Purification of Residual Glycerol from Biodiesel Production as a Value-Added Raw Material for Glycerolysis of Free Fatty Acids in Waste Cooking Oil
Abstract
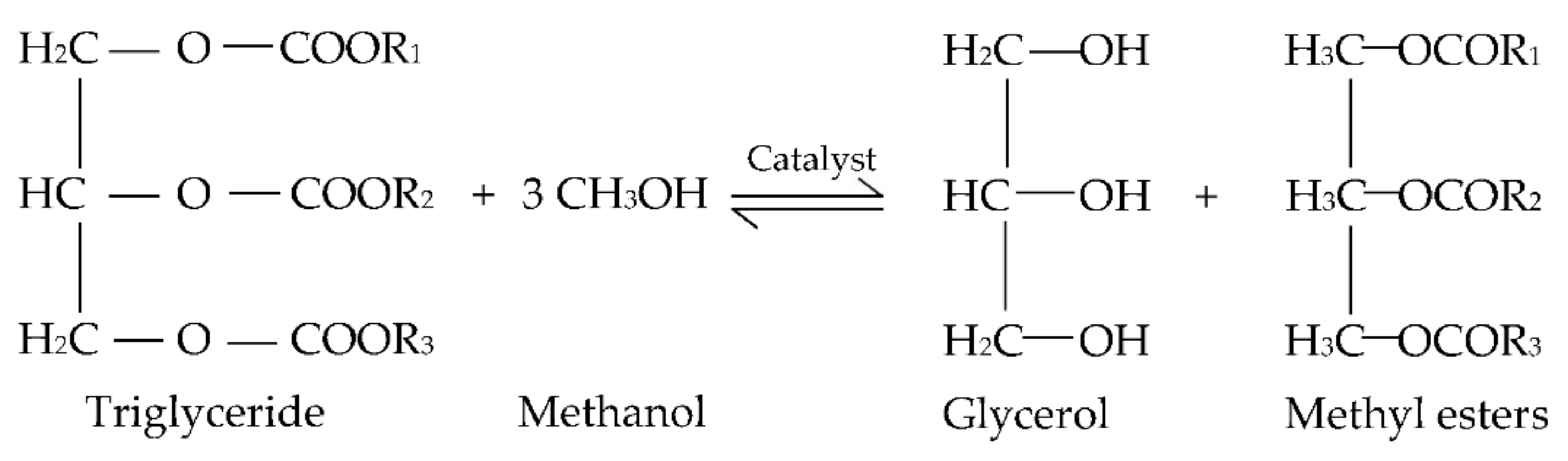
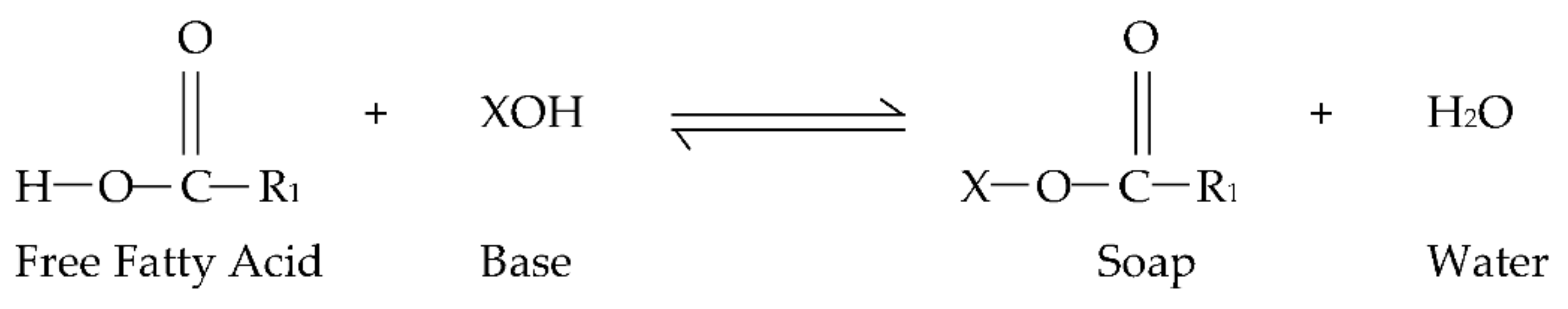
