Tetrabromobisphenol A and Diclazuril Evoke Tissue-Specific Changes of Thyroid Hormone Signaling in Male Thyroid Hormone Action Indicator Mice
Abstract
1. Introduction
2. Results
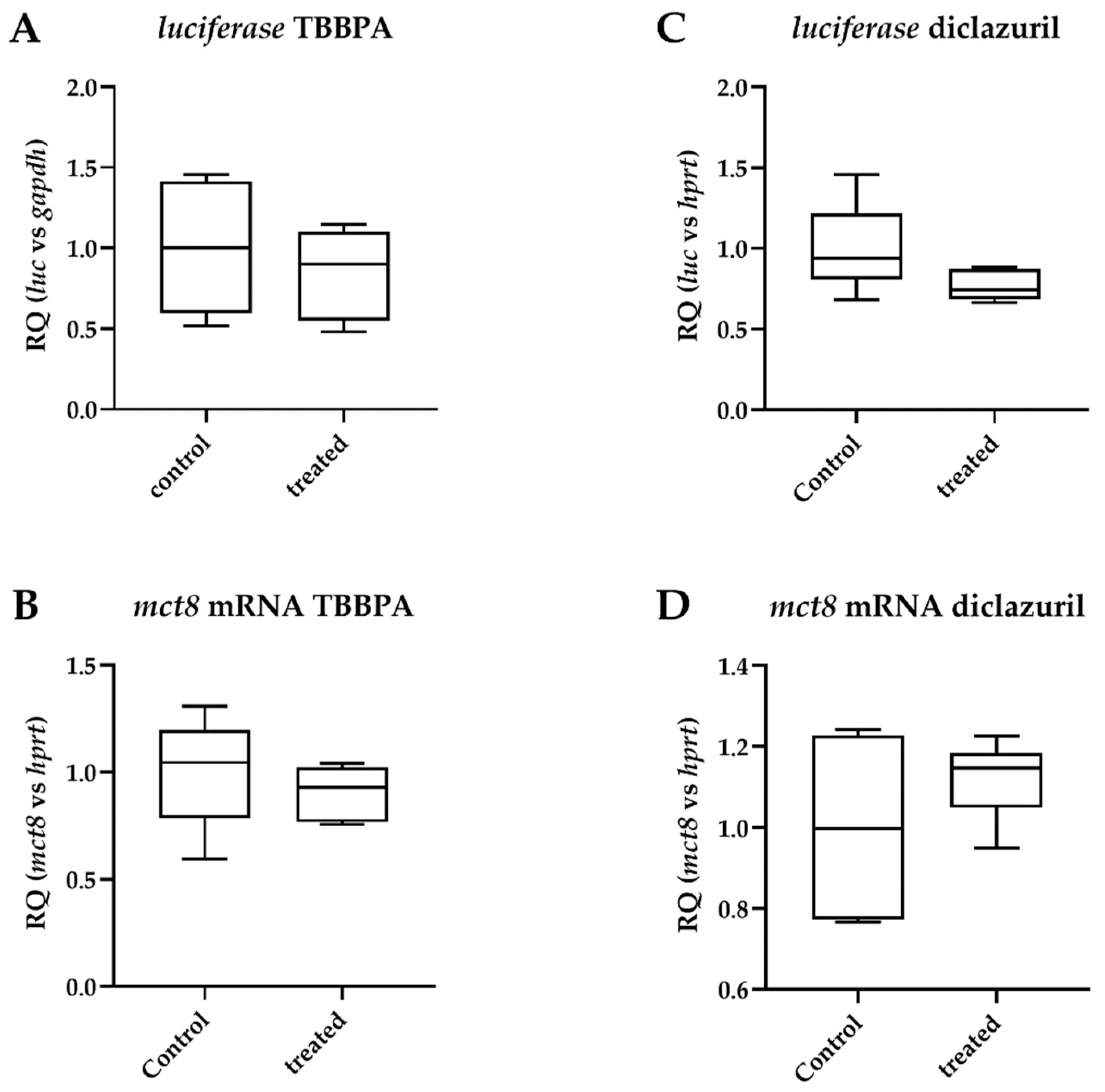
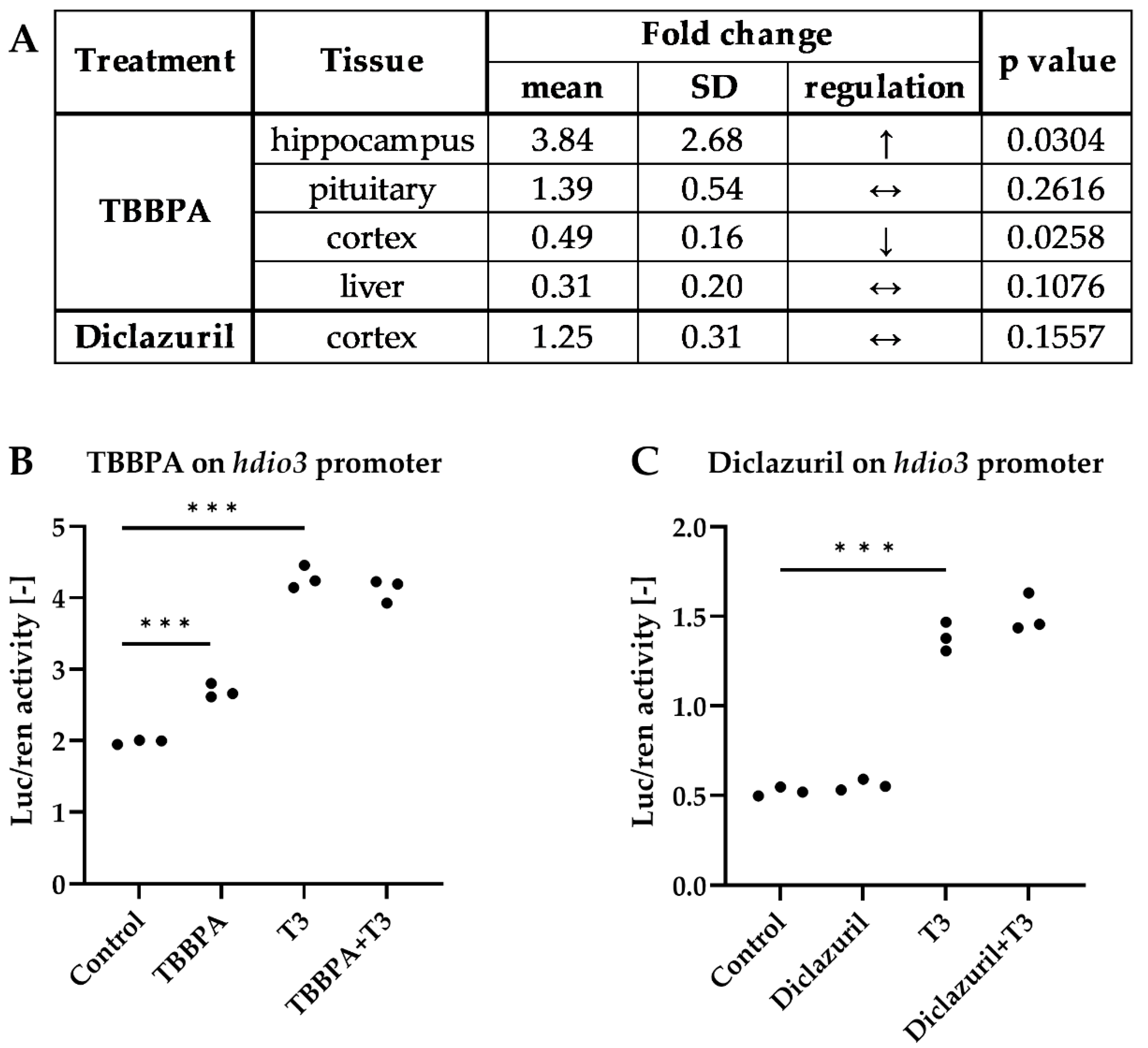
2.1. Tissue-Specific Effects of TBBPA and Diclazuril on Peripheral TH Action
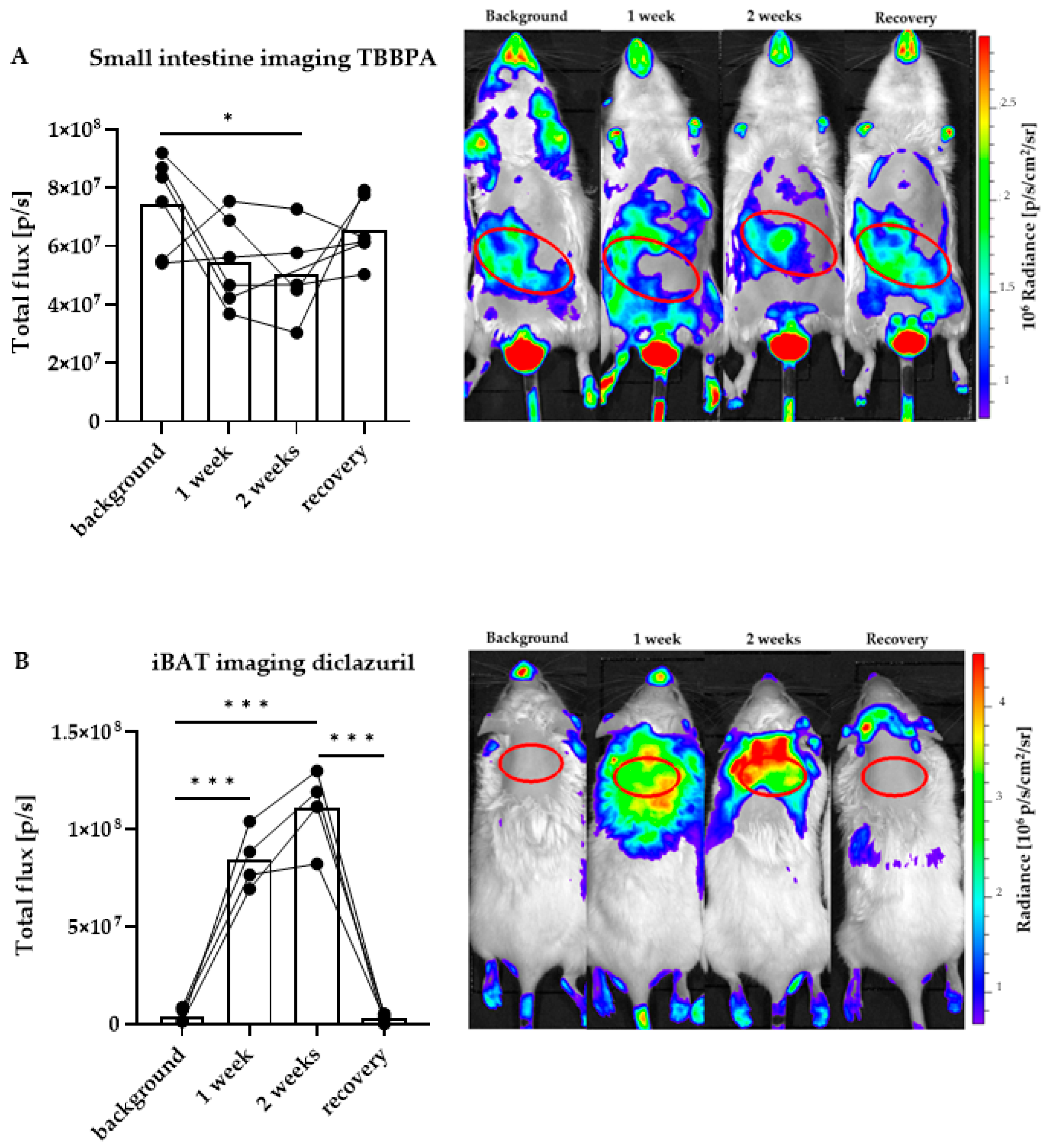
2.2. Distinct Impacts of TBBPA and Diclazuril on Local TH Action in Live THAI Mice
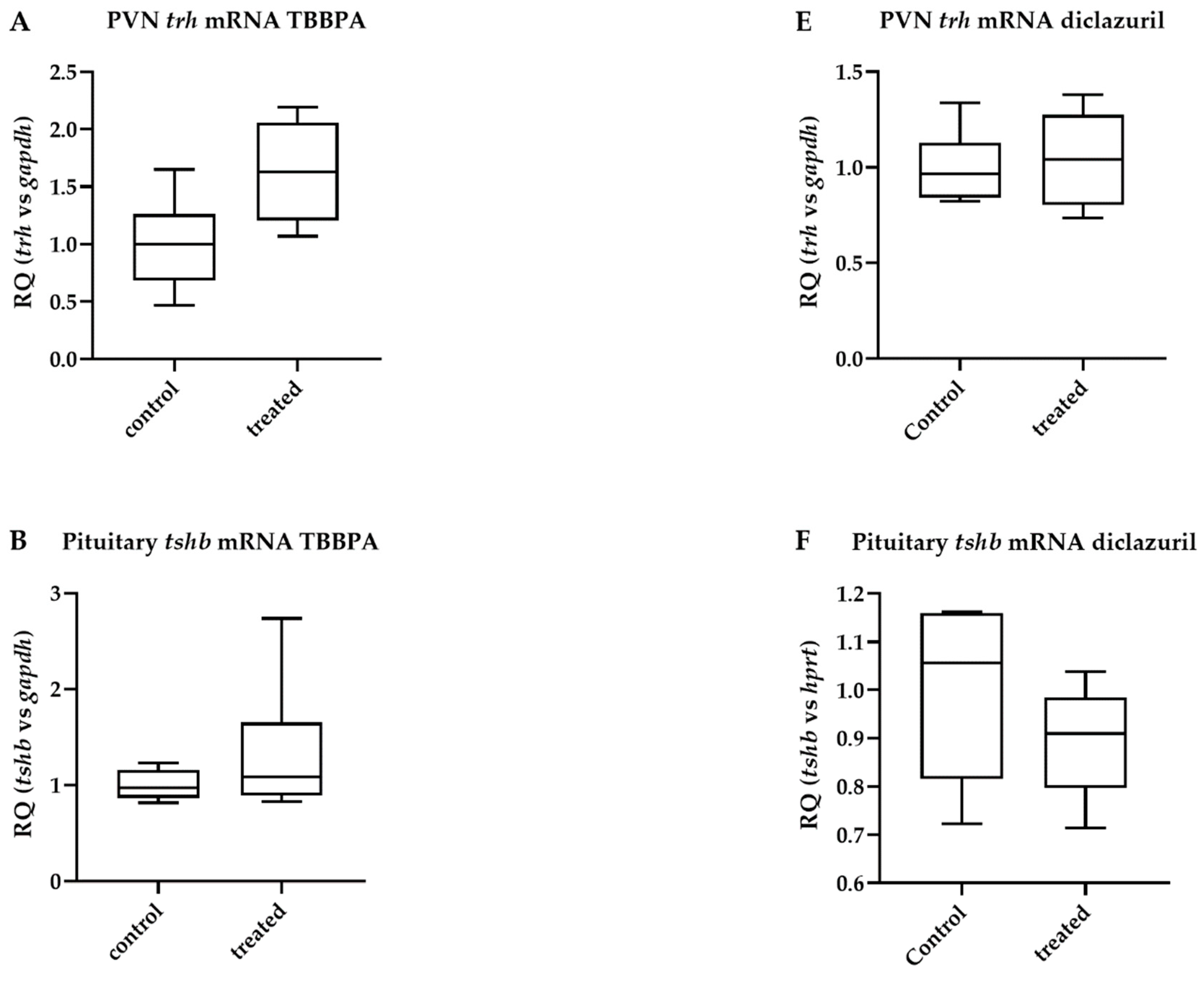
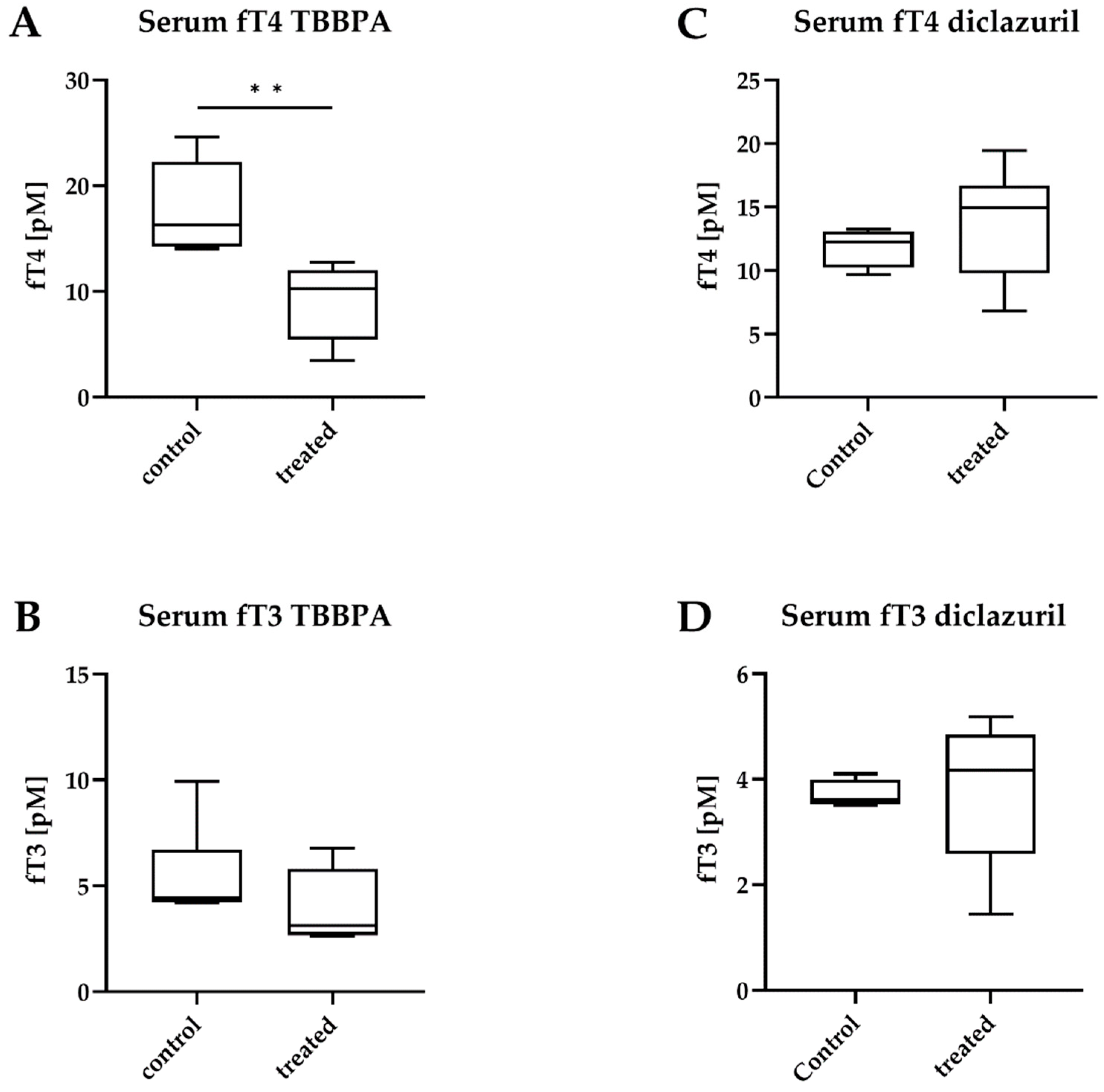
2.3. TBBPA Impacts the Hypothalamo–Pituitary–Thyroid (HPT) Axis, While Diclazuril Does Not
2.4. Diclazuril Does Not Impact the Cerebral Cortex; the Effect of TBBPA on dio3 Expression Is Counterbalanced by Local Mechanisms
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Animal Treatment and Sample Collection
4.3. In Vivo Imaging
4.4. Serum Hormone Measurements
4.5. Taqman qPCR
4.6. Cell Transfection and Luciferase Assay
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larsen, P.R.; Davies, T.F.; Hay, I.D. The Thyroid Gland. In Williams Textbook of Endocrinology, 9th ed.; Wilson, J.D., Foster, D.W., Kronenberg, H.M., Larsen, P.R., Eds.; W.B. Saunders Co.: Philadelphia, PA, USA, 1998; pp. 389–515. [Google Scholar]
- Gereben, B.; Zavacki, A.M.; Ribich, S.; Kim, B.W.; Huang, S.A.; Simonides, W.S.; Zeold, A.; Bianco, A.C. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev. 2008, 29, 898–938. [Google Scholar] [CrossRef] [PubMed]
- Boelen, A.; Kwakkel, J.; Fliers, E. Beyond low plasma T3: Local thyroid hormone metabolism during inflammation and infection. Endocr. Rev. 2011, 32, 670–693. [Google Scholar] [CrossRef] [PubMed]
- Fekete, C.; Lechan, R.M. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 2014, 35, 159–194. [Google Scholar] [CrossRef] [PubMed]
- Gereben, B.; McAninch, E.A.; Ribeiro, M.O.; Bianco, A.C. Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat. Rev. Endocrinol. 2015, 11, 642–652. [Google Scholar] [CrossRef]
