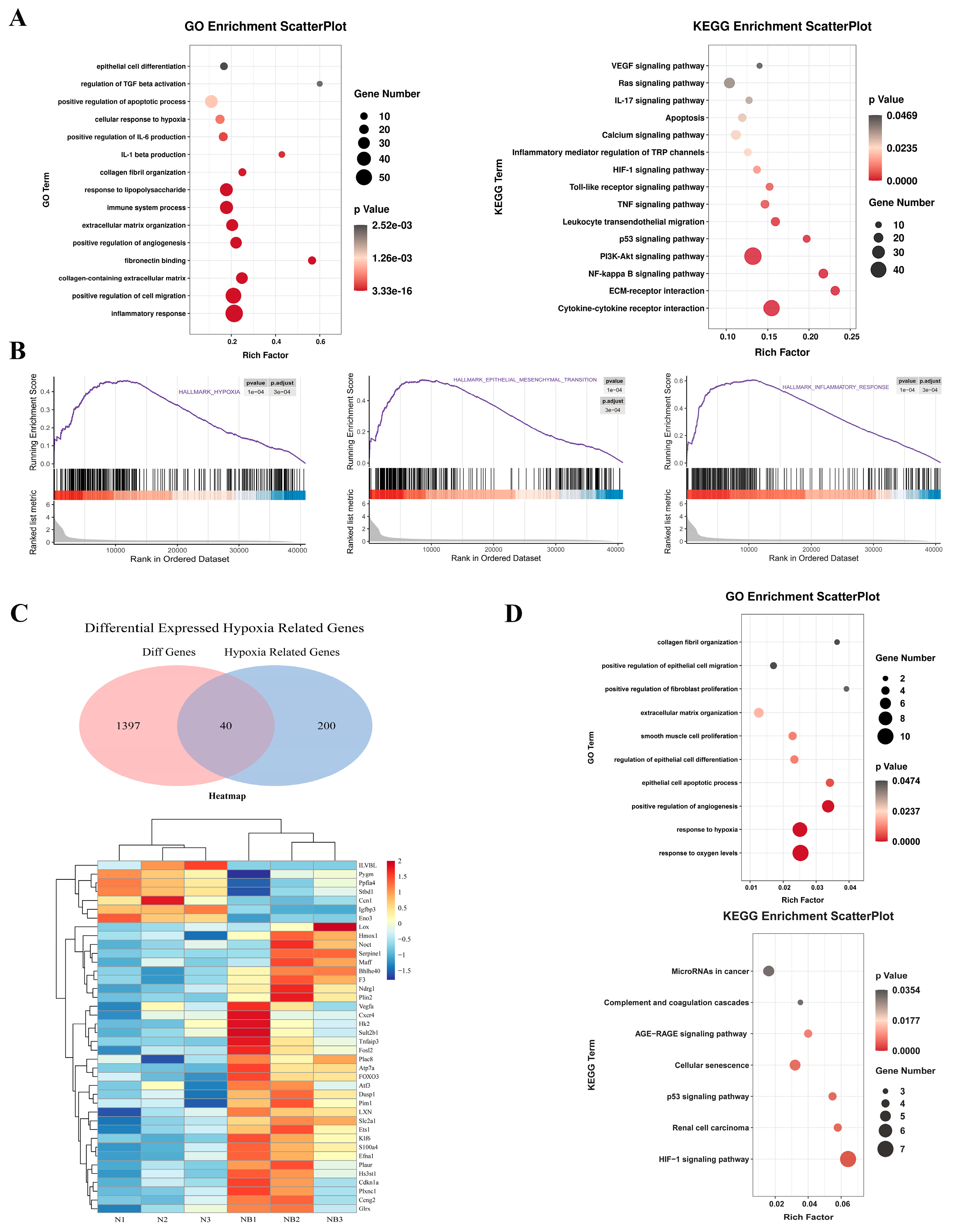
3.2. RNA Sequencing Revealed That Hypoxia Can Induce Fibrosis via EMT and the Inflammatory Response
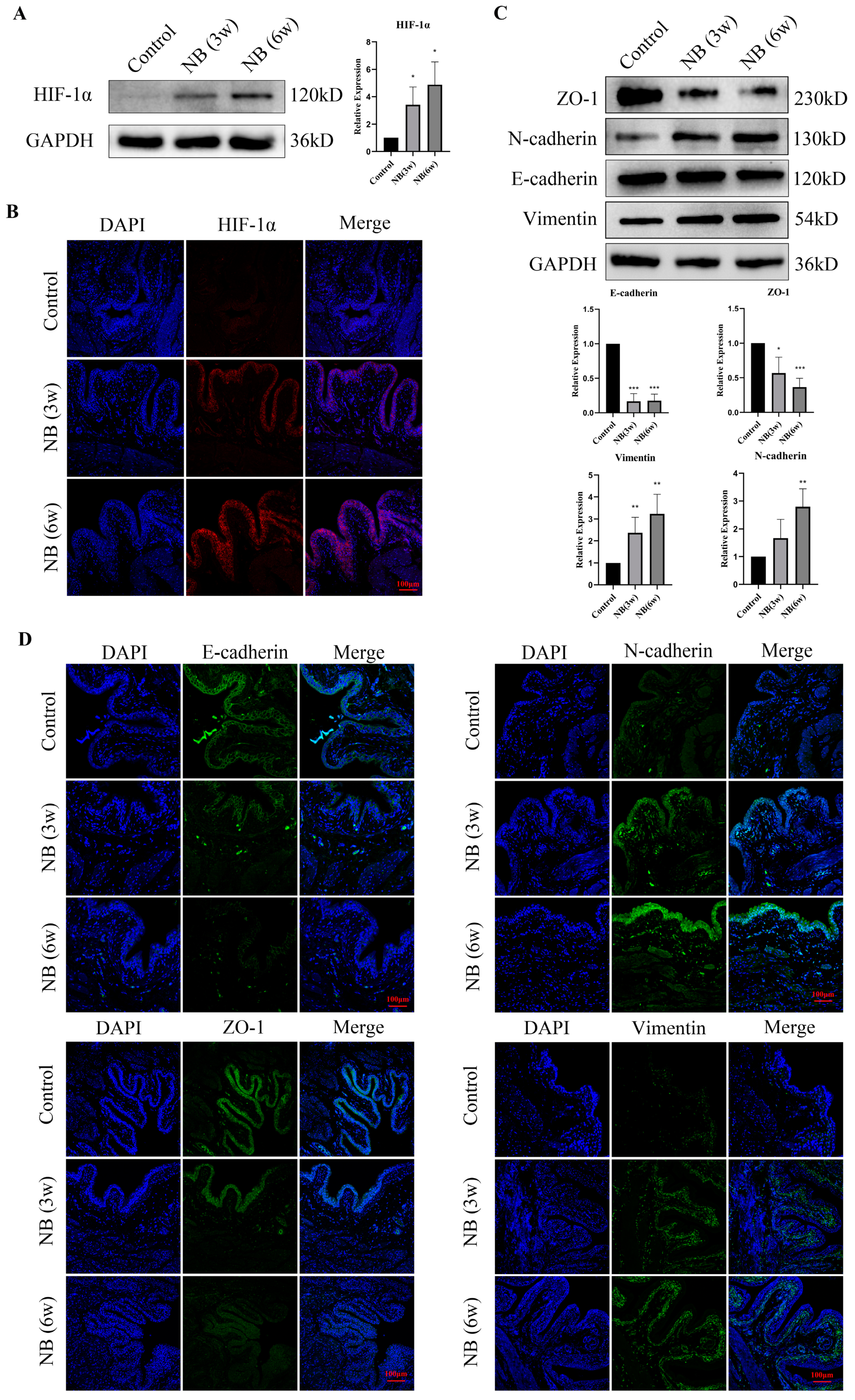
3.3. HIF-1α and EMT-Related Proteins Were Significantly Increased in the Bladder after SCI
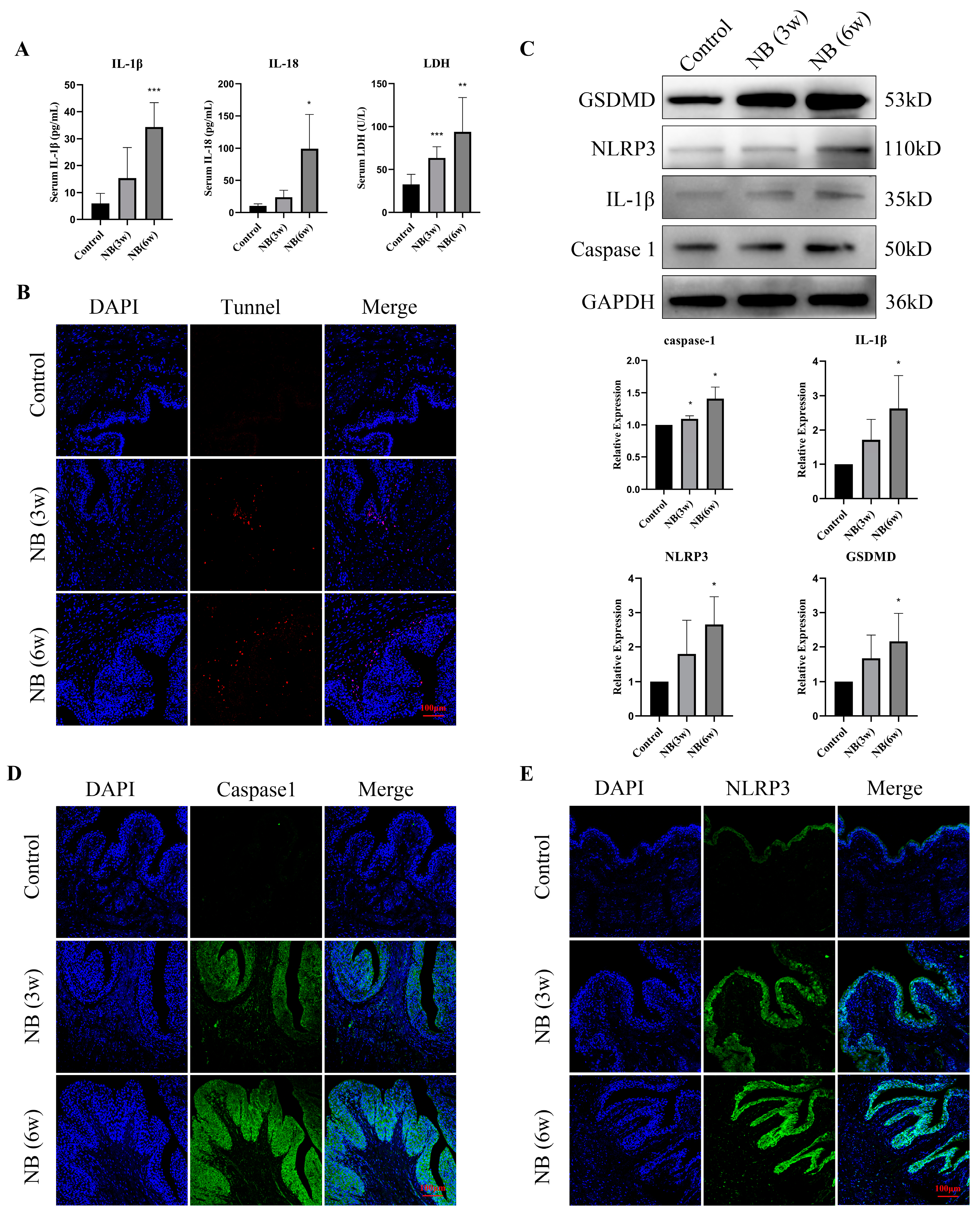
3.4. Inflammatory Factors and Pyroptosis-Related Proteins Were Significantly Increased in the Bladder after SCI
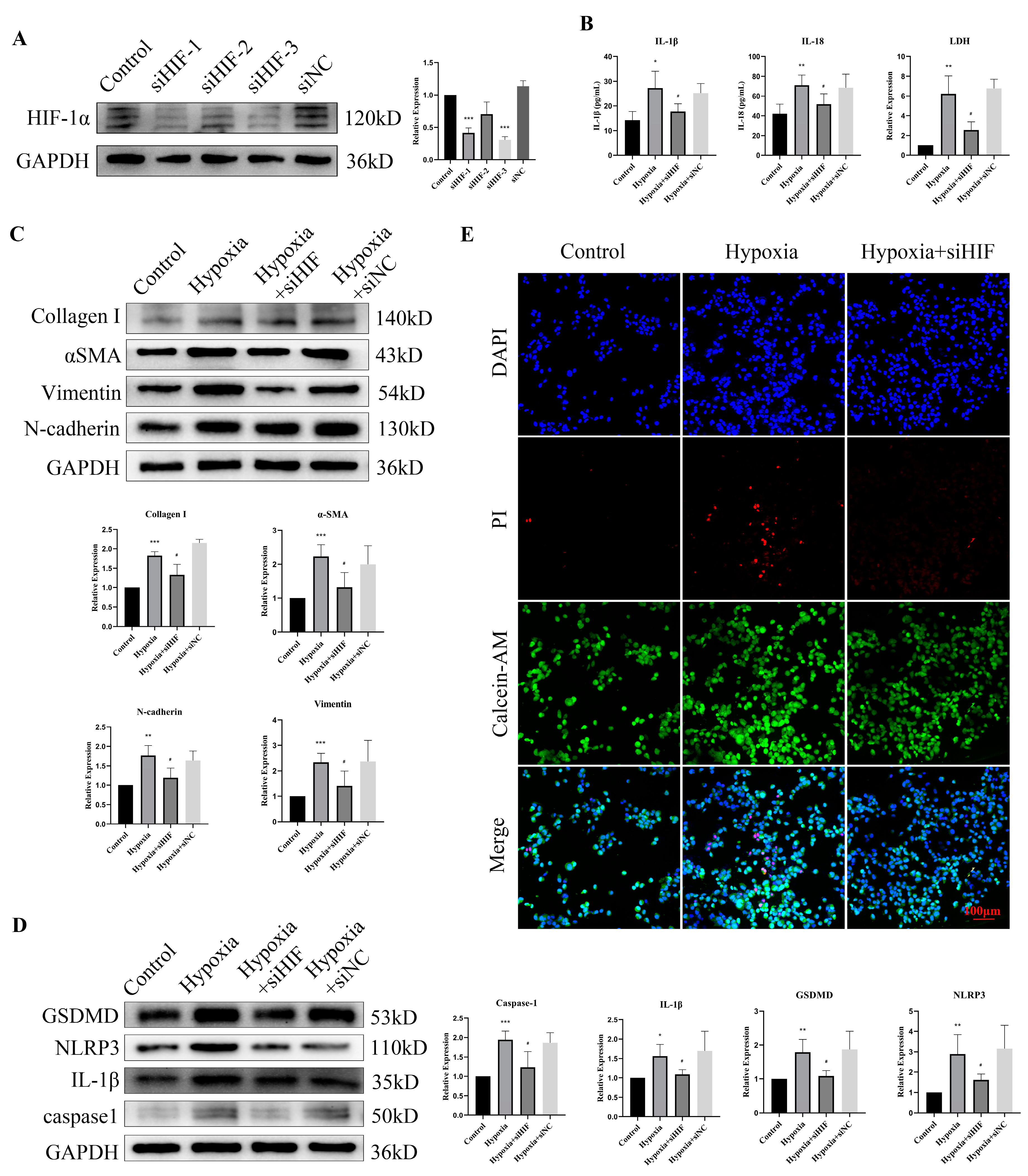
3.5. Hypoxia Increased the Expression of HIF-1α and EMT-Related Proteins in Bladder Epithelial Cells
3.6. Hypoxia Stimulation Induced Pyroptosis and Promoted the Release of Inflammatory Factors in Bladder Epithelial Cells
3.7. HIF-1α Knockdown Rescued Hypoxia-Induced Pyroptosis and EMT
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stein, R.; Bogaert, G.; Dogan, H.S.; Hoen, L.; Kocvara, R.; Nijman, R.J.M.; Quadackers, J.; Rawashdeh, Y.F.; Silay, M.S.; Tekgul, S.; et al. EAU/ESPU guidelines on the management of neurogenic bladder in children and adolescent part I diagnostics and conservative treatment. Neurourol. Urodyn. 2020, 39, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Sripathi, V.; Mitra, A. Management of Neurogenic Bladder. Indian J. Pediatr. 2017, 84, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.T.; Tingskov, S.J.; Djurhuus, J.C.; Nørregaard, R.; Olsen, L.H. Can bladder fibrosis in congenital urinary tract obstruction be reversed? J. Pediatr. Urol. 2017, 13, 574–580. [Google Scholar] [CrossRef]
