Phytochemical Profile and Selective Cytotoxic Activity of a Solanum bulbocastanum Dun. Methanolic Extract on Breast Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Preparation of S. bulbocastanum Extract
2.2. Phenolic and Alkaloid Profile of the S. bulbocastanum Extract
2.3. Volatile Profile of the S. bulbocastanum Extract
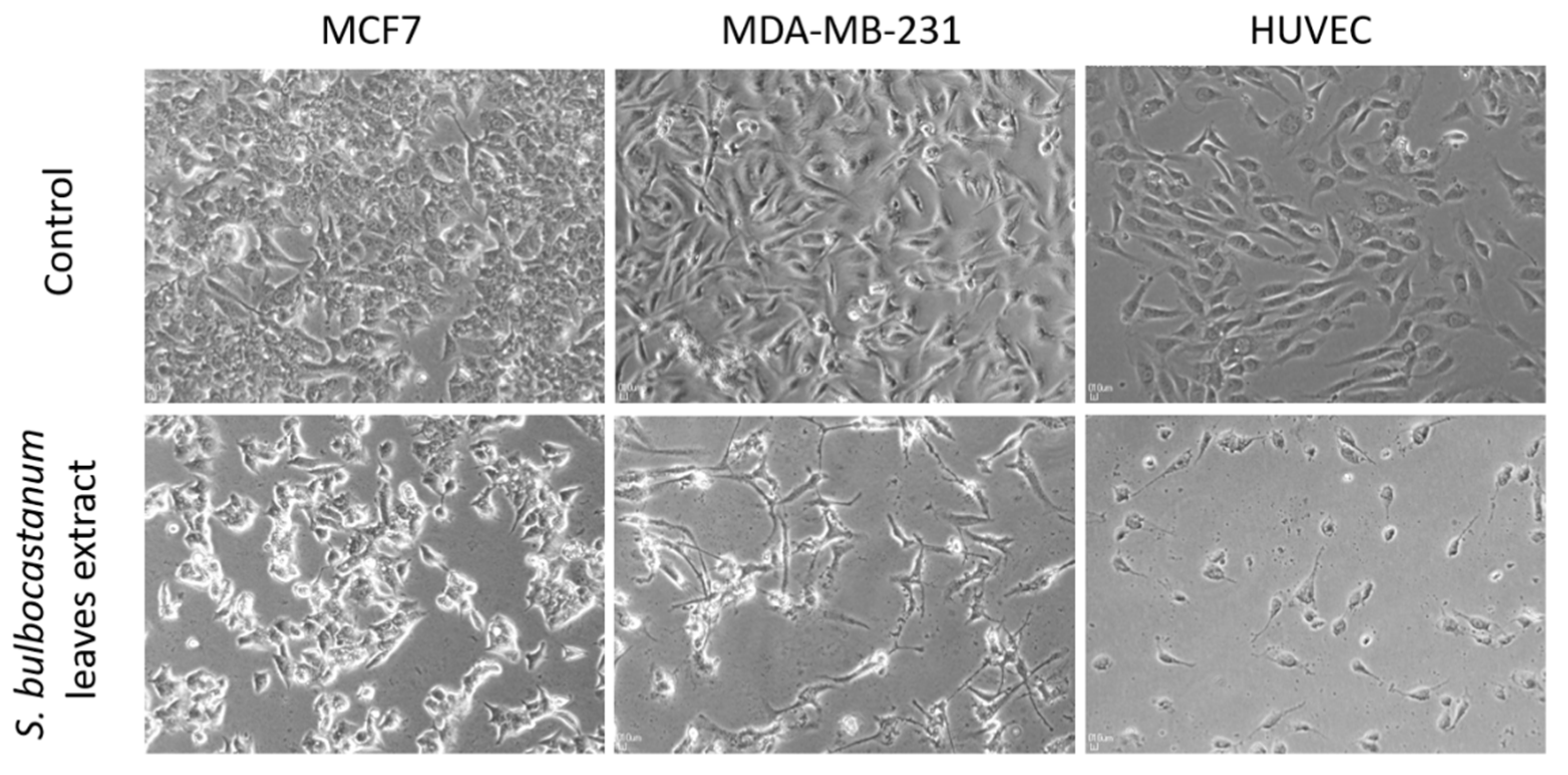
2.4. Selective Cytotoxic Activity of the S. bulbocastanum Extract
3. Discussion
4. Materials and Methods
4.1. Plant Material and Culture Conditions
4.2. Cell Line and Culture Conditions
4.3. Preparation of Plant Extract
4.4. HPLC-PDA/-ESI-MS Identification and Quantification of Phenolic Compounds and Alkaloids
4.5. Non-Targeted ITEX/GC-MS Headspace Analysis of Volatile Compounds
4.6. Selective Cytotoxicity Assay
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saqib, S.; Ullah, F.; Naeem, M.; Younas, M.; Ayaz, A.; Ali, S.; Zaman, W. Mentha: Nutritional and Health Attributes to Treat Various Ailments Including Cardiovascular Diseases. Molecules 2022, 27, 6728. [Google Scholar] [CrossRef]
- Zaman, W.; Ye, J.; Saqib, S.; Liu, Y.; Shan, Z.; Hao, D.; Chen, Z.; Xiao, P. Predicting Potential Medicinal Plants with Phylogenetic Topology: Inspiration from the Research of Traditional Medicine. J. Ethnopharmacol. 2021, 281, 114515. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Kinghorn, A.D.; Pan, L.; Fletcher, J.N.; Chai, H. The Relevance of Higher Plants in Lead Compound Discovery Programs. J. Nat. Prod. 2011, 74, 1539–1555. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-Derived Anticancer Agents: A Green Anticancer Approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Di Modica, M.; Tagliabue, E.; Triulzi, T. Predicting the Efficacy of HER2-Targeted Therapies: A Look at the Host. Dis. Markers 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hon, J.D.C.; Singh, B.; Sahin, A.; Du, G.; Wang, J.; Wang, V.Y.; Zhang, D.Y.; Monaco, M.E.; Lee, P. Breast Cancer Molecular Subtypes: From TNBC to QNBC. Am. J. Cancer Res. 2016, 6, 1864–1872. [Google Scholar]