- Bianco, A.C.; Dumitrescu, A.; Gereben, B.; Ribeiro, M.O.; Fonseca, T.L.; Fernandes, G.W.; Bocco, B. Paradigms of Dynamic Control of Thyroid Hormone Signaling. Endocr. Rev. 2019, 40, 1000–1047. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Palioura, E.; Kandarakis, S.A.; Koutsilieris, M. The impact of endocrine disruptors on endocrine targets. Horm. Metab. Res. 2010, 42, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Feiteiro, J.; Mariana, M.; Cairrao, E. Health toxicity effects of brominated flame retardants: From environmental to human exposure. Environ. Pollut. 2021, 285, 117475. [Google Scholar] [CrossRef]
- Guarnotta, V.; Amodei, R.; Frasca, F.; Aversa, A.; Giordano, C. Impact of Chemical Endocrine Disruptors and Hormone Modulators on the Endocrine System. Int. J. Mol. Sci. 2022, 23, 5710. [Google Scholar] [CrossRef]
- Dong, M.; Li, Y.; Zhu, M.; Li, J.; Qin, Z. Tetrabromobisphenol A Disturbs Brain Development in Both Thyroid Hormone-Dependent and -Independent Manners in Xenopus laevis. Molecules 2021, 27, 249. [Google Scholar] [CrossRef]
- Beck, K.R.; Sommer, T.J.; Schuster, D.; Odermatt, A. Evaluation of tetrabromobisphenol A effects on human glucocorticoid and androgen receptors: A comparison of results from human- with yeast-based in vitro assays. Toxicology 2016, 370, 70–77. [Google Scholar] [CrossRef]
- Mughal, B.B.; Fini, J.B.; Demeneix, B.A. Thyroid-disrupting chemicals and brain development: An update. Endocr. Connect. 2018, 7, R160–R186. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Zhu, M.; Song, S.; Qin, Z. A Multiwell-Based Assay for Screening Thyroid Hormone Signaling Disruptors Using thibz Expression as a Sensitive Endpoint in Xenopus laevis. Molecules 2022, 27, 798. [Google Scholar] [CrossRef]
