Purification and Identification of Peptides from Oyster (Crassostrea hongkongensis) Protein Enzymatic Hydrolysates and Their Anti-Skin Photoaging Effects on UVB-Irradiated HaCaT Cells
Abstract
1. Introduction
2. Results
2.1. Hongkong Oyster (Crassostrea hongkongensis) Protein Hydrolysates (OPH) and Its Ultrafiltration Components Protected against UVB-Induced Damage in HaCaT Cells
2.2. Peptides Separation by Sephadex G-25 Column
2.3. Peptides Separation by RP-HPLC
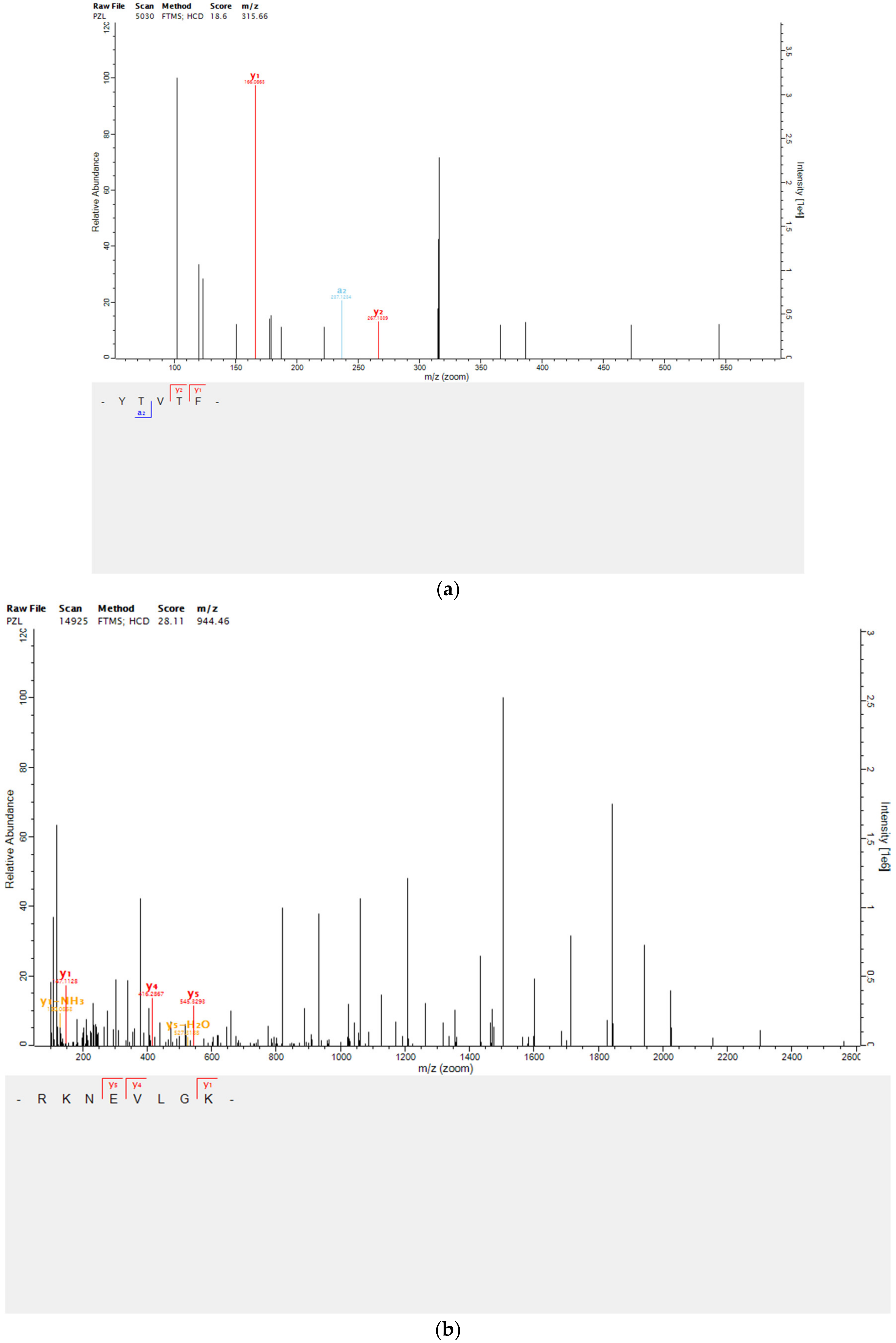
2.4. Peptides Identification by Mass Spectrometry
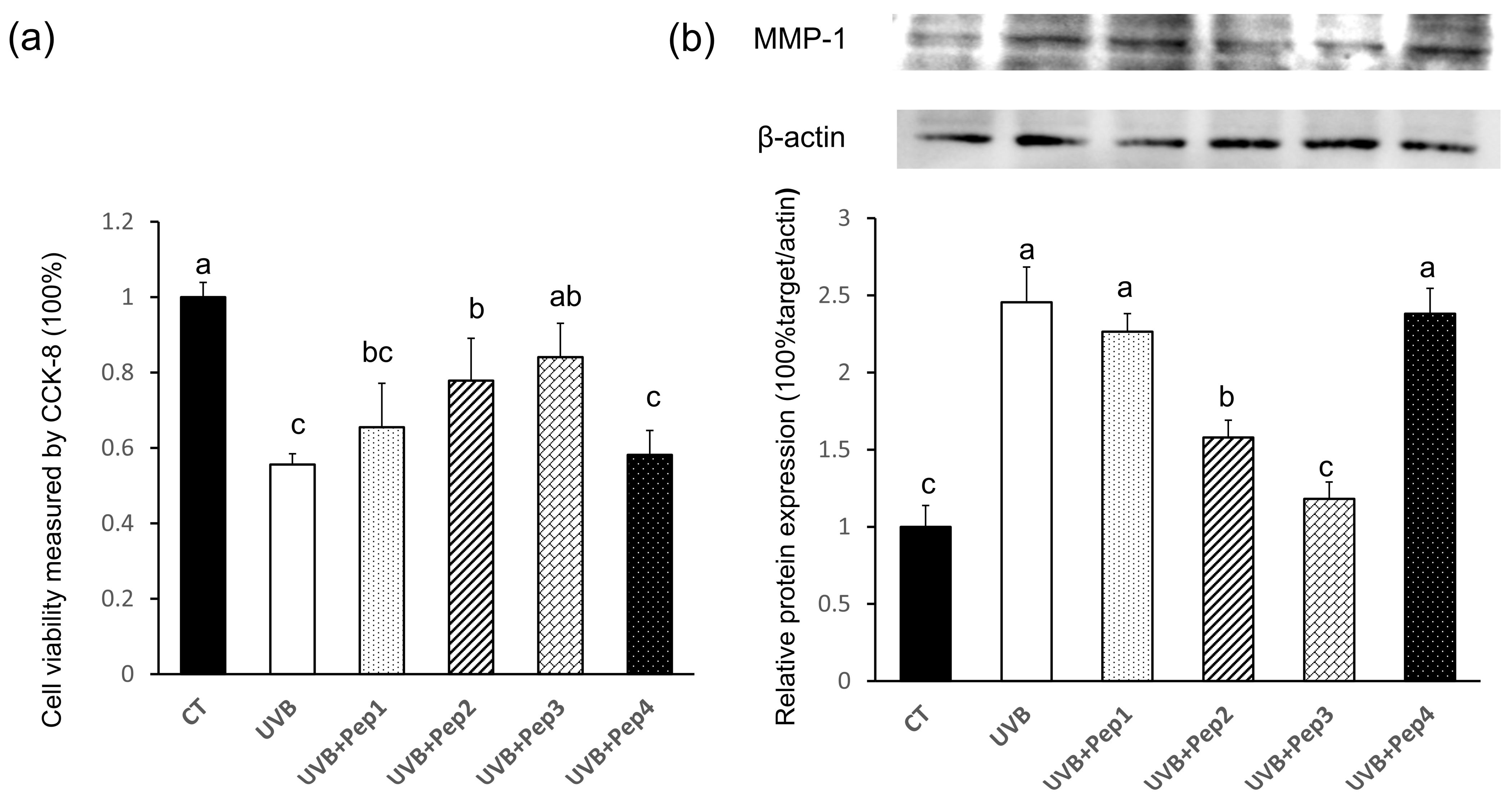
2.5. Validation of the Antiphotoaging Effects of Four Peptides
2.6. Effect of WNLNP on Cell Viability, Oxidative Stress, and Pro-Collagen I Production
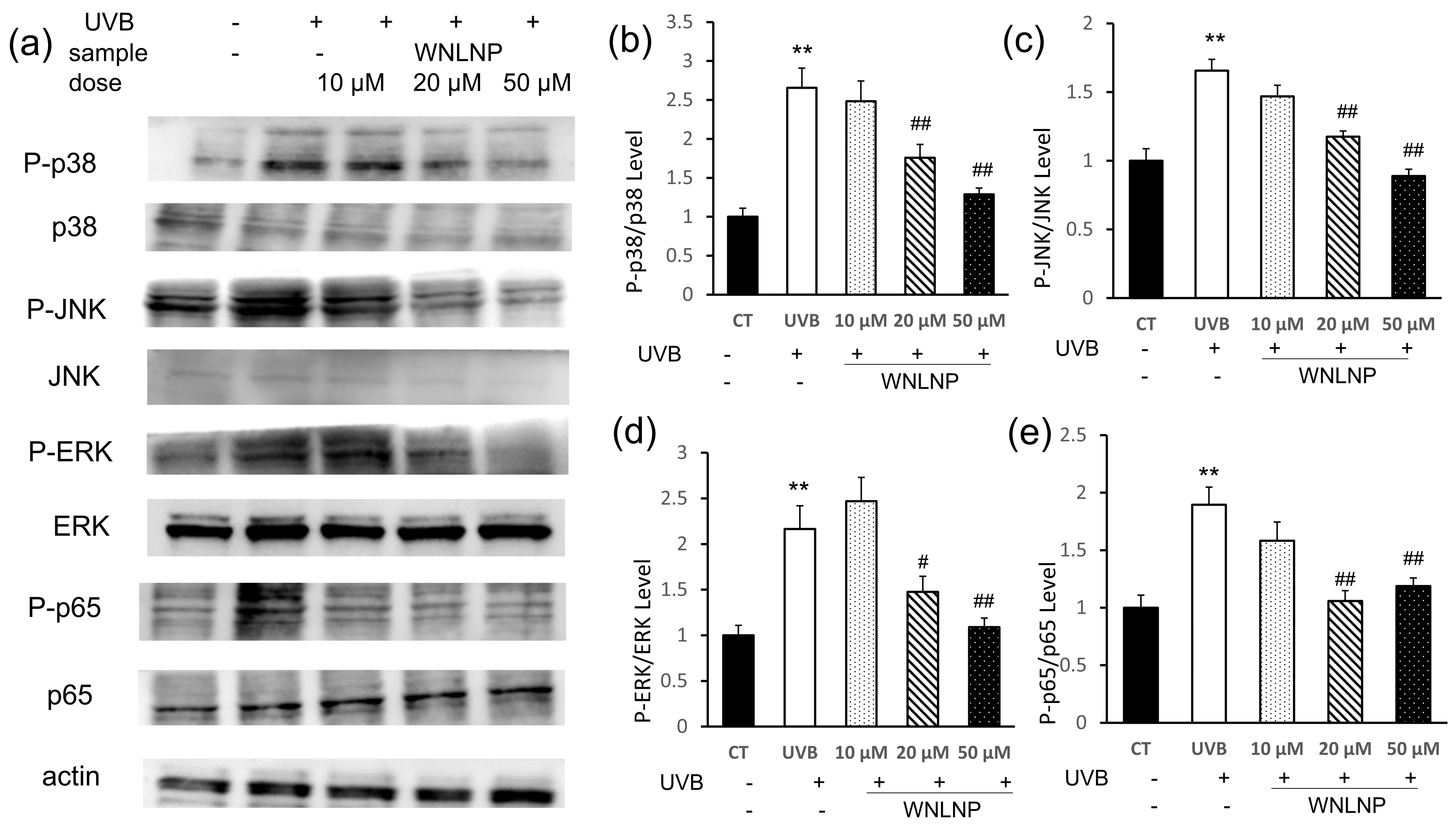
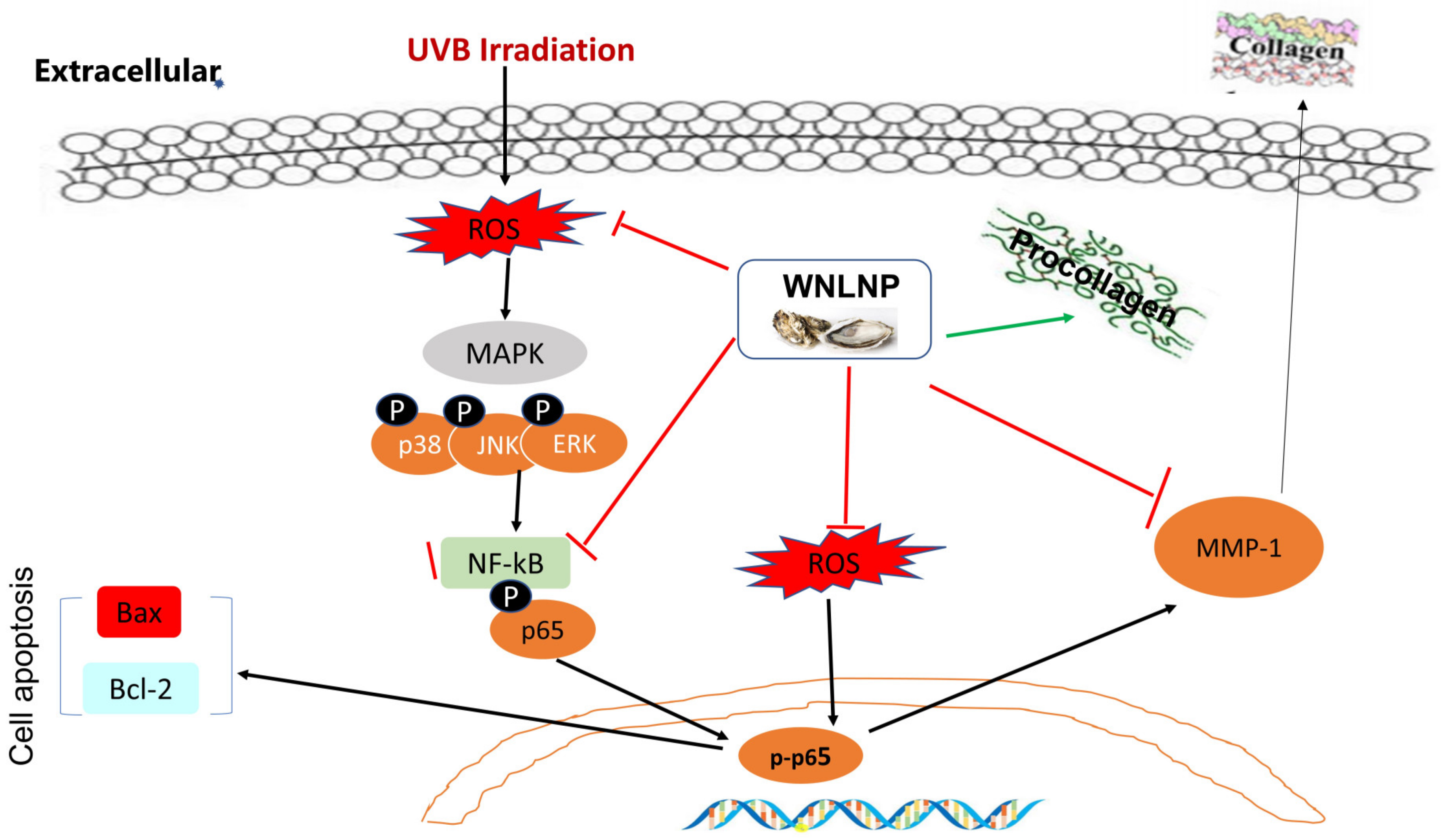
2.7. Regulated Effect of WNLNP on MAPK and NF-κB Signaling Pathways
2.8. WNLNP Regulates the Overexpression of MMP-1 and Apoptosis-Related Signaling Pathway
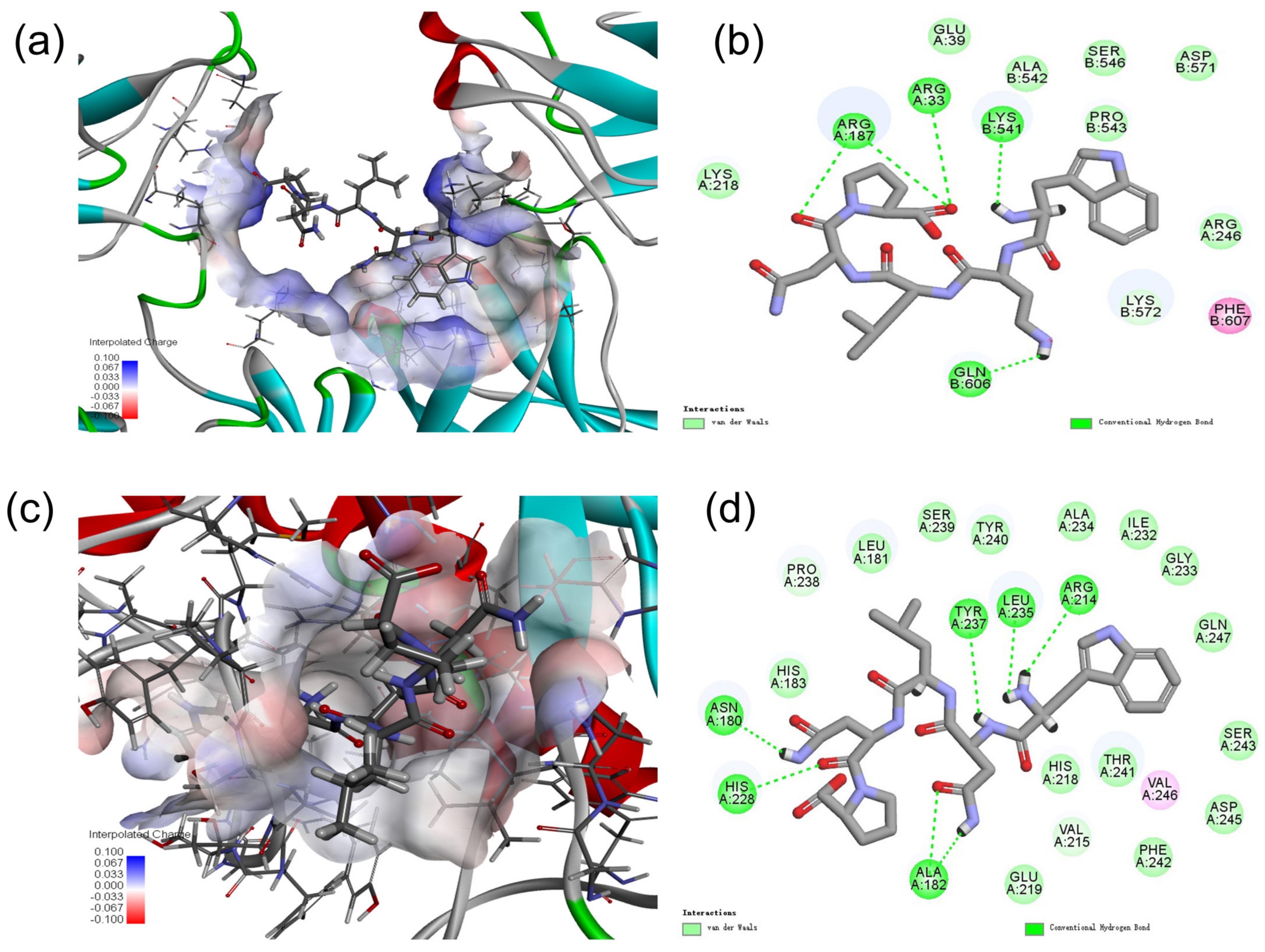
2.9. Molecular Docking Analysis of MMP-1 with WNLNP
3. Discussion
4. Materials and Methods
4.1. Preparation of Oyster Protein Hydrolysate with Low Molecular Weight
4.2. Cell Culture & Cell Viability Test
4.3. Western Blotting
4.4. Sephadex G-25 Purification
4.5. Purification by Reverse Phase Liquid Chromatography
4.6. Sequence Characterization and Chemical Synthesis
4.7. Evaluation of Intracellular ROS Levels in HaCat Cells
4.8. Level of Extracellular Procollagen I Tested by Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Derm.-Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Helfrich, Y.R.; Sachs, D.L.; Voorhees, J.J. Overview of skin aging and photoaging. Dermatol. Nurs. 2008, 20, 177–183. [Google Scholar] [PubMed]