- Malorni, L.; Shetty, P.B.; De Angelis, C.; Hilsenbeck, S.; Rimawi, M.F.; Elledge, R.; Osborne, C.K.; De Placido, S.; Arpino, G. Clinical and Biologic Features of Triple-Negative Breast Cancers in a Large Cohort of Patients with Long-Term Follow-Up. Breast Cancer Res. Treat. 2012, 136, 795–804. [Google Scholar] [CrossRef]
- Boon, H.S.; Olatunde, F.; Zick, S.M. Trends in Complementary/Alternative Medicine Use by Breast Cancer Survivors: Comparing Survey Data from 1998 and 2005. BMC Women’s Health 2007, 7, 4. [Google Scholar] [CrossRef]
- Molassiotis, A.; Scott, J.A.; Kearney, N.; Pud, D.; Magri, M.; Selvekerova, S.; Bruyns, I.; Fernadez-Ortega, P.; Panteli, V.; Margulies, A.; et al. Complementary and Alternative Medicine Use in Breast Cancer Patients in Europe. Support Care Cancer 2006, 14, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Saghatchian, M.; Bihan, C.; Chenailler, C.; Mazouni, C.; Dauchy, S.; Delaloge, S. Exploring Frontiers: Use of Complementary and Alternative Medicine among Patients with Early-Stage Breast Cancer. Breast 2014, 23, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Allen, A.E.; Locasale, J.W. The Molecular Link from Diet to Cancer Cell Metabolism. Mol. Cell 2020, 78, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Radcliffe, E.B.; Thill, C.A.; Ragsdale, D.W. Resistance to Aphids, Late Blight and Viruses in Somatic Fusions and Crosses of Solanum Tuberosum L. and Solanum Bulbocastanum Dun. Am. J. Potato Res. 2012, 89, 489–500. [Google Scholar] [CrossRef]
- Rakosy-Tican, E.; Thieme, R.; König, J.; Nachtigall, M.; Hammann, T.; Denes, T.-E.; Kruppa, K.; Molnár-Láng, M. Introgression of Two Broad-Spectrum Late Blight Resistance Genes, Rpi-Blb1 and Rpi-Blb3, From Solanum Bulbocastanum Dun Plus Race-Specific R Genes Into Potato Pre-Breeding Lines. Front. Plant Sci. 2020, 11, 699. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental Stress and Secondary Metabolites in Plants. In Plant Metabolites and Regulation Under Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2018; pp. 153–167. ISBN 978-0-12-812689-9. [Google Scholar]
- Kowalczyk, T.; Merecz-Sadowska, A.; Rijo, P.; Mori, M.; Hatziantoniou, S.; Górski, K.; Szemraj, J.; Piekarski, J.; Śliwiński, T.; Bijak, M.; et al. Hidden in Plants—A Review of the Anticancer Potential of the Solanaceae Family in In Vitro and In Vivo Studies. Cancers 2022, 14, 1455. [Google Scholar] [CrossRef]
