Interplay between Myokine Profile and Glycemic Control in Type 2 Diabetes Mellitus Patients with Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Cohorts of Participants
2.2. Determination of Co-Morbidities and Measurement of Anthropometric Parameters
2.3. Examination of Hemodynamics
2.4. Diet and Medications
2.5. Blood Sampling, Storage and Measurement of Biomarkers
2.6. Statistics
3. Results
3.1. General Characteristics of the Patients
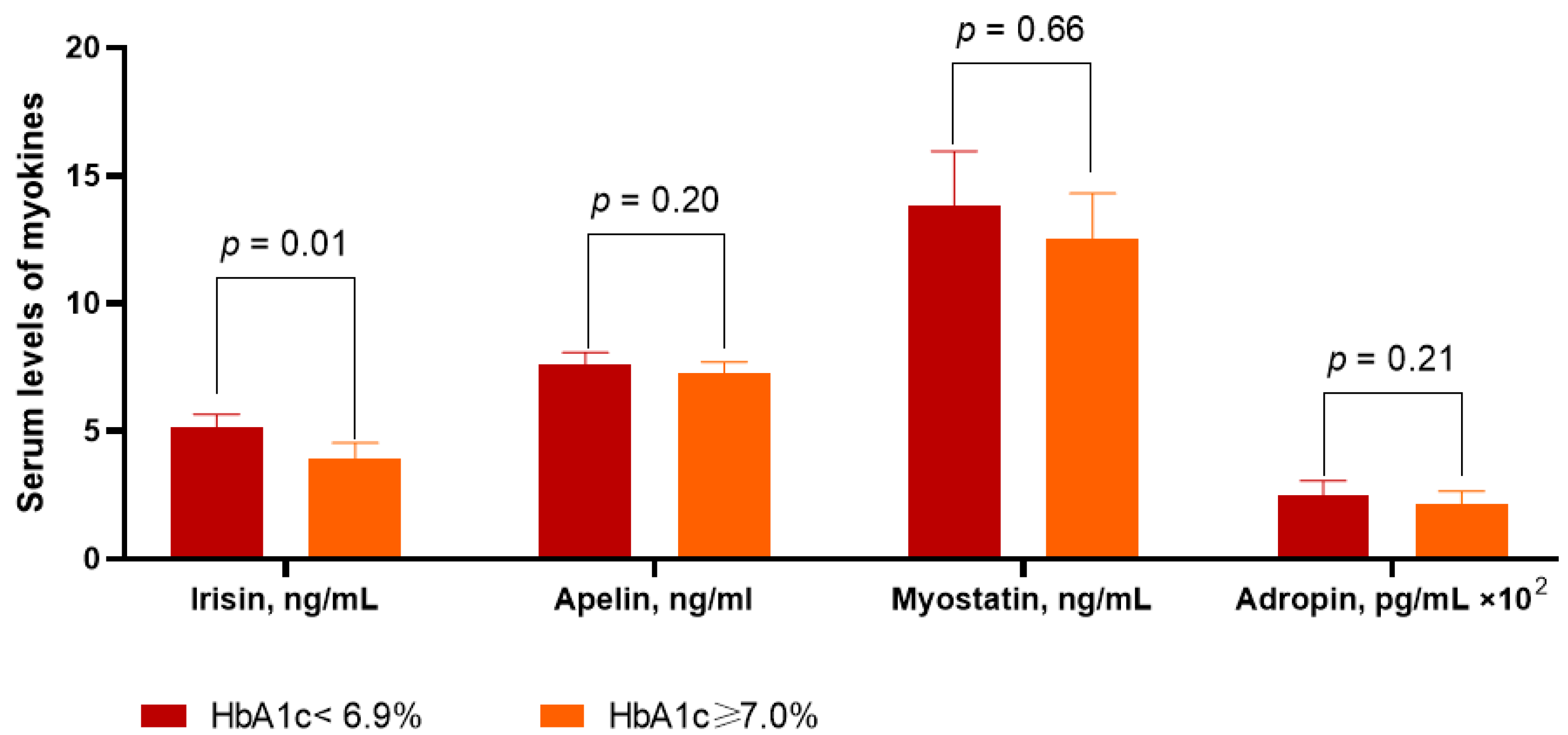
3.2. Circulating Levels of Myokines in the Patients Included in the Study
3.3. Spearman’s Correlation between Circulating Levels of Myokines and Other Parameters
3.4. The Predictors of Poor Glycemic Control in T2DM Patients with HF: The Univariate and Multivariate Linear Regression
3.5. Comparison of the Models
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.-H.; Yoo, B.-S. Current Prevalence, Incidence, and Outcomes of Heart Failure with Preserved Ejection Fraction. Heart Fail. Clin. 2021, 17, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Bouthoorn, S.; Valstar, G.B.; Gohar, A.; Ruijter, H.D.; Reitsma, H.B.; Hoes, A.W.; Rutten, F.H. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis. Diabetes Vasc. Dis. Res. 2018, 15, 477–493. [Google Scholar] [CrossRef] [PubMed]
- van Riet, E.E.; Hoes, A.W.; Wagenaar, K.P.; Limburg, A.; Landman, M.A.; Rutten, F.H. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur. J. Heart Fail. 2016, 18, 242–252. [Google Scholar] [CrossRef]
- Gohar, A.; Kievit, R.F.; Valstar, G.B.; Hoes, A.W.; E Van Riet, E.; Van Mourik, Y.; Bertens, L.C.; Boonman-Winter, L.J.; Bots, M.L.; Ruijter, H.D.; et al. Opportunistic screening models for high-risk men and women to detect diastolic dysfunction and heart failure with preserved ejection fraction in the community. Eur. J. Prev. Cardiol. 2018, 26, 613–623. [Google Scholar] [CrossRef]
- Paolillo, S.; Scardovi, A.B.; Campodonico, J. Role of comorbidities in heart failure prognosis Part I: Anaemia, iron deficiency, diabetes, atrial fibrillation. Eur. J. Prev. Cardiol. 2020, 27 (Suppl. 2), 27–34. [Google Scholar] [CrossRef]
- Kenny, H.C.; Abel, E.D. Heart Failure in Type 2 Diabetes Mellitus. Circ. Res. 2019, 124, 121–141. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Omote, K.; Reddy, Y.N.V.; Sorimachi, H.; Obokata, M.; A Borlaug, B. Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. Eur. Heart J. 2022, 43, 1941–1951. [Google Scholar] [CrossRef]
- Maciorkowska, M.; Musiałowska, D.; Małyszko, J. Adropin and irisin in arterial hypertension, diabetes mellitus and chronic kidney disease. Adv. Clin. Exp. Med. 2019, 28, 1571–1575. [Google Scholar] [CrossRef]