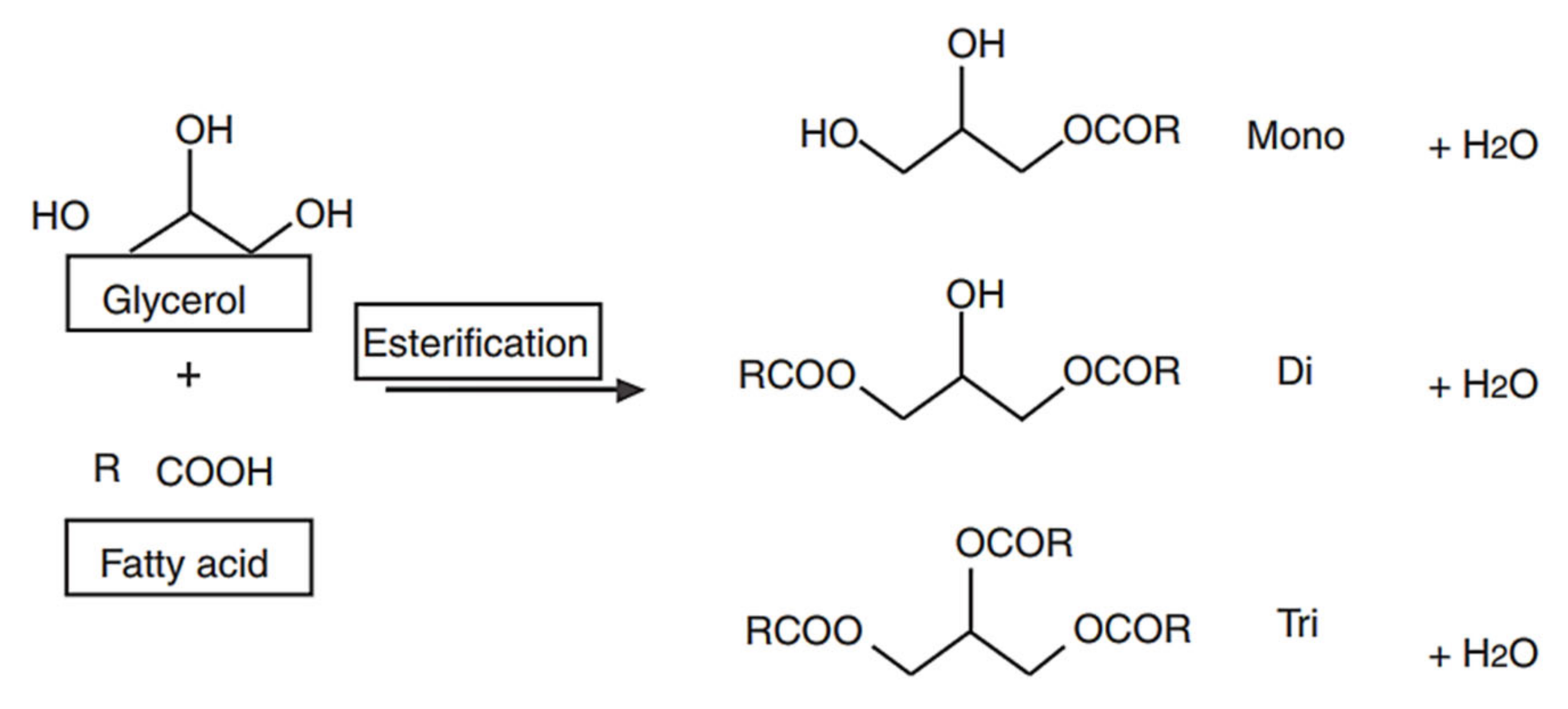
1. Introduction
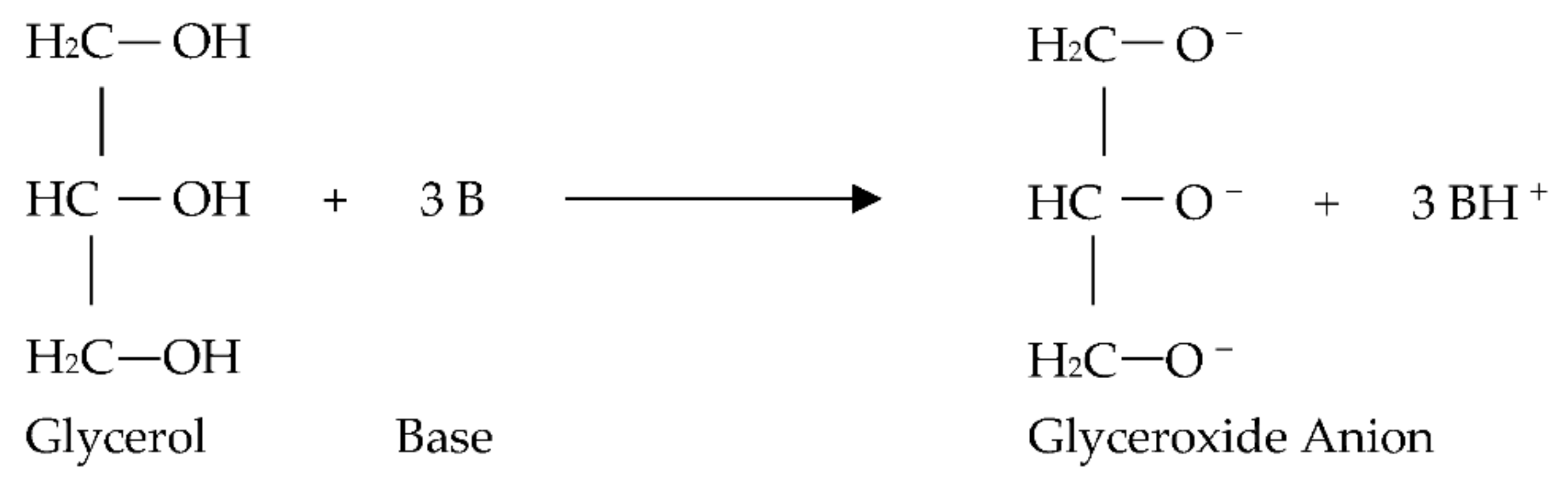
- The glycerol interacts with three moles of the base catalyst to generate free radical anion and three moles of the protonated catalyst, as shown in Figure 4.