- Distl, M.; Wink, M. Identification and Quantification of Steroidal Alkaloids from Wild Tuber-Bearing Solanum Species by HPLC and LC-ESI-MS. Potato Res. 2009, 52, 79–104. [Google Scholar] [CrossRef]
- Sedlák, P.; Sedláková, V.; Vašek, J.; Zeka, D.; Čílová, D.; Melounová, M.; Orsák, M.; Domkářová, J.; Doležal, P.; Vejl, P. Phenotypic, Molecular and Biochemical Evaluation of Somatic Hybrids between Solanum Tuberosum and S. Bulbocastanum. Sci. Rep. 2022, 12, 4484. [Google Scholar] [CrossRef]
- Savarese, S.; Andolfi, A.; Cimmino, A.; Carputo, D.; Frusciante, L.; Evidente, A. Glycoalkaloids as Biomarkers for Recognition of Cultivated, Wild, and Somatic Hybrids of Potato. Chem. Biodivers. 2009, 6, 437–446. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansoub, M.A.; Asmawi, M.Z.; Murugaiyah, V. Effect of Extraction Solvents and Plant Parts Used on the Antihyperlipidemic and Antioxidant Effects of Garcinia Atroviridis: A Comparative Study: Antihyperlipidemic and Antioxidant Effects of Garcinia Atroviridis. J. Sci. Food Agric. 2014, 94, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Supriatno; Seow, E.M. Effect of Solvents in Extracting Polyphenols and Antioxidants of Selected Raw Vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Soares, V.; Taujale, R.; Garrett, R.; da Silva, A.J.R.; Borges, R.M. Extending Compound Identification for Molecular Network Using the LipidXplorer Database Independent Method: A Proof of Concept Using Glycoalkaloids from Solanum Pseudoquina A. St.-Hil. Phytochem. Anal. 2019, 30, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Cruceriu, D.; Diaconeasa, Z.; Socaci, S.; Socaciu, C.; Balacescu, O.; Rakosy-Tican, E. Extracts of the Wild Potato Species Solanum Chacoense on Breast Cancer Cells: Biochemical Characterization, In Vitro Selective Cytotoxicity and Molecular Effects. Nutr. Cancer 2020, 73, 630–641. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Bullo, S.; Buskaran, K.; Baby, R.; Dorniani, D.; Fakurazi, S.; Hussein, M.Z. Dual Drugs Anticancer Nanoformulation Using Graphene Oxide-PEG as Nanocarrier for Protocatechuic Acid and Chlorogenic Acid. Pharm. Res. 2019, 36, 91. [Google Scholar] [CrossRef]