- Du, X.-L.; Jiang, W.-X.; Lv, Z.-T. Lower Circulating Irisin Level in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2016, 48, 644–652. [Google Scholar] [CrossRef]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovic, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In Vivo and In Vitro studies. J. Physiol. 2014, 592, 1091–1107. [Google Scholar] [CrossRef]
- Noori-Zadeh, A.; Bakhtiyari, S.; Khanjari, S.; Haghani, K.; Darabi, S. Elevated blood apelin levels in type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2019, 148, 43–53. [Google Scholar] [CrossRef]
- Soriguer, F.; Garrido-Sanchez, L.; Garcia-Serrano, S.; Garcia-Almeida, J.M.; Garcia-Arnes, J.; Tinahones, F.J.; Garcia-Fuentes, E. Apelin Levels Are Increased in Morbidly Obese Subjects with Type 2 Diabetes Mellitus. Obes. Surg. 2009, 19, 1574–1580. [Google Scholar] [CrossRef]
- Berezin, A.A.; Lichtenauer, M.; Boxhammer, E.; Stöhr, E.; Berezin, A.E. Discriminative Value of Serum Irisin in Prediction of Heart Failure with Different Phenotypes among Patients with Type 2 Diabetes Mellitus. Cells 2022, 11, 2794. [Google Scholar] [CrossRef]
- Berezin, A.A.; Fushtey, I.M.; Berezin, A.E. Discriminative Utility of Apelin-to-NT-Pro-Brain Natriuretic Peptide Ratio for Heart Failure with Preserved Ejection Fraction among Type 2 Diabetes Mellitus Patients. J. Cardiovasc. Dev. Dis. 2022, 9, 23. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016, 253, 281–344. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, P.M.; Ponikowski, P.; Anker, S.D.; Bauersachs, J.; Chioncel, O.; Cleland, J.G.; de Boer, R.A.; Drexel, H.; Ben Gal, T.; Hill, L.; et al. Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 1169–1186. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R.; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American association of clinical endocrinologists and american college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pract. 2016, 22, 1–203. [Google Scholar] [CrossRef]
- Toto, R.D. Microalbuminuria: Definition, Detection, and Clinical Significance. J. Clin. Hypertens. 2004, 6 (Suppl. 3), 2–7. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F.; Otto, C.M. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 372–392. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Nielsen, R.; Jorsal, A.; Tougaard, R.S.; Rasmussen, J.J.; Schou, M.; Videbæk, L.; Gustafsson, I.; Faber, J.; Flyvbjerg, A.; Wiggers, H.; et al. The impact of the glucagon-like peptide-1 receptor agonist liraglutide on natriuretic peptides in heart failure patients with reduced ejection fraction with and without type 2 diabetes. Diabetes Obes. Metab. 2020, 22, 2141–2150. [Google Scholar] [CrossRef]