- Myosho, T.; Ishibashi, A.; Fujimoto, S.; Miyagawa, S.; Iguchi, T.; Kobayashi, T. Preself-Feeding Medaka Fry Provides a Suitable Screening System for in Vivo Assessment of Thyroid Hormone-Disrupting Potential. Environ. Sci. Technol. 2022, 56, 6479–6490. [Google Scholar] [CrossRef]
- Mohacsik, P.; Erdelyi, F.; Baranyi, M.; Botz, B.; Szabo, G.; Toth, M.; Haltrich, I.; Helyes, Z.; Sperlagh, B.; Toth, Z.; et al. A Transgenic Mouse Model for Detection of Tissue-Specific Thyroid Hormone Action. Endocrinology 2018, 159, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shen, S.; Yan, Y.; Sun, C.; Lu, Z.; Feng, H.; Ma, Y.; Tang, Z.; Yu, J.; Wu, Y.; et al. Triiodothyronine (T3) promotes brown fat hyperplasia via thyroid hormone receptor alpha mediated adipocyte progenitor cell proliferation. Nat. Commun. 2022, 13, 3394. [Google Scholar] [CrossRef]
- Sinkó, R.; Mohácsik, P.; Kővári, D.; Penksza, V.; Wittmann, G.; Mácsai, L.; Fonseca, T.L.; Bianco, A.C.; Fekete, C.; Gereben, B. Different hypothalamic mechanisms control decreased circulating thyroid hormone levels in infection and fasting-induced Non-Thyroidal Illness Syndrome in male Thyroid Hormone Action Indicator Mice. Thyroid 2022, in press. [CrossRef]
- Tong, F.; Gu, X.; Gu, C.; Xie, J.; Xie, X.; Jiang, B.; Wang, Y.; Ertunc, T.; Schaffer, A.; Ji, R. Stimulation of Tetrabromobisphenol A Binding to Soil Humic Substances by Birnessite and the Chemical Structure of the Bound Residues. Environ. Sci. Technol. 2016, 50, 6257–6266. [Google Scholar] [CrossRef] [PubMed]
- Sunday, O.E.; Bin, H.; Guanghua, M.; Yao, C.; Zhengjia, Z.; Xian, Q.; Xiangyang, W.; Weiwei, F. Review of the environmental occurrence, analytical techniques, degradation and toxicity of TBBPA and its derivatives. Environ. Res. 2022, 206, 112594. [Google Scholar] [CrossRef]
- Yuan, X.; Li, T.; He, Y.; Xue, N. Degradation of TBBPA by nZVI activated persulfate in soil systems. Chemosphere 2021, 284, 131166. [Google Scholar] [CrossRef]