- Diffey, B.L. Solar ultraviolet radiation effects on biological systems. Phys. Med. Biol. 1991, 36, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Guerra, K.C.; Zafar, N.; Crane, J.S. Skin Cancer Prevention; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Awad, F.; Assrawi, E.; Louvrier, C.; Jumeau, C.; Giurgea, I.; Amselem, S.; Karabina, S.A. Photoaging and skin cancer: Is the inflammasome the missing link? Mech. Ageing Dev. 2018, 172, 131–137. [Google Scholar] [CrossRef]
- Halder, R.M.; Ara, C.J. Skin cancer and photoaging in ethnic skin. Dermatol. Clin. 2003, 21, 725–732. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.-Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.-W.; Yi, T.H. Gallic Acid Regulates Skin Photoaging in UVB-exposed Fibroblast and Hairless Mice. Phytother. Res. 2014, 28, 1778–1788. [Google Scholar] [CrossRef]
- Liu, S.; You, L.; Zhao, Y.; Chang, X. Hawthorn Polyphenol Extract Inhibits UVB-Induced Skin Photoaging by Regulating MMP Expression and Type I Procollagen Production in Mice. J. Agric. Food Chem. 2018, 66, 8537–8546. [Google Scholar] [CrossRef]
- Muzaffer, U.; Paul, V.; Prasad, R.; Karthikeyan, R.; Agilan, B. Protective effect of Juglans regia L. against ultraviolet B radiation induced inflammatory responses in human epidermal keratinocytes. Phytomedicine 2018, 42, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Calcabrini, C.; De Bellis, R.; Mancini, U.; Cucchiarini, L.; Stocchi, V.; Potenza, L. Protective Effect of Juglans regia L. Walnut Extract Against Oxidative DNA Damage. Plant Foods Hum. Nutr. 2017, 72, 192–197. [Google Scholar] [CrossRef]