- Lin, A.T.; Monson, F.C.; Kato, K.; Haugaard, N.; Wein, A.J.; Levin, R.M. Effect of chronic ischemia on glucose metabolism of rabbit urinary bladder. J. Urol. 1989, 142, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef]
- Zhu, Y.; Tan, J.; Xie, H.; Wang, J.; Meng, X.; Wang, R. HIF-1α regulates EMT via the Snail and β-catenin pathways in paraquat poisoning-induced early pulmonary fibrosis. J. Cell. Mol. Med. 2016, 20, 688–697. [Google Scholar] [CrossRef]
- Yu, L.M.; Zhang, W.H.; Han, X.X.; Li, Y.Y.; Lu, Y.; Pan, J.; Mao, J.Q.; Zhu, L.Y.; Deng, J.J.; Huang, W.; et al. Hypoxia-Induced ROS Contribute to Myoblast Pyroptosis during Obstructive Sleep Apnea via the NF-κB/HIF-1α Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 4596368. [Google Scholar] [CrossRef]
- Huang, R.Y.; Guilford, P.; Thiery, J.P. Early events in cell adhesion and polarity during epithelial-mesenchymal transition. J. Cell Sci. 2012, 125, 4417–4422. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Byrne, N.J.; Soni, S.; Takahara, S.; Ferdaoussi, M.; Al Batran, R.; Darwesh, A.M.; Levasseur, J.L.; Beker, D.; Vos, D.Y.; Schmidt, M.A.; et al. Chronically Elevating Circulating Ketones Can Reduce Cardiac Inflammation and Blunt the Development of Heart Failure. Circ. Heart Fail. 2020, 13, e006573. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; He, Y.; Xing, D.; Liu, E.; Yang, X.; Zhu, W.; Wang, Q.; Wen, J.G. The TGF-β1 pathway is early involved in neurogenic bladder fibrosis of juvenile rats. Pediatr. Res. 2021, 90, 759–767. [Google Scholar] [CrossRef]
- Wang, Q.W.; Wen, J.G.; Song, D.K.; Su, J.; Che, Y.Y.; Zhang, P.; Du, A.M.; Wang, D.X.; Zhu, Q.H.; Wei, J.X. Is it possible to use urodynamic variables to predict upper urinary tract dilatation in children with neurogenic bladder-sphincter dysfunction? BJU Int. 2006, 98, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Koch, L. Development: Transcriptomic blueprints. Nat. Rev. Genet. 2017, 18, 639. [Google Scholar] [CrossRef]
- Floriddia, E. Transcriptomics predicts activity. Nat. Neurosci. 2022, 25, 977. [Google Scholar] [CrossRef]
- Austin, P.F.; Bauer, S.B.; Bower, W.; Chase, J.; Franco, I.; Hoebeke, P.; Rittig, S.; Walle, J.V.; von Gontard, A.; Wright, A.; et al. The standardization of terminology of lower urinary tract function in children and adolescents: Update report from the standardization committee of the International Children’s Continence Society. Neurourol. Urodyn. 2016, 35, 471–481. [Google Scholar] [CrossRef]