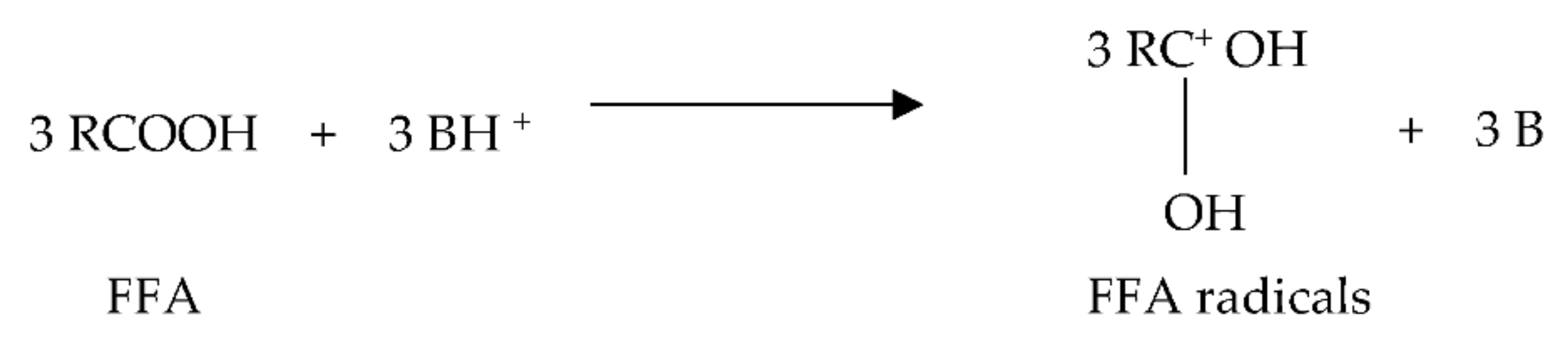
- Three molecules of protonated catalyst attack three molecules of substrate FFAs to generate free radicals, as seen in Figure 5.
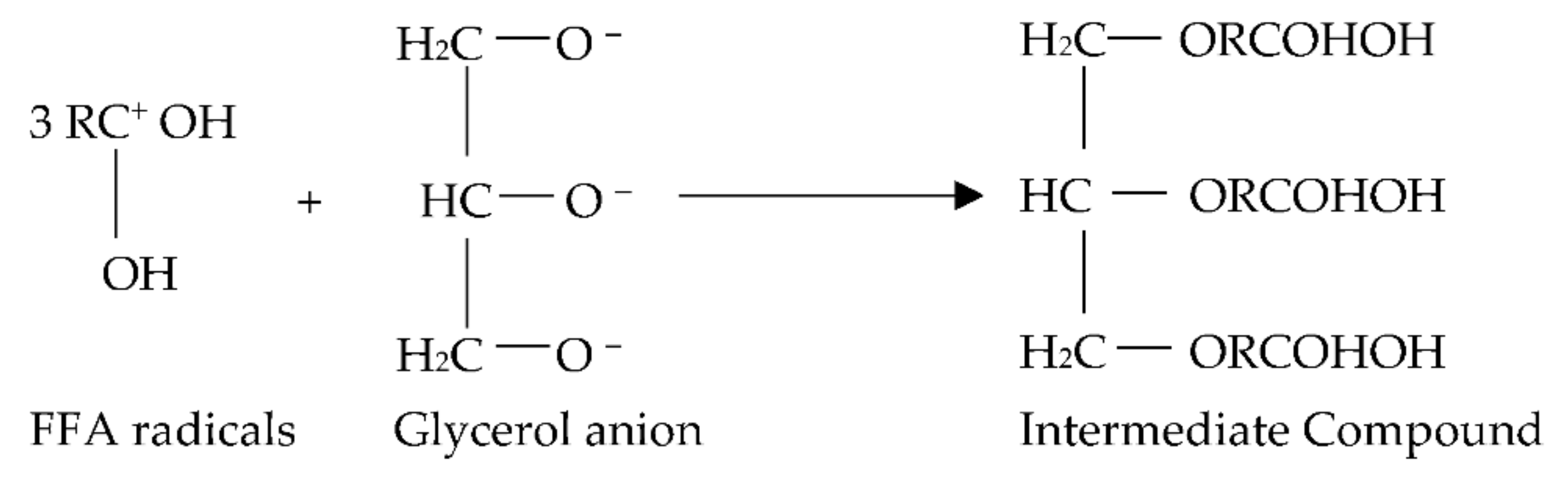
- The glyceroxide anion reacts with the FFAs cations to generate an intermediate molecule, as seen in Figure 6.
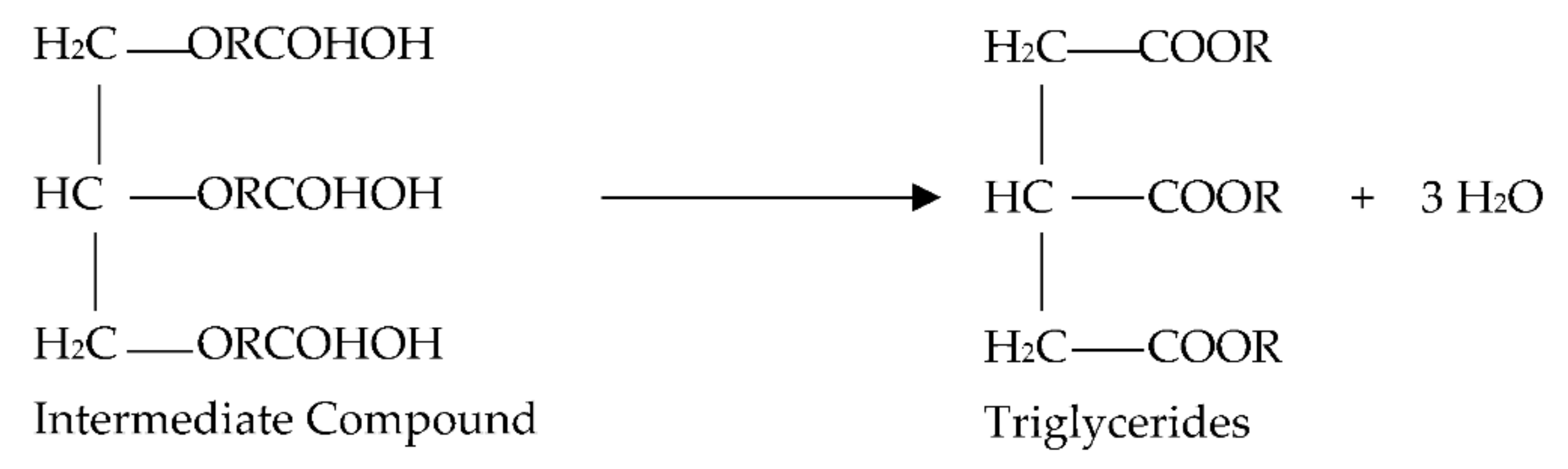
- As demonstrated in the equation, the intermediate chemical decomposes into water and triglycerides, as shown in Figure 7.