- Yoo, J.H.; Kim, J.K.; Yang, H.J.; Park, K.M. Effects of Egg Shell Membrane Hydrolysates on UVB-radiation-induced Wrinkle Formation in SKH-1 Hairless Mice. Korean J. Food Sci. Anim. Resour. 2015, 35, 58–70. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, B.; Zheng, Q.; Zhu, G.; Cao, W.; Qin, X.; Zhang, C. Ameliorative Effects of Peptides from the Oyster (Crassostrea hongkongensis) Protein Hydrolysates against UVB-Induced Skin Photodamage in Mice. Mar. Drugs 2020, 18, 288. [Google Scholar] [CrossRef]
- Liang, J.; Pei, X.; Zhang, Z.; Wang, N.; Wang, J.; Li, Y. The Protective Effects of Long-Term Oral Administration of Marine Collagen Hydrolysate from Chum Salmon on Collagen Matrix Homeostasis in the Chronological Aged Skin of Sprague-Dawley Male Rats. J. Food Sci. 2010, 75, H230–H238. [Google Scholar] [CrossRef]
- Xiao, Z.; Liang, P.; Chen, J.; Chen, M.; Gong, F.; Li, C.; Zhou, C.; Hong, P.; Yang, P.; Qian, Z. A Peptide YGDEY from Tilapia Gelatin Hydrolysates Inhibits UVB-mediated Skin Photoaging by Regulating MMP-1 and MMP-9 Expression in HaCaT Cells. Photochem. Photobiol. 2019, 95, 1424–1432. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef]
- Fuda, H.; Watanabe, M.; Hui, S.-P.; Joko, S.; Okabe, H.; Jin, S.; Takeda, S.; Miki, E.; Watanabe, T.; Chiba, H. Anti-apoptotic effects of novel phenolic antioxidant isolated from the Pacific oyster (Crassostrea gigas) on cultured human hepatocytes under oxidative stress. Food Chem. 2015, 176, 226–233. [Google Scholar] [CrossRef]