- Lieb, J.I.; Chichester, P.; Kogan, B.; Das, A.K.; Leggett, R.E.; Schröder, A.; Levin, R.M. Rabbit urinary bladder blood flow changes during the initial stage of partial outlet obstruction. J. Urol. 2000, 164, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Monson, F.C.; Wein, A.J.; Ruggieri, M.R.; Levin, R.M. The effects of short-term in-vivo ischemia on the contractile function of the rabbit urinary bladder. J. Urol. 1988, 139, 1350–1354. [Google Scholar] [CrossRef]
- Wu, Q.; You, L.; Nepovimova, E.; Heger, Z.; Wu, W.; Kuca, K.; Adam, V. Hypoxia-inducible factors: Master regulators of hypoxic tumor immune escape. J. Hematol. Oncol. 2022, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- De Heer, E.C.; Jalving, M.; Harris, A.L. HIFs, angiogenesis, and metabolism: Elusive enemies in breast cancer. J. Clin. Investig. 2020, 130, 5074–5087. [Google Scholar] [CrossRef]
- Koritsiadis, G.; Stravodimos, K.; Koutalellis, G.; Agrogiannis, G.; Koritsiadis, S.; Lazaris, A.; Constantinides, C. Immunohistochemical estimation of hypoxia in human obstructed bladder and correlation with clinical variables. BJU Int. 2008, 102, 328–332. [Google Scholar] [CrossRef]
- Panicker, J.N. Neurogenic Bladder: Epidemiology, Diagnosis, and Management. Semin. Neurol. 2020, 40, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Wang, F.; Wang, Y.; Ju, S. CSN8 is a key regulator in hypoxia-induced epithelial-mesenchymal transition and dormancy of colorectal cancer cells. Mol. Cancer 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.F.; Kimura, K.; Iwano, M.; Haase, V.H. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle 2008, 7, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.F.; Kimura, K.; Bernhardt, W.M.; Shrimanker, N.; Akai, Y.; Hohenstein, B.; Saito, Y.; Johnson, R.S.; Kretzler, M.; Cohen, C.D.; et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Investig. 2007, 117, 3810–3820. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.Y.; Wu, V.W.C.; Law, H.K.W. Hypoxia-Induced Epithelial-Mesenchymal Transition in Cancers: HIF-1α and Beyond. Front. Oncol. 2020, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Che, Y.; Zhang, Q.; Huang, H.; Ding, C.; Wang, Y.; Wang, G.; Cao, L.; Hao, H. Apaf-1 Pyroptosome Senses Mitochondrial Permeability Transition. Cell Metab. 2021, 33, 424–436.e410. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.H.; Yoon, J.E.; Ko, M.S.; Leem, J.; Yun, J.Y.; Hong, C.H.; Cho, Y.K.; Lee, S.E.; Jang, J.E.; Baek, J.Y.; et al. Sphingomyelin synthase 1 mediates hepatocyte pyroptosis to trigger non-alcoholic steatohepatitis. Gut 2021, 70, 1954–1964. [Google Scholar] [CrossRef] [PubMed]
- Kadono, K.; Kageyama, S.; Nakamura, K.; Hirao, H.; Ito, T.; Kojima, H.; Dery, K.J.; Li, X.; Kupiec-Weglinski, J.W. Myeloid Ikaros-SIRT1 signaling axis regulates hepatic inflammation and pyroptosis in ischemia-stressed mouse and human liver. J. Hepatol. 2022, 76, 896–909. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Gong, Q.; Guo, J. Pyroptosis: Mechanisms and Links with Fibrosis. Cells 2021, 10, 3509. [Google Scholar] [CrossRef]