- Lyche, J.L.; Rosseland, C.; Berge, G.; Polder, A. Human health risk associated with brominated flame-retardants (BFRs). Environ. Int. 2015, 74, 170–180. [Google Scholar] [CrossRef]
- Kitamura, S.; Kato, T.; Iida, M.; Jinno, N.; Suzuki, T.; Ohta, S.; Fujimoto, N.; Hanada, H.; Kashiwagi, K.; Kashiwagi, A. Anti-thyroid hormonal activity of tetrabromobisphenol A, a flame retardant, and related compounds: Affinity to the mammalian thyroid hormone receptor, and effect on tadpole metamorphosis. Life Sci. 2005, 76, 1589–1601. [Google Scholar] [CrossRef]
- Liu, A.F.; Qu, G.B.; Yu, M.; Liu, Y.W.; Shi, J.B.; Jiang, G.B. Tetrabromobisphenol-A/S and Nine Novel Analogs in Biological Samples from the Chinese Bohai Sea: Implications for Trophic Transfer. Environ. Sci. Technol. 2016, 50, 4203–4211. [Google Scholar] [CrossRef] [PubMed]
- Barghi, M.; Shin, E.S.; Kim, J.C.; Choi, S.D.; Chang, Y.S. Human exposure to HBCD and TBBPA via indoor dust in Korea: Estimation of external exposure and body burden. Sci. Total Environ. 2017, 593–594, 779–786. [Google Scholar] [CrossRef]
- Cariou, R.; Antignac, J.P.; Zalko, D.; Berrebi, A.; Cravedi, J.P.; Maume, D.; Marchand, P.; Monteau, F.; Riu, A.; Andre, F.; et al. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: Occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere 2008, 73, 1036–1041. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Xiang, Y.; Li, L.; Qie, H.; Ren, M.; Lin, A.; Qi, F. Effects of tetrabromobisphenol A (TBBPA) on the reproductive health of male rodents: A systematic review and meta-analysis. Sci. Total Environ. 2021, 781, 146745. [Google Scholar] [CrossRef]
- Decherf, S.; Seugnet, I.; Fini, J.B.; Clerget-Froidevaux, M.S.; Demeneix, B.A. Disruption of thyroid hormone-dependent hypothalamic set-points by environmental contaminants. Mol. Cell Endocrinol. 2010, 323, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liang, J.; Tang, P.; Yu, C.; Fan, H.; Liao, Q.; Long, J.; Pan, D.; Zeng, X.; Liu, S.; et al. Associations of bisphenol exposure with thyroid hormones in pregnant women: A prospective birth cohort study in China. Environ. Sci. Pollut. Res. Int. 2022, 29, 87170–87183. [Google Scholar] [CrossRef]