- Watanabe, M.; Fuda, H.; Jin, S.; Sakurai, T.; Hui, S.-P.; Takeda, S.; Watanabe, T.; Koike, T.; Chiba, H. A phenolic antioxidant from the Pacific oyster (Crassostrea gigas) inhibits oxidation of cultured human hepatocytes mediated by diphenyl-1-pyrenylphosphine. Food Chem. 2012, 134, 2086–2089. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Zhang, C.; Cao, W.; Qin, X.; Gao, J.; Zheng, H. The purification and identification of immunoregulatory peptides from oyster (Crassostrea hongkongensis) enzymatic hydrolysate. RSC Adv. 2019, 9, 32854–32863. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2017, 245, 205–222. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, W.; Zhang, Z.; Han, X.; Bu, G.; Meng, F.; Kong, F.; Cao, X.; Huang, A.; Feng, Z.; et al. Oral oyster polypeptides protect ovary against d-galactose-induced premature ovarian failure in C57BL/6 mice. J. Sci. Food Agric. 2020, 100, 92–101. [Google Scholar] [CrossRef]
- Han, J.H.; Bang, J.S.; Choi, Y.J.; Choung, S.-Y. Anti-melanogenic effects of oyster hydrolysate in UVB-irradiated C57BL/6J mice and B16F10 melanoma cells via downregulation of cAMP signaling pathway. J. Ethnopharmacol. 2018, 229, 137–144. [Google Scholar] [CrossRef]
- Han, J.-H.; Bang, J.S.; Choi, Y.J.; Choung, S.-Y. Oral administration of oyster (Crassostrea gigas) hydrolysates protects against wrinkle formation by regulating the MAPK pathway in UVB-irradiated hairless mice. Photochem. Photobiol. Sci. 2019, 18, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. Protective Effect of Garlic on Cellular Senescence in UVB-Exposed HaCaT Human Keratinocytes. Nutrients 2016, 8, 464. [Google Scholar] [CrossRef]