- Damman, K.; Beusekamp, J.C.; Boorsma, E.M.; Swart, H.P.; Smilde, T.D.; Elvan, A.; van Eck, J.M.; Heerspink, H.J.; Voors, A.A. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur. J. Heart Fail. 2020, 22, 713–722. [Google Scholar] [CrossRef]
- Ejiri, K.; Miyoshi, T.; Kihara, H.; Hata, Y.; Nagano, T.; Takaishi, A.; Toda, H.; Nanba, S.; Nakamura, Y.; Akagi, S.; et al. Effect of Luseogliflozin on Heart Failure with Preserved Ejection Fraction in Patients with Diabetes Mellitus. J. Am. Heart Assoc. 2020, 9, e015103. [Google Scholar] [CrossRef]
- Nassif, M.E.; Windsor, S.L.; Tang, F.; Khariton, Y.; Husain, M.; Inzucchi, S.E.; Mc-Guire, D.K.; Pitt, B.; Scirica, B.M.; Austin, B.; et al. Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients With Heart Failure With Reduced Ejection Fraction. Circulation 2019, 140, 1463–1476. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A.; Lichtenauer, M. Myokines and Heart Failure: Challenging Role in Adverse Cardiac Remodeling, Myopathy, and Clinical Outcomes. Dis. Markers 2021, 2021, 6644631. [Google Scholar] [CrossRef] [PubMed]
- Springer, J.; Springer, J.-I.; Anker, S.D. Muscle wasting and sarcopenia in heart failure and beyond: Update 2017. ESC Heart Fail. 2017, 4, 492–498. [Google Scholar] [CrossRef]
- Biesemann, N.; Mendler, L.; Wietelmann, A.; Hermann, S.; Schäfers, M.; Krüger, M.; Boettger, T.; Borchardt, T.; Braun, T. Myostatin Regulates Energy Homeostasis in the Heart and Prevents Heart Failure. Circ. Res. 2014, 115, 296–310. [Google Scholar] [CrossRef]
- Chandrasekaran, B.; Dar, O.; McDonagh, T. The role of apelin in cardiovascular function and heart failure. Eur. J. Heart Fail. 2008, 10, 725–732. [Google Scholar] [CrossRef]
- Yosaee, S.; Soltani, S.; Sekhavati, E.; Jazayeri, S. Adropin- A Novel Biomarker of Heart Disease: A Systematic Review Article. Iran. J. Public Health 2016, 45, 1568–1576. [Google Scholar]
- Ishida, J.; Konishi, M.; Saitoh, M.; Anker, M.; Anker, S.D.; Springer, J. Myostatin signaling is up-regulated in female patients with advanced heart failure. Int. J. Cardiol. 2017, 238, 37–42. [Google Scholar] [CrossRef]
- Lian, W.; Gu, X.; Qin, Y.; Zheng, X. Elevated Plasma Levels of Adropin in Heart Failure Patients. Intern. Med. 2011, 50, 1523–1527. [Google Scholar] [CrossRef]
- Breitbart, A.; Auger-Messier, M.; Molkentin, J.; Heineke, J. Myostatin from the heart: Local and systemic actions in cardiac failure and muscle wasting. Am. J. Physiol. Circ. Physiol. 2011, 300, H1973–H1982. [Google Scholar] [CrossRef]
- Goidescu, C.M.; Chiorescu, R.M.; Diana, M.-H.L.; Mocan, M.; Stoia, M.A.; Anton, F.P.; Farcaş, A.D. ACE2 and Apelin-13: Biomarkers with a Prognostic Value in Congestive Heart Failure. Dis. Markers 2021, 2021, 5569410. [Google Scholar] [CrossRef]