- Clayton, E.M.; Todd, M.; Dowd, J.B.; Aiello, A.E. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003-2006. Environ. Health Perspect. 2011, 119, 390–396. [Google Scholar] [CrossRef]
- Darnerud, P.O. Toxic effects of brominated flame retardants in man and in wildlife. Environ. Int. 2003, 29, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Hackstein, J.H.; Mackenstedt, U.; Mehlhorn, H.; Meijerink, J.P.; Schubert, H.; Leunissen, J.A. Parasitic apicomplexans harbor a chlorophyll a-D1 complex, the potential target for therapeutic triazines. Parasitol. Res. 1995, 81, 207–216. [Google Scholar] [CrossRef]
- Stock, M.L.; Elazab, S.T.; Hsu, W.H. Review of triazine antiprotozoal drugs used in veterinary medicine. J. Vet. Pharmacol. Ther. 2018, 41, 184–194. [Google Scholar] [CrossRef]
- European Medicines Agency, V.M.E.U. Committee for Veterinary Medicinal Products. In Diclazuril. Summary Report (1); EMEA/MRL/086/96-FINAL; European Medicines Agency: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Peek, H.W.; Landman, W.J. Coccidiosis in poultry: Anticoccidial products, vaccines and other prevention strategies. Vet. Q. 2011, 31, 143–161. [Google Scholar] [CrossRef]
- Oz, H.S. Novel Synergistic Protective Efficacy of Atovaquone and Diclazuril on Fetal-Maternal Toxoplasmosis. Int. J. Clin. Med. 2014, 5, 921–932. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, Y.; Park, J.; Lee, H.S. Endocrine disrupting potential of veterinary drugs by in vitro stably transfected human androgen receptor transcriptional activation assays. Environ. Pollut. 2021, 286, 117201. [Google Scholar] [CrossRef] [PubMed]