- Park, C.; Lee, M.; Kim, K.; Cho, K.; Eun, H.; Yoo, I.; Chung, J. Prevention of ultraviolet radiation-induced premature skin aging by a novel antioxidant, Melanocin A, in hairless mice. J. Investig. Dermatol. 2005, 124, A132. [Google Scholar]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Chen, M.-F.; Gong, F.; Zhang, Y.Y.; Li, C.-Y.; Zhou, C.-X.; Hong, P.-Z.; Sun, S.-L.; Qian, Z.-J. Preventive Effect of YGDEY from Tilapia Fish Skin Gelatin Hydrolysates against Alcohol-Induced Damage in HepG2 Cells through ROS-Mediated Signaling Pathways. Nutrients 2019, 11, 392. [Google Scholar] [CrossRef]
- Kang, K.H.; Qian, Z.J.; Ryu, B.; Kim, D.; Kim, S.K. Protective effects of protein hydrolysate from marine microalgae Navicula incerta on ethanol-induced toxicity in HepG2/CYP2E1 cells. Food Chem. 2012, 132, 677–685. [Google Scholar] [CrossRef]
- Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Bonina, F.; Cardile, V. Hydroxytyrosol from olive fruits prevents blue-light-induced damage in human keratinocytes and fibroblasts. J. Cell. Physiol. 2018, 234, 9065–9076. [Google Scholar] [CrossRef]
- Peres, P.; Terra, V.; Guarnier, F.; Cecchini, R.; Cecchini, A. Photoaging and chronological aging profile: Understanding oxidation of the skin. J. Photochem. Photobiol. B Biol. 2011, 103, 93–97. [Google Scholar] [CrossRef]
- Thyss, R.; Virolle, V.; Imbert, V.; Peyron, J.F.; Aberdam, D.; Virolle, T. NF-KB promotes death in UVB irradiated keratinocytes through Egr-1 induction. J. Investig. Dermatol. 2004, 123, A98. [Google Scholar]
- Bigot, N.; Boumediene, K.; Galera, P.; Oddos, T. NF-kB inhibition of collagen synthesis in aging human dermal fibroblasts. J. Am. Acad. Dermatol. 2012, 66, Ab11. [Google Scholar]