- Shao, P.; Zhang, J.F.; Chen, X.X.; Sun, P.L. Microwave-Assisted Extraction and Purification of Chlorogenic Acid from by-Products of Eucommia Ulmoides Oliver and Its Potential Anti-Tumor Activity. J. Food Sci. Technol. 2015, 52, 4925–4934. [Google Scholar] [CrossRef]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Wang, S.; Zhang, Q.; Zhao, J.; Song, L. Chlorogenic Acid Induces Apoptosis, Inhibits Metastasis and Improves Antitumor Immunity in Breast Cancer via the NF-κB Signaling Pathway. Oncol. Rep. 2020, 45, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Farha, A.K.; Gan, R.-Y.; Li, H.-B.; Wu, D.-T.; Atanasov, A.G.; Gul, K.; Zhang, J.-R.; Yang, Q.-Q.; Corke, H. The Anticancer Potential of the Dietary Polyphenol Rutin: Current Status, Challenges, and Perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 832–859. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A Review on Extraction, Identification and Purification Methods, Biological Activities and Approaches to Enhance Its Bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Orfali, G.d.C.; Duarte, A.C.; Bonadio, V.; Martinez, N.P.; de Araújo, M.E.M.B.; Priviero, F.B.M.; Carvalho, P.O.; Priolli, D.G. Review of Anticancer Mechanisms of Isoquercitin. WJCO 2016, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Shakya, R.; Navarre, D.A. LC-MS Analysis of Solanidane Glycoalkaloid Diversity among Tubers of Four Wild Potato Species and Three Cultivars (Solanum Tuberosum). J. Agric. Food Chem. 2008, 56, 6949–6958. [Google Scholar] [CrossRef] [PubMed]
- Boukhatem, M.N.; Sudha, T.; Darwish, N.H.E.; Chader, H.; Belkadi, A.; Rajabi, M.; Houche, A.; Benkebailli, F.; Oudjida, F.; Mousa, S.A. A New Eucalyptol-Rich Lavender (Lavandula Stoechas L.) Essential Oil: Emerging Potential for Therapy against Inflammation and Cancer. Molecules 2020, 25, 3671. [Google Scholar] [CrossRef] [PubMed]
- Leighton, X.; Bera, A.; Eidelman, O.; Eklund, M.; Puthillathu, N.; Pollard, H.B.; Srivastava, M. High ANXA7 Potentiates Eucalyptol Toxicity in Hormone-Refractory Prostate Cancer. Anticancer Res. 2018, 38, 3831–3842. [Google Scholar] [CrossRef]