- Paul-Friedman, K.; Martin, M.; Crofton, K.M.; Hsu, C.W.; Sakamuru, S.; Zhao, J.; Xia, M.; Huang, R.; Stavreva, D.A.; Soni, V.; et al. Limited Chemical Structural Diversity Found to Modulate Thyroid Hormone Receptor in the Tox21 Chemical Library. Environ. Health Perspect. 2019, 127, 97009. [Google Scholar] [CrossRef]
- Bianco, A.C.; Salvatore, D.; Gereben, B.; Berry, M.J.; Larsen, P.R. Biochemistry, cellular and molecular biology and physiological roles of the iodothyronine selenodeiodinases. Endo. Rev. 2002, 23, 38–89. [Google Scholar] [CrossRef]
- Demeneix, B.A. Evidence for Prenatal Exposure to Thyroid Disruptors and Adverse Effects on Brain Development. Eur. Thyroid J. 2019, 8, 283–292. [Google Scholar] [CrossRef]
- Mughal, B.B.; Demeneix, B.A. Endocrine disruptors: Flame retardants and increased risk of thyroid cancer. Nat. Rev. Endocrinol. 2017, 13, 627–628. [Google Scholar] [CrossRef]
- Caporale, N.; Leemans, M.; Birgersson, L.; Germain, P.L.; Cheroni, C.; Borbely, G.; Engdahl, E.; Lindh, C.; Bressan, R.B.; Cavallo, F.; et al. From cohorts to molecules: Adverse impacts of endocrine disrupting mixtures. Science 2022, 375, eabe8244. [Google Scholar] [CrossRef]
- Zoeller, R.T. Endocrine disrupting chemicals and thyroid hormone action. Adv. Pharmacol. 2021, 92, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Sarvari, M.; Kallo, I.; Hrabovszky, E.; Solymosi, N.; Rodolosse, A.; Liposits, Z. Long-Term Estrogen Receptor Beta Agonist Treatment Modifies the Hippocampal Transcriptome in Middle-Aged Ovariectomized Rats. Front. Cell Neurosci. 2016, 10, 149. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Blagburn, B.L. Activity of diclazuril against Toxoplasma gondii in cultured cells and mice. Am. J. Vet. Res. 1994, 55, 530–533. [Google Scholar] [PubMed]
- Guyot, R.; Chatonnet, F.; Gillet, B.; Hughes, S.; Flamant, F. Toxicogenomic analysis of the ability of brominated flame retardants TBBPA and BDE-209 to disrupt thyroid hormone signaling in neural cells. Toxicology 2014, 325, 125–132. [Google Scholar] [CrossRef]