- Xu, D.; Wang, W.; Liao, J.; Liao, L.; Li, C.; Zhao, M. Walnut protein hydrolysates, rich with peptide fragments of WSREEQEREE and ADIYTEEAGR ameliorate UV-induced photoaging through inhibition of the NF-kappaB/MMP-1 signaling pathway in female rats. Food Funct. 2020, 11, 10601–10616. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Lee, H.-J.; Lee, H.; Kim, J.-H.; Hwang, J.; Koo, J.I.; Kim, S.-H. The Anti-Wrinkle Mechanism of Melatonin in UVB Treated HaCaT Keratinocytes and Hairless Mice via Inhibition of ROS and Sonic Hedgehog Mediated Inflammatory Proteins. Int. J. Mol. Sci. 2018, 19, 1995. [Google Scholar] [CrossRef]
- Han, A.-R.; Nam, M.-H.; Lee, K.-W. Plantamajoside Inhibits UVB and Advanced Glycation End Products-Induced MMP-1 Expression by Suppressing the MAPK and NF-κB Pathways in HaCaT Cells. Photochem. Photobiol. 2016, 92, 708–719. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, T.H.; Wahedi, H.M.; Baek, S.H.; Kim, S.Y. Resveratrol-Enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades. Oxid. Med. Cell. Longev. 2017, 2017, 8379539. [Google Scholar] [CrossRef]
- Zeng, Q.; Jiang, J.; Wang, J.; Zhou, Q.; Zhang, X. N-Terminal Acetylation and C-Terminal Amidation of Spirulina platensis-Derived Hexapeptide: Anti-Photoaging Activity and Proteomic Analysis. Mar. Drugs 2019, 17, 520. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, Q.; Yuan, L.; Zhuang, Y. Protective Effects of LSGYGP from Fish Skin Gelatin Hydrolysates on UVB-Induced MEFs by Regulation of Oxidative Stress and Matrix Metalloproteinase Activity. Nutrients 2018, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Xiao, Z.; He, Y.-L.; Tang, Y.; Li, L.; Zhou, C.; Hong, P.; Luo, H.; Qian, Z.-J. Heptapeptide Isolated from Isochrysis zhanjiangensis Exhibited Anti-Photoaging Potential via MAPK/AP-1/MMP Pathway and Anti-Apoptosis in UVB-Irradiated HaCaT Cells. Mar Drugs 2021, 19, 626. [Google Scholar] [CrossRef]