2. Materials and Methods
2.1. Pre-Treatment
2.2. Determination of Acid Value and FFA Content
2.3. Transesterification
2.4. Methanol Recovery
2.5. Glycerol Purification
2.6. Glycerolysis
2.7. Acid Esterification
2.8. Statistical Analysis
3. Results and Discussion
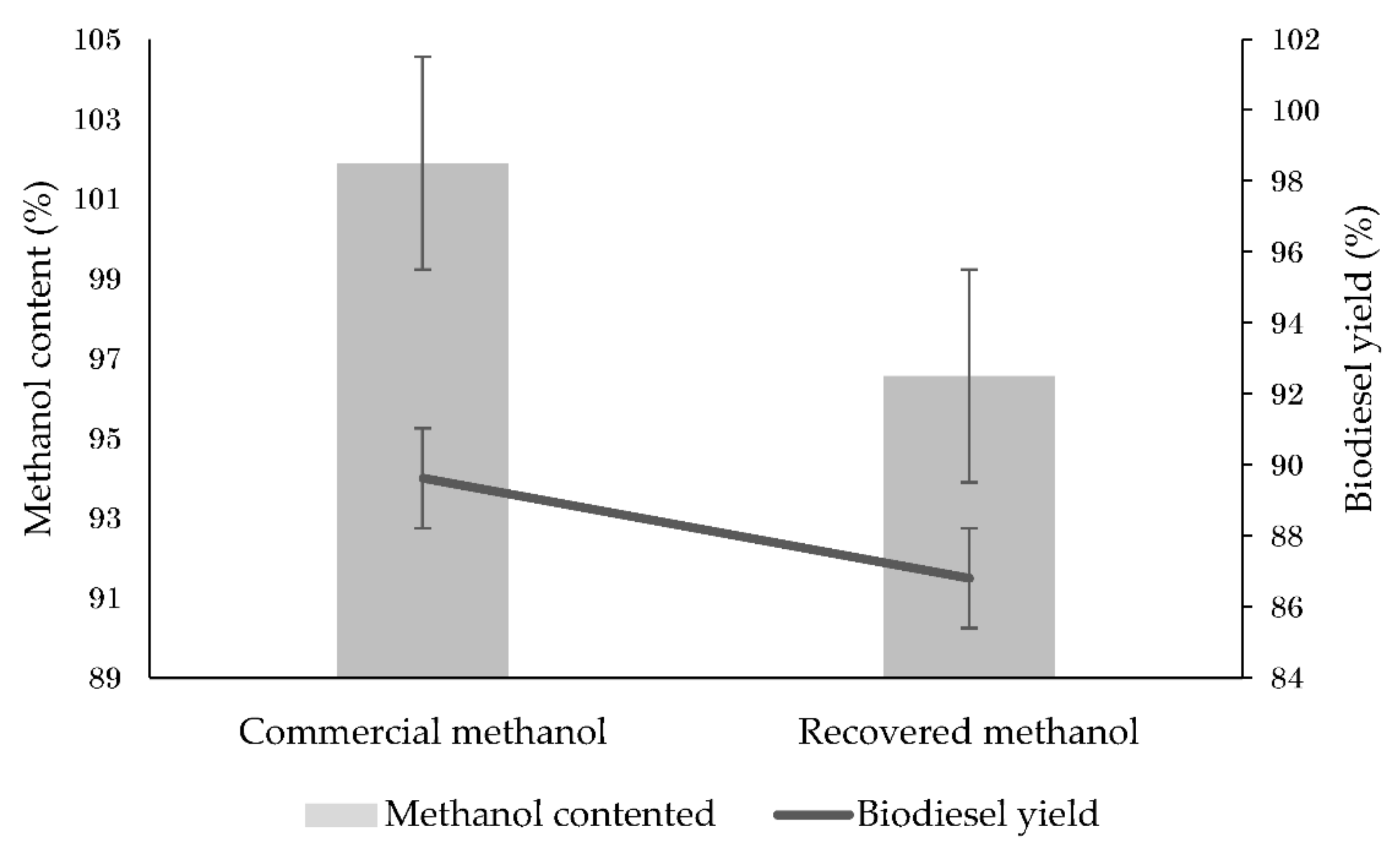
3.1. Methanol Recovery
3.2. Crude Glycerol and Methanol Removed Glycerol
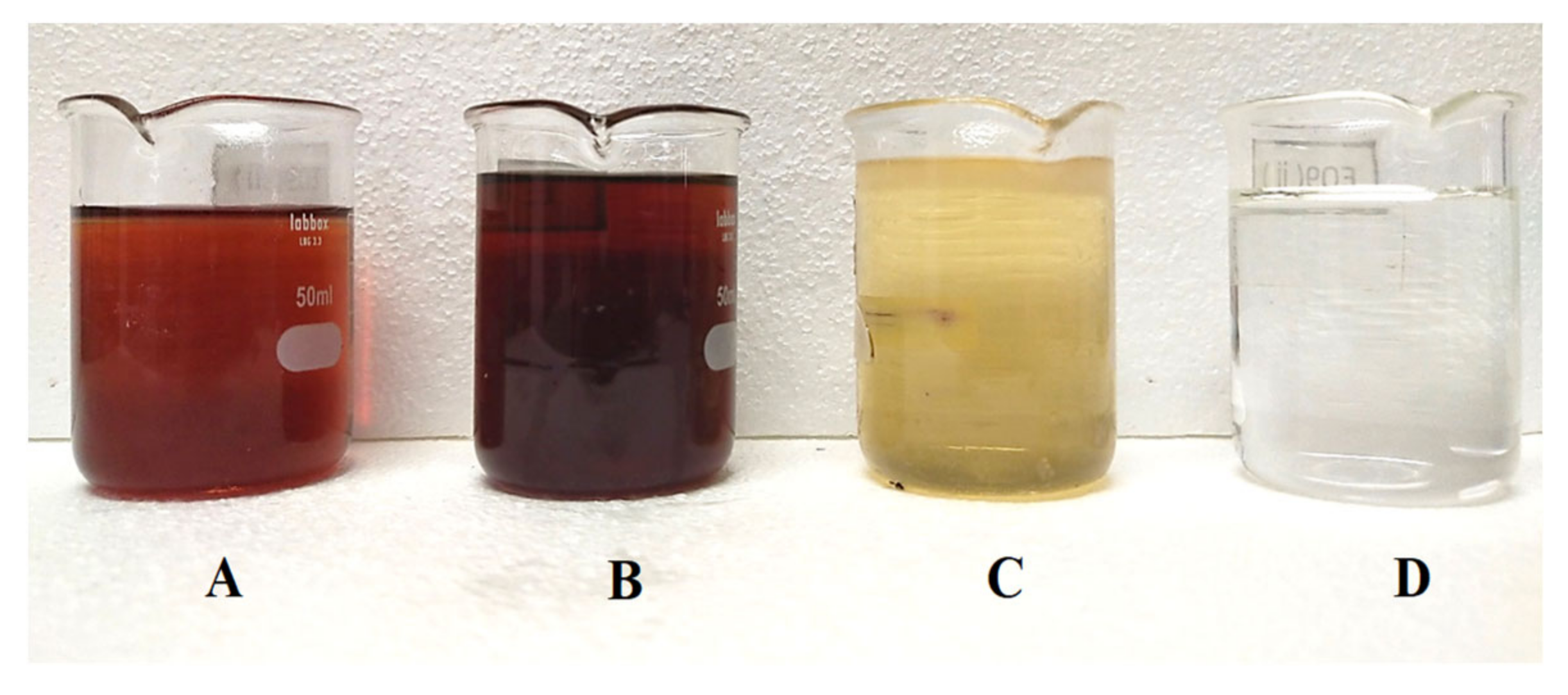