- Cope, R.B.; Kacew, S.; Dourson, M. A reproductive, developmental and neurobehavioral study following oral exposure of tetrabromobisphenol A on Sprague-Dawley rats. Toxicology 2015, 329, 49–59. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Zhang, M.; Qiu, J.; Li, X.; Zhang, W.; Fan, J.; Zhou, H.; He, L. Determination of residual enantiomers of diclazuril in chicken edible tissues by high performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1118–1119, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Sotome, R.; Hirasawa, A.; Kikusato, M.; Amo, T.; Furukawa, K.; Kuriyagawa, A.; Watanabe, K.; Collin, A.; Shirakawa, H.; Hirakawa, R.; et al. In vivo emergence of beige-like fat in chickens as physiological adaptation to cold environments. Amino Acids 2021, 53, 381–393. [Google Scholar] [CrossRef]
- Asadi Iraee, H.; Asadi Iraee, M.; Youssefi, M.R.; Abouhosseini Tabari, M. Growth performance parameters in chicken experimental coccidiosis treated with Diclazuril and Clopidol: The need for assessing new anticoccidial resources. Iran. J. Vet. Med. 2015, 9, 189–194. [Google Scholar]
- Dentice, M.; Luongo, C.; Huang, S.; Ambrosio, R.; Elefante, A.; Mirebeau-Prunier, D.; Zavacki, A.M.; Fenzi, G.; Grachtchouk, M.; Hutchin, M.; et al. Sonic hedgehog-induced type 3 deiodinase blocks thyroid hormone action enhancing proliferation of normal and malignant keratinocytes. Proc. Natl. Acad. Sci. USA 2007, 104, 14466–14471. [Google Scholar] [CrossRef] [PubMed]
- Egri, P.; Fekete, C.; Denes, A.; Reglodi, D.; Hashimoto, H.; Fulop, B.D.; Gereben, B. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Regulates the Hypothalamo-Pituitary-Thyroid (HPT) Axis via Type 2 Deiodinase in Male Mice. Endocrinology 2016, 157, 2356–2366. [Google Scholar] [CrossRef]



| Gene Symbol | Gene Name | Assay ID |