- Bang, J.S.; Jin, Y.J.; Choung, S.-Y. Low molecular polypeptide from oyster hydrolysate recovers photoaging in SKH-1 hairless mice. Toxicol. Appl. Pharmacol. 2019, 386, 114844. [Google Scholar] [CrossRef]
- Chen, C.-L.; Liou, S.-F.; Chen, S.-J.; Shih, M.-F. Protective effects of Chlorella-derived peptide on UVB-induced production of MMP-1 and degradation of procollagen genes in human skin fibroblasts. Regul. Toxicol. Pharmacol. 2011, 60, 112–119. [Google Scholar] [CrossRef]
- Chen, T.; Hou, H.; Fan, Y.; Wang, S.; Chen, Q.; Si, L.; Li, B. Protective effect of gelatin peptides from pacific cod skin against photoaging by inhibiting the expression of MMPs via MAPK signaling pathway. J. Photochem. Photobiol. B Biol. 2016, 165, 34–41. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2010, 1802, 396–405. [Google Scholar] [CrossRef]
- Ryeom, G.G.M.; Bang, W.J.; Kim, Y.B.; Lee, G.E. Gallotannin Improves the Photoaged-Related Proteins by Extracellular Signal-Regulated Kinases/c-Jun N-Terminal Kinases Signaling Pathway in Human Epidermal Keratinocyte Cells. J. Med. Food 2018, 21, 785–792. [Google Scholar] [CrossRef]
- Han, S.H.; Ballinger, E.; Choung, S.-Y.; Kwon, J.Y. Anti-Photoaging Effect of Hydrolysates from Pacific Whiting Skin via MAPK/AP-1, NF-κB, TGF-β/Smad, and Nrf-2/HO-1 Signaling Pathway in UVB-Induced Human Dermal Fibroblasts. Mar. Drugs 2022, 20, 308. [Google Scholar] [CrossRef] [PubMed]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Photoprotective Effects of a Hyperoside-Enriched Fraction Prepared from Houttuynia cordata Thunb. on Ultraviolet B-Induced Skin Aging in Human Fibroblasts through the MAPK Signaling Pathway. Plants 2021, 10, 2628. [Google Scholar] [CrossRef]