- Holliday, D.L.; Speirs, V. Choosing the Right Cell Line for Breast Cancer Research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef]
- Kiokias, S.; Oreopoulou, V. A Review of the Health Protective Effects of Phenolic Acids against a Range of Severe Pathologic Conditions (Including Coronavirus-Based Infections). Molecules 2021, 26, 5405. [Google Scholar] [CrossRef]
- Uluwaduge, D.I. Glycoalkaloids, Bitter Tasting Toxicants in Potatoes: A Review. Int. J. Food Sci. Nutr. 2018, 3, 188–193. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient Requirements of Suspension Cultures of Soybean Root Cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef] [PubMed]
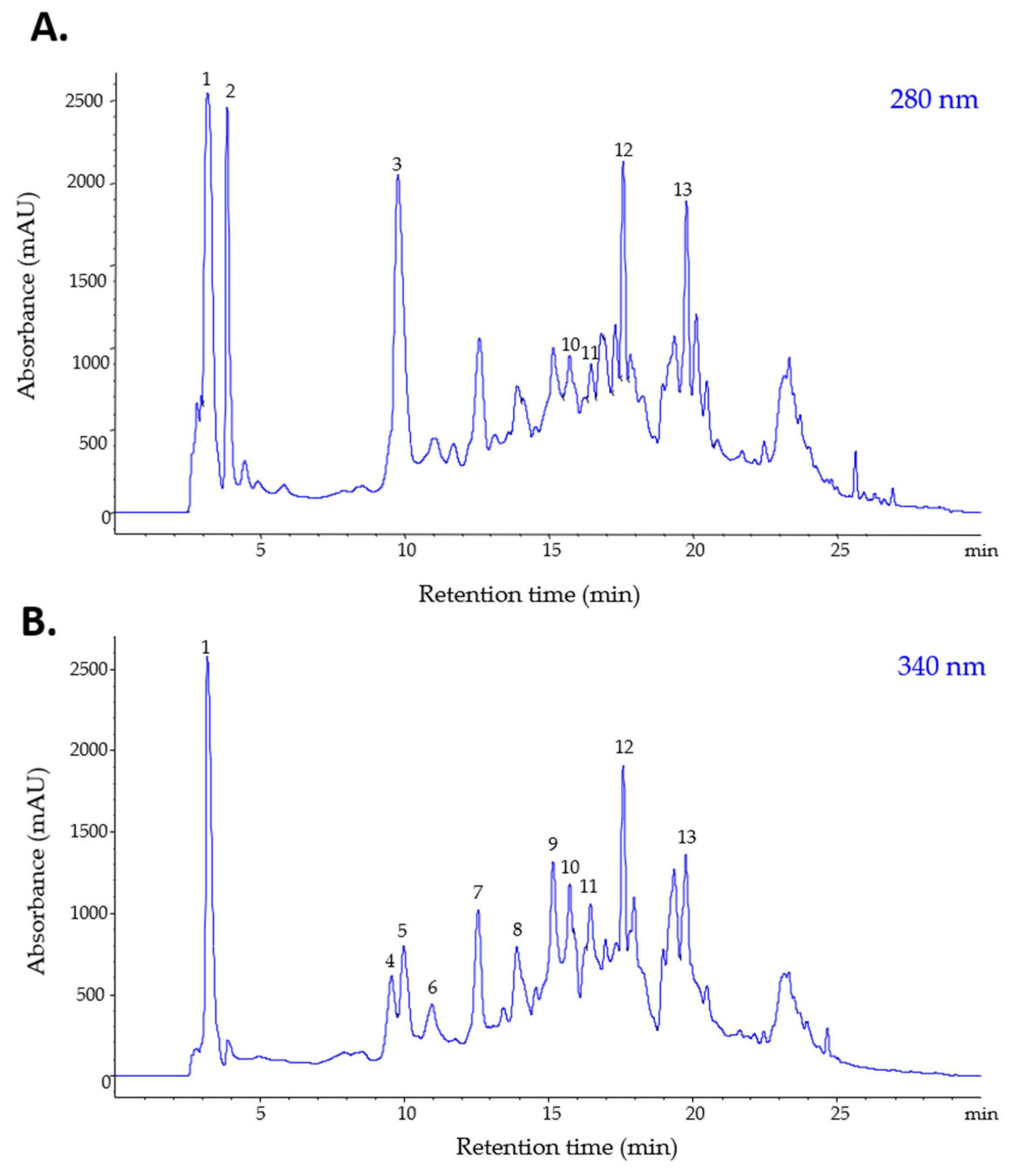

| Class of Compounds | Peak No. | Rt (min) | [M + H]+ (m/z) | UV λmax (nm) | Compound | Concentration (µg/mL Extract) |
|---|---|---|---|---|---|---|
| Hydroxybenzoic acid (BA) | 1 | 3.21 | 139 | 265 | Hydroxybenzoic acid | 1238.708 |
| 2 | 3.86 | 156 | 260 | Dihydroxybenzoic acid | 596.786 | |
| 4 | 9.77 | 156 | 260 | Protocatechuic acid | 998.314 | |
| Total BA 1 | 2833.808 | |||||
| Hydroxycinnamic acid (HBA) | 3 | 9.56 | 369 | 330 | 3-Feruloyquinic acid | 337.601 |
| 5 | 9.98 | 355 | 322 | 3-Caffeoylquinic acid (Neochlorogenic acid) | 644.398 | |
| 6 | 10.96 | 355 | 322 | 4-Caffeoylquinic acid (Cryptochlorogenic acid) | 305.146 | |
| 7 | 12.55 | 355 | 322 | 5-Caffeoylquinic acid (Chlorogenic acid) | 671.983 | |
| 8 | 13.89 | 181 | 322 | Caffeic acid | 476.366 | |
| 9 | 15.15 | 369 | 330 | 5-Feruloyquinic acid | 690.313 | |
| Total HBA 2 | 3125.807 | |||||
| Flavonols (FL) | 10 | 15.75 | 611 | 360, 255 | Quercetin-rutinoside (Rutin) | 452.452 |
| 11 | 16.29 | 465 | 360, 255 | Quercetin-glucoside | 366.467 | |
| Total FL 3 | 818.919 | |||||
| Alkaloid (ALK) | 12 | 17.61 | 467 | 370,320,230 | Alkaloid precursor | 441.431 |
| 13 | 19.76 | 450 | 420,310,240 | Alkaloid precursor | 372.093 | |
| Total ALK 2 | 813.524 | |||||
| Peak No. | Compound | Rt (min) | Relative Abundance (%) | Area | Height | SI |
|---|---|---|---|---|---|---|