|---|---|---|
| actinb | β actin | Mm02619580_g1 |
| dCpG luciferase | dCpG luciferase reporter (custom made) | AIY9ZTZ |
| dio3 | deiodinase, iodothyronine type III | Mm00548953_s1 |
| gapdh | glyceraldehyde-3-phosphate dehydrogenase | Mm99999915_g1 |
| hprt1 | hypoxanthine guanine phosphoribosyl transferase | Mm01545399_m1 |
| slc16a2 | MCT8, monocarboxylate transporter 8 | Mm01232724_m1 |
| trh | Thyrotropin releasing hormone | Mm01963590_s1 |
| tshb | thyroid stimulating hormone, beta subunit | Mm03990915_g1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinkó, R.; Rada, K.; Kollár, A.; Mohácsik, P.; Tenk, M.; Fekete, C.; Gereben, B. Tetrabromobisphenol A and Diclazuril Evoke Tissue-Specific Changes of Thyroid Hormone Signaling in Male Thyroid Hormone Action Indicator Mice. Int. J. Mol. Sci. 2022, 23, 14782. https://doi.org/10.3390/ijms232314782
Sinkó R, Rada K, Kollár A, Mohácsik P, Tenk M, Fekete C, Gereben B. Tetrabromobisphenol A and Diclazuril Evoke Tissue-Specific Changes of Thyroid Hormone Signaling in Male Thyroid Hormone Action Indicator Mice. International Journal of Molecular Sciences. 2022; 23(23):14782. https://doi.org/10.3390/ijms232314782
Chicago/Turabian StyleSinkó, Richárd, Kristóf Rada, Anna Kollár, Petra Mohácsik, Miklós Tenk, Csaba Fekete, and Balázs Gereben. 2022. "Tetrabromobisphenol A and Diclazuril Evoke Tissue-Specific Changes of Thyroid Hormone Signaling in Male Thyroid Hormone Action Indicator Mice" International Journal of Molecular Sciences 23, no. 23: 14782. https://doi.org/10.3390/ijms232314782
APA StyleSinkó, R., Rada, K., Kollár, A., Mohácsik, P., Tenk, M., Fekete, C., & Gereben, B. (2022). Tetrabromobisphenol A and Diclazuril Evoke Tissue-Specific Changes of Thyroid Hormone Signaling in Male Thyroid Hormone Action Indicator Mice. International Journal of Molecular Sciences, 23(23), 14782. https://doi.org/10.3390/ijms232314782