- Li, J.; Fu, A.; Zhang, L. An Overview of Scoring Functions Used for Protein–Ligand Interactions in Molecular Docking. Interdiscip. Sci. Comput. Life Sci. 2019, 11, 320–328. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Z.; Gao, J.; Su, W.; Cao, W.; Zhu, G.; Qin, X.; Zhang, C.; Qi, Y. Purification and Identification of Peptides from Oyster (Crassostrea hongkongensis) Protein Enzymatic Hydrolysates and Their Anti-Skin Photoaging Effects on UVB-Irradiated HaCaT Cells. Mar. Drugs 2022, 20, 749. https://doi.org/10.3390/md20120749
Peng Z, Gao J, Su W, Cao W, Zhu G, Qin X, Zhang C, Qi Y. Purification and Identification of Peptides from Oyster (Crassostrea hongkongensis) Protein Enzymatic Hydrolysates and Their Anti-Skin Photoaging Effects on UVB-Irradiated HaCaT Cells. Marine Drugs. 2022; 20(12):749. https://doi.org/10.3390/md20120749
Chicago/Turabian StylePeng, Zhilan, Jialong Gao, Weimin Su, Wenhong Cao, Guoping Zhu, Xiaoming Qin, Chaohua Zhang, and Yi Qi. 2022. "Purification and Identification of Peptides from Oyster (Crassostrea hongkongensis) Protein Enzymatic Hydrolysates and Their Anti-Skin Photoaging Effects on UVB-Irradiated HaCaT Cells" Marine Drugs 20, no. 12: 749. https://doi.org/10.3390/md20120749
APA StylePeng, Z., Gao, J., Su, W., Cao, W., Zhu, G., Qin, X., Zhang, C., & Qi, Y. (2022). Purification and Identification of Peptides from Oyster (Crassostrea hongkongensis) Protein Enzymatic Hydrolysates and Their Anti-Skin Photoaging Effects on UVB-Irradiated HaCaT Cells. Marine Drugs, 20(12), 749. https://doi.org/10.3390/md20120749