- Jorgensen, I.; Rayamajhi, M.; Miao, E.A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 2017, 17, 151–164. [Google Scholar] [CrossRef]
- Anders, H.J.; Suarez-Alvarez, B.; Grigorescu, M.; Foresto-Neto, O.; Steiger, S.; Desai, J.; Marschner, J.A.; Honarpisheh, M.; Shi, C.; Jordan, J.; et al. The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int. 2018, 93, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.M.; Yang, R.Q.; Li, Y.; Ning, Z.W.; Zhang, L.L.; Zhou, G.S.; Luo, W.; Li, D.H.; Chen, Y.; Pan, M.X.; et al. Angiotensin-(1-7) Improves Liver Fibrosis by Regulating the NLRP3 Inflammasome via Redox Balance Modulation. Antioxid. Redox Signal. 2016, 24, 795–812. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Portugall, I.; Bartok, E.; Dhana, E.; Evers, B.D.; Primiano, M.J.; Hall, J.P.; Franklin, B.S.; Knolle, P.A.; Hornung, V.; Hartmann, G.; et al. An NLRP3-specific inflammasome inhibitor attenuates crystal-induced kidney fibrosis in mice. Kidney Int. 2016, 90, 525–539. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Yeh, H.-C.; Chiu, F.-Y.; Wu, H.-E.; Fang, H.-C.; Li, C.-Y. The role of HIF-1α in regulating NLRP3 inflammasome activation in bladder cancer. J. Clin. Oncol. 2020, 38, e17028. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Chun, J.; Vilaysane, A.; Clark, S.; French, G.; Bracey, N.A.; Trpkov, K.; Bonni, S.; Duff, H.J.; et al. Inflammasome-independent NLRP3 augments TGF-β signaling in kidney epithelium. J. Immunol. 2013, 190, 1239–1249. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Hong, Y.; Chen, J.; Zhou, X.; Tian, X.; Yu, Y.; Shen, L.; Long, C.; Cai, M.; Wu, S.; et al. Hypoxia-Induced HIF-1α Expression Promotes Neurogenic Bladder Fibrosis via EMT and Pyroptosis. Cells 2022, 11, 3836. https://doi.org/10.3390/cells11233836
Li Q, Hong Y, Chen J, Zhou X, Tian X, Yu Y, Shen L, Long C, Cai M, Wu S, et al. Hypoxia-Induced HIF-1α Expression Promotes Neurogenic Bladder Fibrosis via EMT and Pyroptosis. Cells. 2022; 11(23):3836. https://doi.org/10.3390/cells11233836
Chicago/Turabian StyleLi, Qi, Yifan Hong, Jing Chen, Xiazhu Zhou, Xiaomao Tian, Yihang Yu, Lianju Shen, Chunlan Long, Miao Cai, Shengde Wu, and et al. 2022. "Hypoxia-Induced HIF-1α Expression Promotes Neurogenic Bladder Fibrosis via EMT and Pyroptosis" Cells 11, no. 23: 3836. https://doi.org/10.3390/cells11233836
APA StyleLi, Q., Hong, Y., Chen, J., Zhou, X., Tian, X., Yu, Y., Shen, L., Long, C., Cai, M., Wu, S., & Wei, G. (2022). Hypoxia-Induced HIF-1α Expression Promotes Neurogenic Bladder Fibrosis via EMT and Pyroptosis. Cells, 11(23), 3836. https://doi.org/10.3390/cells11233836