| 1 | Acetic acid, butyl ester | 6.081 | 85.23 | 42,403,774 | 7,960,501 | 99 |
| 2 | Benzaldehyde | 11.723 | 0.26 | 130,981 | 82,780 | 78 |
| 3 | Phenol | 12.368 | 0.97 | 484,938 | 130,120 | 99 |
| 4 | Eucalyptol | 14.249 | 0.43 | 212,757 | 77,371 | 95 |
| 5 | Acetophenone | 15.464 | 1.98 | 987,044 | 296,437 | 99 |
| 6 | Butanoic acid, 2-methyl-, 3-methylbutyl ester | 16.654 | 0.26 | 129,757 | 56,950 | 95 |
| 7 | Butanoic acid, 3-methyl-, 3-methylbutyl ester | 16.874 | 1.48 | 738,417 | 288,419 | 100 |
| 8 | n-Amyl isovalerate | 16.941 | 0.62 | 309,632 | 135,954 | 99 |
| 9 | Ethanone, 1-[4-(1-methylethyl)phenyl]- | 22.789 | 8.76 | 4,356,596 | 1,174,963 | 100 |
| Cell Line | S. bulbocastanum Leaves Extract | |
|---|---|---|
| IC50 (µg/mL) | Selectivity Coefficient | |
| MCF7 | 139.1 | 4.96 |
| MDA-MB-231 | 336.4 | 2.05 |
| Hs578T | 351.6 | 1.96 |
| HUVEC | 689.9 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraschiv, M.; Csiki, M.; Diaconeasa, Z.; Socaci, S.; Balacescu, O.; Rakosy-Tican, E.; Cruceriu, D. Phytochemical Profile and Selective Cytotoxic Activity of a Solanum bulbocastanum Dun. Methanolic Extract on Breast Cancer Cells. Plants 2022, 11, 3262. https://doi.org/10.3390/plants11233262
Paraschiv M, Csiki M, Diaconeasa Z, Socaci S, Balacescu O, Rakosy-Tican E, Cruceriu D. Phytochemical Profile and Selective Cytotoxic Activity of a Solanum bulbocastanum Dun. Methanolic Extract on Breast Cancer Cells. Plants. 2022; 11(23):3262. https://doi.org/10.3390/plants11233262
Chicago/Turabian StyleParaschiv, Mihnea, Magda Csiki, Zorita Diaconeasa, Sonia Socaci, Ovidiu Balacescu, Elena Rakosy-Tican, and Daniel Cruceriu. 2022. "Phytochemical Profile and Selective Cytotoxic Activity of a Solanum bulbocastanum Dun. Methanolic Extract on Breast Cancer Cells" Plants 11, no. 23: 3262. https://doi.org/10.3390/plants11233262
APA StyleParaschiv, M., Csiki, M., Diaconeasa, Z., Socaci, S., Balacescu, O., Rakosy-Tican, E., & Cruceriu, D. (2022). Phytochemical Profile and Selective Cytotoxic Activity of a Solanum bulbocastanum Dun. Methanolic Extract on Breast Cancer Cells. Plants, 11(23), 3262. https://doi.org/10.3390/plants11233262