3.3. Crude Glycerol Purification
3.4. Economic Analysis of Glycerol Purification and Commercially Available Glycerol
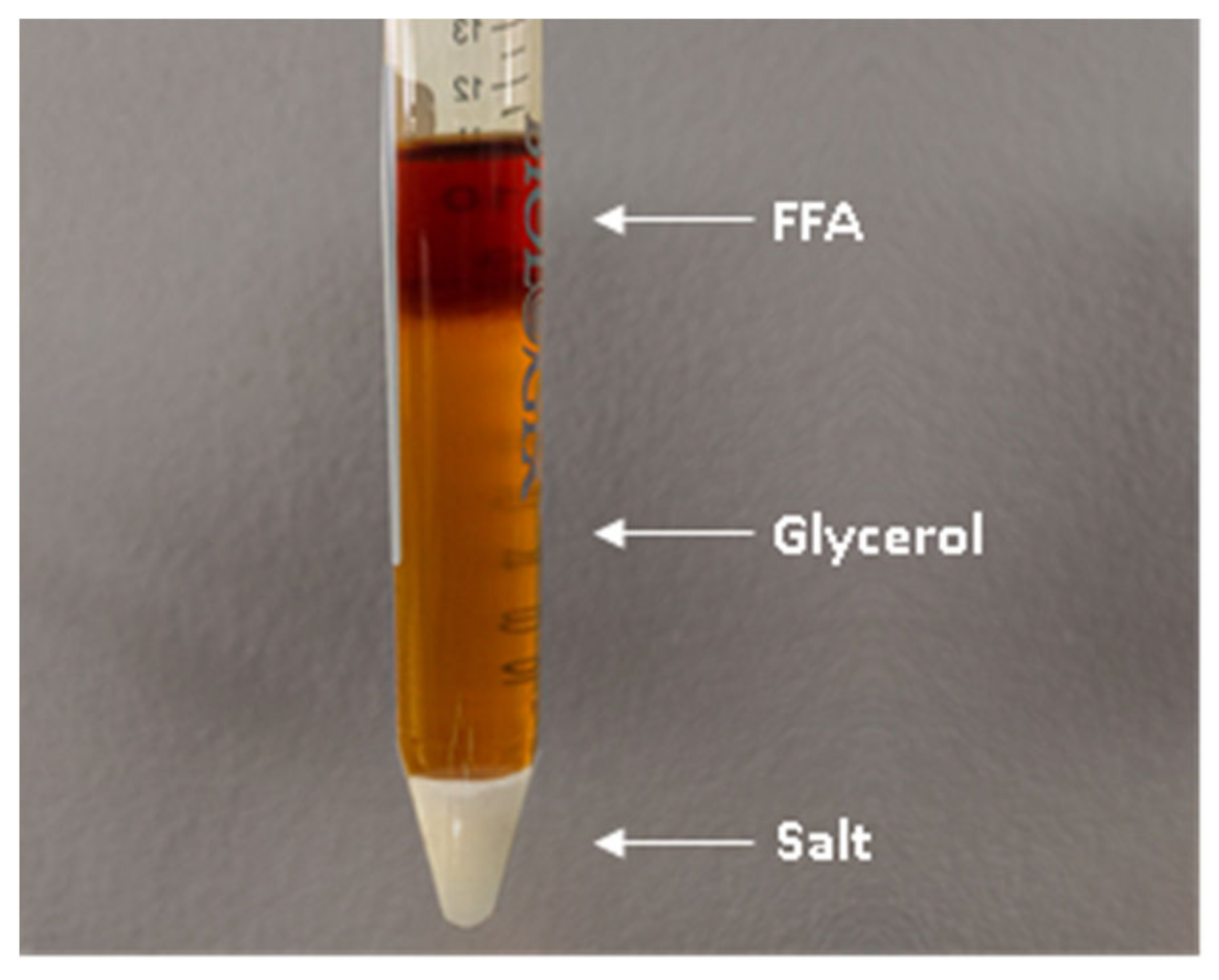
3.5. Glycerolysis