- Kalkan, A.K.; Cakmak, H.A.; Erturk, M.; Kalkan, K.E.; Uzun, F.; Tasbulak, O.; Diker, V.O.; Aydin, S.; Celik, A. Adropin and Irisin in Patients with Cardiac Cachexia. Arq. Bras. Cardiol. 2018, 111, 39–47. [Google Scholar] [CrossRef]
- Berezin, A.A.; Lichtenauer, M.; Boxhammer, E.; Fushtey, I.M. Serum Levels of Irisin Predict Cumulative Clinical Outcomes in Heart Failure Patients With Type 2 Diabetes Mellitus. Front. Physiol. 2022, 13, 957. [Google Scholar] [CrossRef]
- Silvestrini, A.; Bruno, C.; Vergani, E.; Venuti, A.; Favuzzi, A.M.R.; Guidi, F.; Nicolotti, N.; Meucci, E.; Mordente, A.; Mancini, A. Circulating irisin levels in heart failure with preserved or reduced ejection fraction: A pilot study. PLoS ONE 2019, 14, e0210320. [Google Scholar] [CrossRef]
- Shen, S.; Gao, R.; Bei, Y.; Li, J.; Zhang, H.; Zhou, Y.; Yao, W.; Xu, D.; Zhou, F.; Jin, M.; et al. Serum Irisin Predicts Mortality Risk in Acute Heart Failure Patients. Cell. Physiol. Biochem. 2017, 42, 615–622. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Kansakar, U.; Varzideh, F.; Wilson, S.; Mone, P.; Lombardi, A.; Gambardella, J.; Santulli, G. Heart failure in diabetes. Metabolism 2021, 125. [Google Scholar] [CrossRef]
- Li, R.-L.; Wu, S.-S.; Wu, Y.; Wang, X.-X.; Chen, H.-Y.; Xin, J.-J.; Li, H.; Lan, J.; Xue, K.-Y.; Li, X.; et al. Irisin alleviates pressure overload-induced cardiac hypertrophy by inducing protective autophagy via mTOR-independent activation of the AMPK-ULK1 pathway. J. Mol. Cell. Cardiol. 2018, 121, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, B.; Zhao, C.; Wang, Y.; Zhou, Y.; Lin, J.; Zhao, R. Irisin Regulates Cardiac Responses to Exercise in Health and Diseases: A Narrative Review. J. Cardiovasc. Transl. Res. 2022, 1–13. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Wu, S.; Wu, Y.; Chen, H.; Xin, J.; Li, H.; Lan, J.; Xue, K.; Li, X.; et al. Irisin ameliorates angiotensin II-induced cardiomyocyte apoptosis through autophagy. J. Cell. Physiol. 2019, 234, 17578–17588. [Google Scholar] [CrossRef]
- Lecker, S.H.; Zavin, A.; Cao, P.; Arena, R.; Allsup, K.; Daniels, K.M.; Joseph, J.; Schulze, P.C.; Forman, D.E. Expression of the Irisin Precursor FNDC5 in Skeletal Muscle Correlates with Aerobic Exercise Performance in Patients with Heart Failure. Circ. Heart Fail. 2012, 5, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Halade, G.V.; Lee, D.H. Inflammation and resolution signaling in cardiac repair and heart failure. eBioMedicine 2022, 79, 103992. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, X.; Wu, K.; Liu, K.; Wang, S.; Chen, X. Irisin attenuates H2O2-induced apoptosis in cardiomyocytes via microRNA-19b/AKT/mTOR signaling pathway. Int. J. Clin. Exp. Pathol. 2017, 10, 7707–7717. [Google Scholar]

| Variables | Entire T2DM Patient Cohort with HF (n = 314) | Patients with HbA1c < 6.9% (n = 183) | Patients with HbA1c ≥ 7.0% (n = 131) | p-Value |
|---|---|---|---|---|
| Age, year | 52 (40–62) | 51 (41–62) | 52 (40–60) | 0.82 |
| Male, n (%) | 205 (65.3) | 118 (64.5) | 87 (66.4) | 0.86 |
| Dyslipidemia, n (%) | 268 (85.3) | 152 (83.1) | 116 (88.5) | 0.82 |
| Hypertension, n (%) | 267 (85.0) | 158 (86.3) | 109 (83.2) | 0.88 |
| Stable CAD, n (%) | 135 (43.0) | 74 (40.4) | 61 (45.6) | 0.62 |
| Paroxysmal/persistent AF, n (%) | 72 (22.9) | 35 (19.1) | 37 (28.2) | 0.01 |
| Smoking, n (%) | 157 (50.0) | 89 (48.6) | 68 (51.9) | 0.58 |
| Abdominal obesity, n (%) | 158 (50.3) | 84 (45.9) | 74 (56.5) | 0.046 |
| Microalbuminuria, n (%) | 109 (34.7) | 56 (30.6) | 53 (40.5) | 0.042 |
| LVH, n (%) | 266 (84.7) | 144 (78.7) | 122 (93.1) | 0.04 |
| BMI, kg/m2 | 26.1 ± 1.5 | 25.8 ± 2.1 | 26.7 ± 2.0 | 0.86 |
| Waist circumference, sm | 86.5 ± 2.0 | 85.6 ± 2.9 | 87.2 ± 2.2 | 0.84 |
| WHR, units | 0.86 ± 0.04 | 0.86 ± 0.03 | 0.88 ± 0.02 | 0.82 |
| HFpEF, n (%) | 124 (39.5) | 72 (39.3) | 52 (39.7) | 0.84 |
| HFmrEF, n (%) | 105 (33.4) | 58 (31.7) | 47 (35.9) | 0.78 |
| HFrEF, n (%) | 85 (27.1) | 53 (29.0) | 32 (24.4) | 0.86 |
| I/II/III NYHA class, n | 46/165/103 | 30/103/50 | 16/62/53 | 0.24 |
| SBP, mm Hg | 131 ± 5 | 132 ± 5 | 130 ± 4 | 0.92 |
| DBP, mm Hg | 79 ± 4 | 80 ± 4 | 78 ± 3 | 0.90 |
| LVEDV, mL | 161 ± 7 | 154 ± 9 | 168 ± 6 | 0.72 |
| LVESV, mL | 84 ± 4 | 74 ± 7 | 86 ± 2 | 0.73 |
| LVEF, % | 49 ± 5 | 51 ± 6 | 48 ± 4 | 0.44 |
| LVMMI, g/m2 | 164 ± 5.60 | 151 ± 6.12 | 172 ± 4.42 | 0.044 |
| LAVI, mL/m2 | 46 ± 6 | 39 ± 8 | 54 ± 5 | 0.046 |
| E/e’, unit | 14.5 ± 0.8 | 13.9 ± 0.5 | 15.2 ± 0.7 | 0.01 |
| eGFR, mL/min/1.73 m2 | 81 ± 5.1 | 83 ± 6.0 | 79 ± 5.6 | 0.82 |
| HOMA-IR, units | 9.74 ± 3.1 | 7.65 ± 3.7 | 11.40 ± 2.4 | 0.05 |
| NT-proBNP, pmol/mL | 2855 (1430–4140) | 2718 (1380–3720) | 2982 (1640–4527) | 0.12 |
| Fasting glucose, mmol/L | 6.65 ± 1.93 | 5.84 ± 1.22 | 7.34 ± 1.38 | 0.01 |
| Creatinine, µmol/L | 110.4 ± 14.2 | 108.8 ± 12.0 | 114.2 ± 10.3 | 0.28 |
| HbA1c, % | 7.01 ± 0.5 | 6.47 ± 0.3 | 7.42 ± 0.4 | 0.04 |
| TC, mmol/L | 6.53 ± 0.06 | 6.41 ± 0.05 | 6.62 ± 0.07 | 0.88 |
| HDL-C, mmol/L | 0.94 ± 0.19 | 0.95 ± 0.21 | 0.93 ± 0.18 | 0.84 |
| LDL-C, mmol/L | 4.50 ± 0.15 | 4.43 ± 0.20 | 4.51 ± 0.16 | 0.88 |
| TG, mmol/L | 2.27 ± 0.05 | 2.26 ± 0.04 | 2.31 ± 0.03 | 0.88 |
| SGLT2i, n (%) | 293 (93.3) | 171 (93.4) | 122 (93.1) | 0.90 |
| ACEIs/ARBs/ARNI, n (%) | 274 (87.3) | 158 (86.3) | 116 (88.5) | 0.89 |
| MRA, n (%) | 85 (27.1) | 53 (29.0) | 32 (24.4) | 0.86 |
| Statins, n (%) | 268 (85.3) | 152 (83.1) | 116 (88.5) | 0.82 |
| Beta-blockers, n (%) | 292 (93.0) | 174 (95.0) | 118 (90.1) | 0.22 |
| Ivabradin, n (%) | 37 (11.8) | 23 (12.6) | 14 (10.7) | 0.68 |
| Dependent Variable: HbA1c ≥ 7.0% | ||||
|---|---|---|---|---|
| Variables | Univariate Linear Regression | Multivariate Linear Regression | ||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| III NYHA class vs. I/II NYHA class | 1.03 (1.00–1.07) | 0.050 | - | |
| AF versus sinus rhythm | 1.08 (0.93–1.17) | 0.82 | - | |
| HFrEF vs. HFpEF/HFmrEF | 1.04 (1.02–1.07) | 0.042 | 1.05 (1.01–1.09) | 0.050 |
| LVEF | 1.03 (1.01–1.05) | 0.048 | 1.02 (1.00–1.05) | 0.28 |
| LAVI | 1.05 (1.03–1.09) | 0.044 | 1.03 (1.00–1.07) | 0.050 |
| E/e’ | 1.02 (0.98–1.05) | 0.86 | - | |
| NT-proBNP | 1.07 (1.03–1.12) | 0.01 | 1.07 (1.02–1.10) | 0.04 |
| Irisin | 1.09 (1.05–1.16) | 0.001 | 1.09 (1.04–1.17) | 0.001 |
| Apelin | 1.07 (1.02–1.11) | 0.024 | 1.05 (1.00–1.11) | 0.050 |
| Myostatin | 1.04 (1.01–1.06) | 0.044 | 1.02 (1.00–1.04) | 0.052 |
| Adropin | 1.07 (0.99–1.12) | 0.850 | - | |
| Models | AUC | NRI | IDI | |||
|---|---|---|---|---|---|---|
| M (95% CI) | p-value | M (95% CI) | p-value | M (95% CI) | p-value | |
| NT-proBNP | 0.66 (0.60–0.74) | - | Reference | - | Reference | - |
| Irisin | 0.81 (0.73–0.89) | 0.001 | 0.34 (0.31–0.37) | 0.01 | 0.45 (0.40–0.52) | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezin, A.A.; Obradovic, Z.; Novikov, E.V.; Boxhammer, E.; Lichtenauer, M.; Berezin, A.E. Interplay between Myokine Profile and Glycemic Control in Type 2 Diabetes Mellitus Patients with Heart Failure. Diagnostics 2022, 12, 2940. https://doi.org/10.3390/diagnostics12122940
Berezin AA, Obradovic Z, Novikov EV, Boxhammer E, Lichtenauer M, Berezin AE. Interplay between Myokine Profile and Glycemic Control in Type 2 Diabetes Mellitus Patients with Heart Failure. Diagnostics. 2022; 12(12):2940. https://doi.org/10.3390/diagnostics12122940
Chicago/Turabian StyleBerezin, Alexander A., Zeljko Obradovic, Evgen V. Novikov, Elke Boxhammer, Michael Lichtenauer, and Alexander E. Berezin. 2022. "Interplay between Myokine Profile and Glycemic Control in Type 2 Diabetes Mellitus Patients with Heart Failure" Diagnostics 12, no. 12: 2940. https://doi.org/10.3390/diagnostics12122940
APA StyleBerezin, A. A., Obradovic, Z., Novikov, E. V., Boxhammer, E., Lichtenauer, M., & Berezin, A. E. (2022). Interplay between Myokine Profile and Glycemic Control in Type 2 Diabetes Mellitus Patients with Heart Failure. Diagnostics, 12(12), 2940. https://doi.org/10.3390/diagnostics12122940