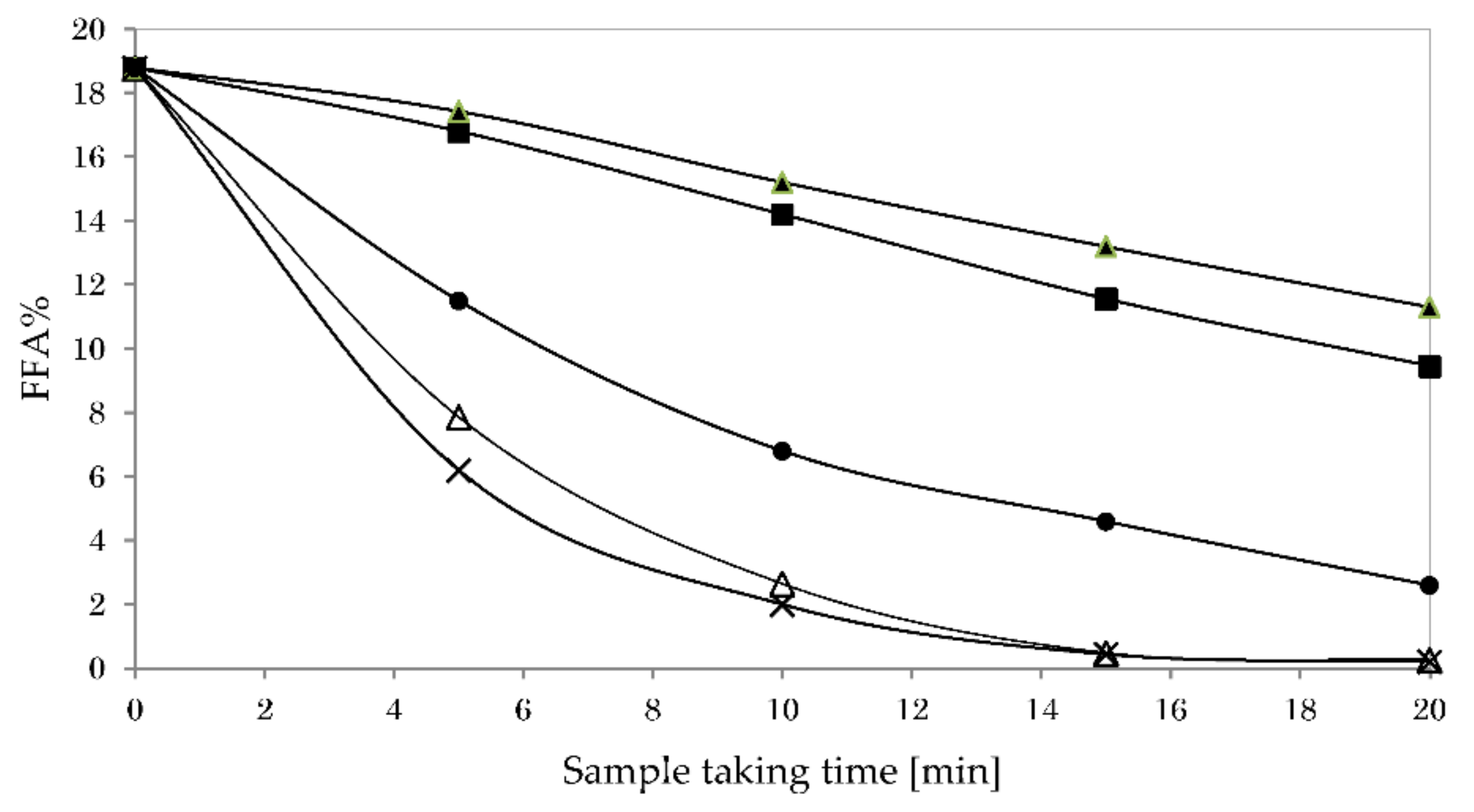
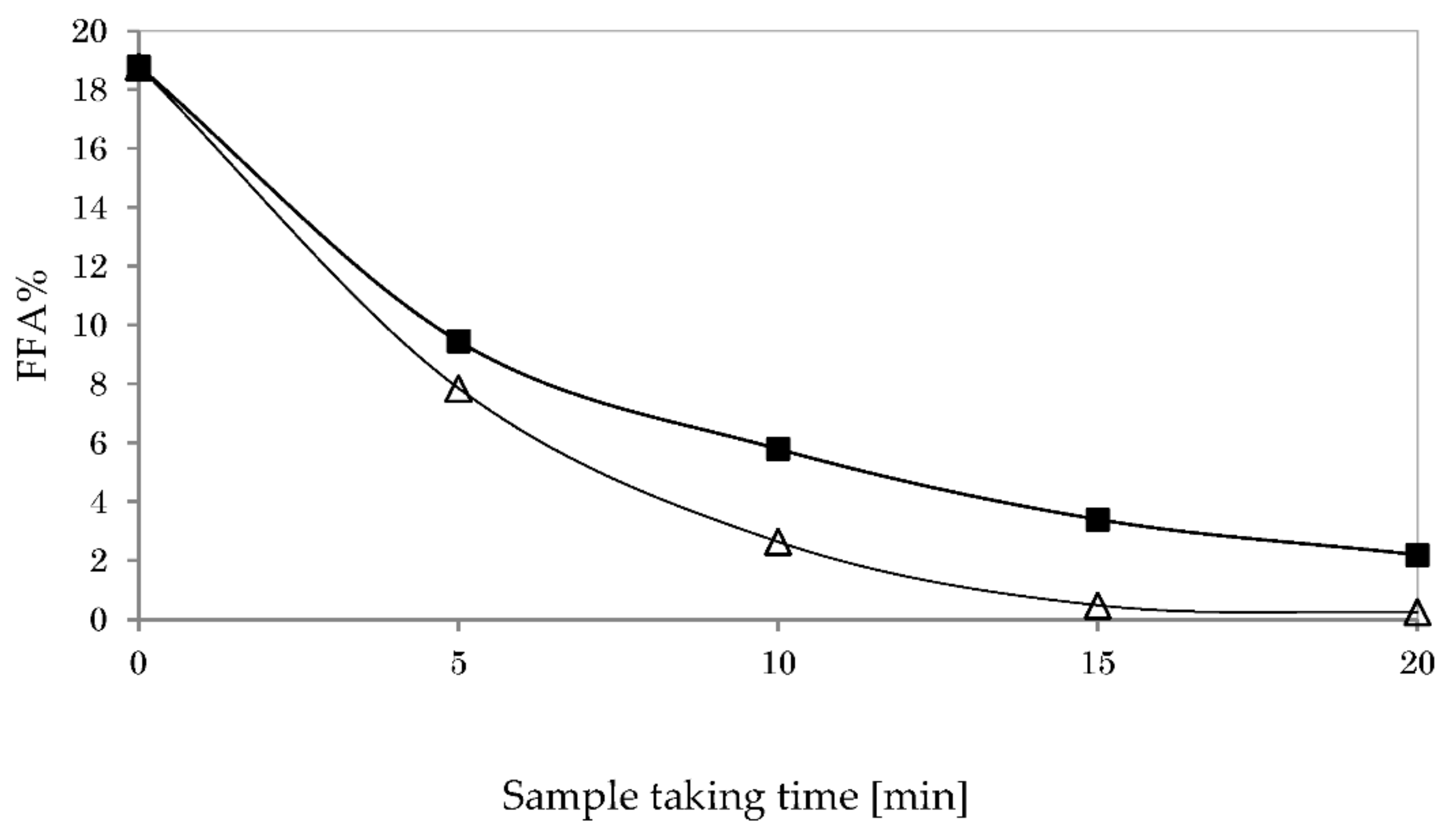
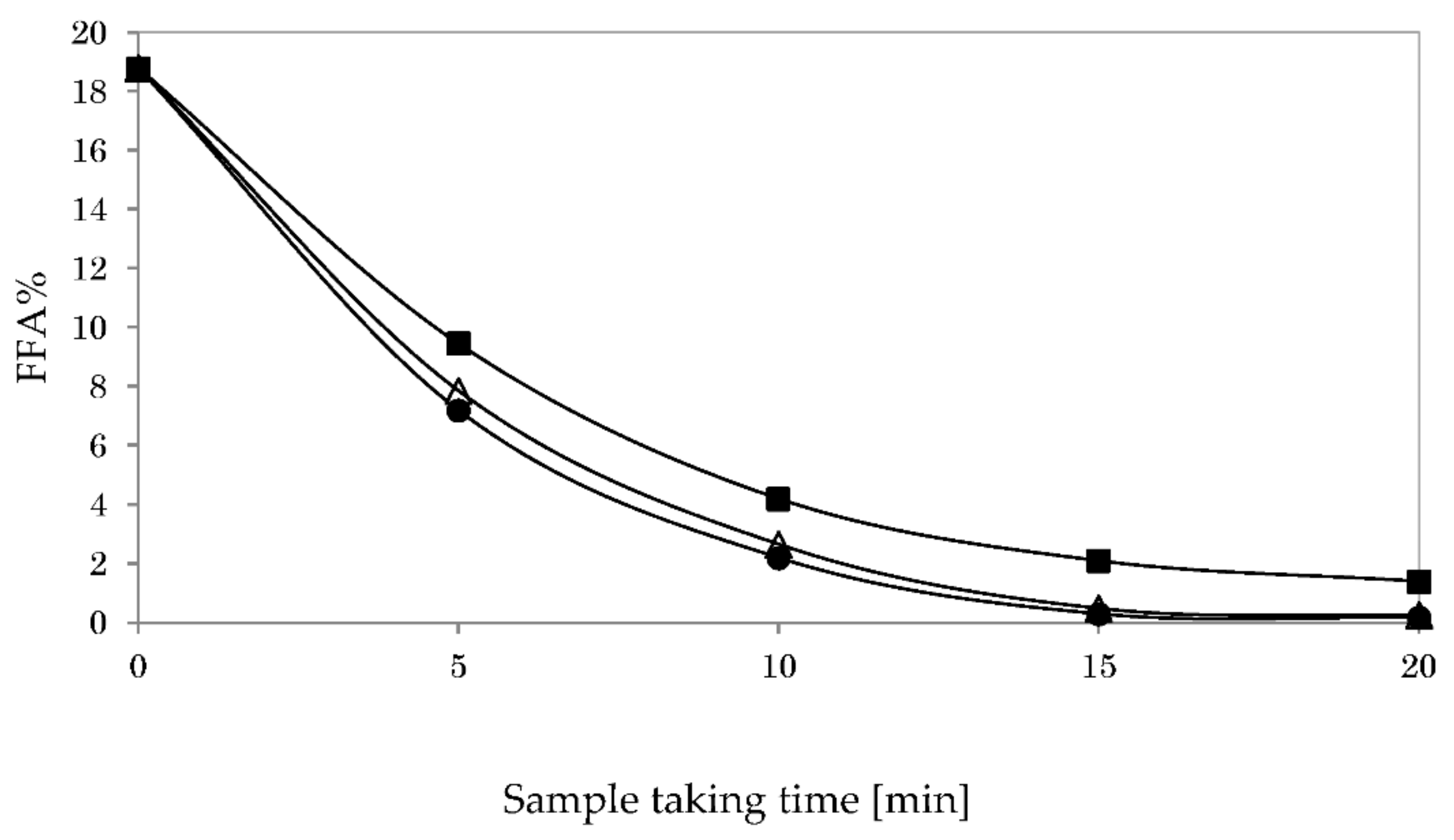
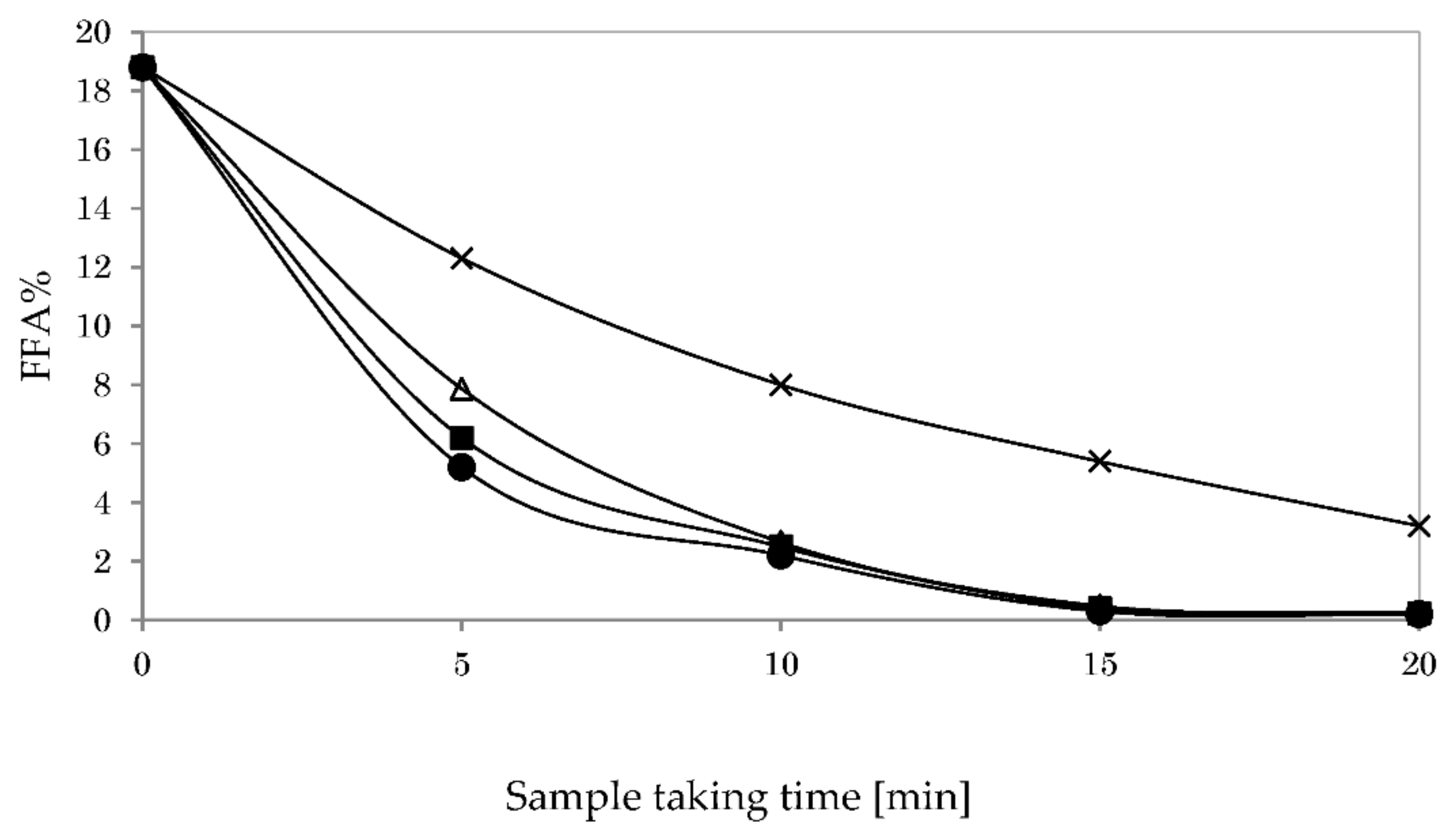
3.5.1. Impact of Reaction Temperature on Glycerolysis
3.5.2. Impact of Stirring Speed on Glycerolysis
3.5.3. Impact of Catalyst Type on Glycerolysis
3.5.4. Impact of Co-Solvent Type on Glycerolysis
3.5.5. Impact of Oil-To-Hexane Ratio on Glycerolysis
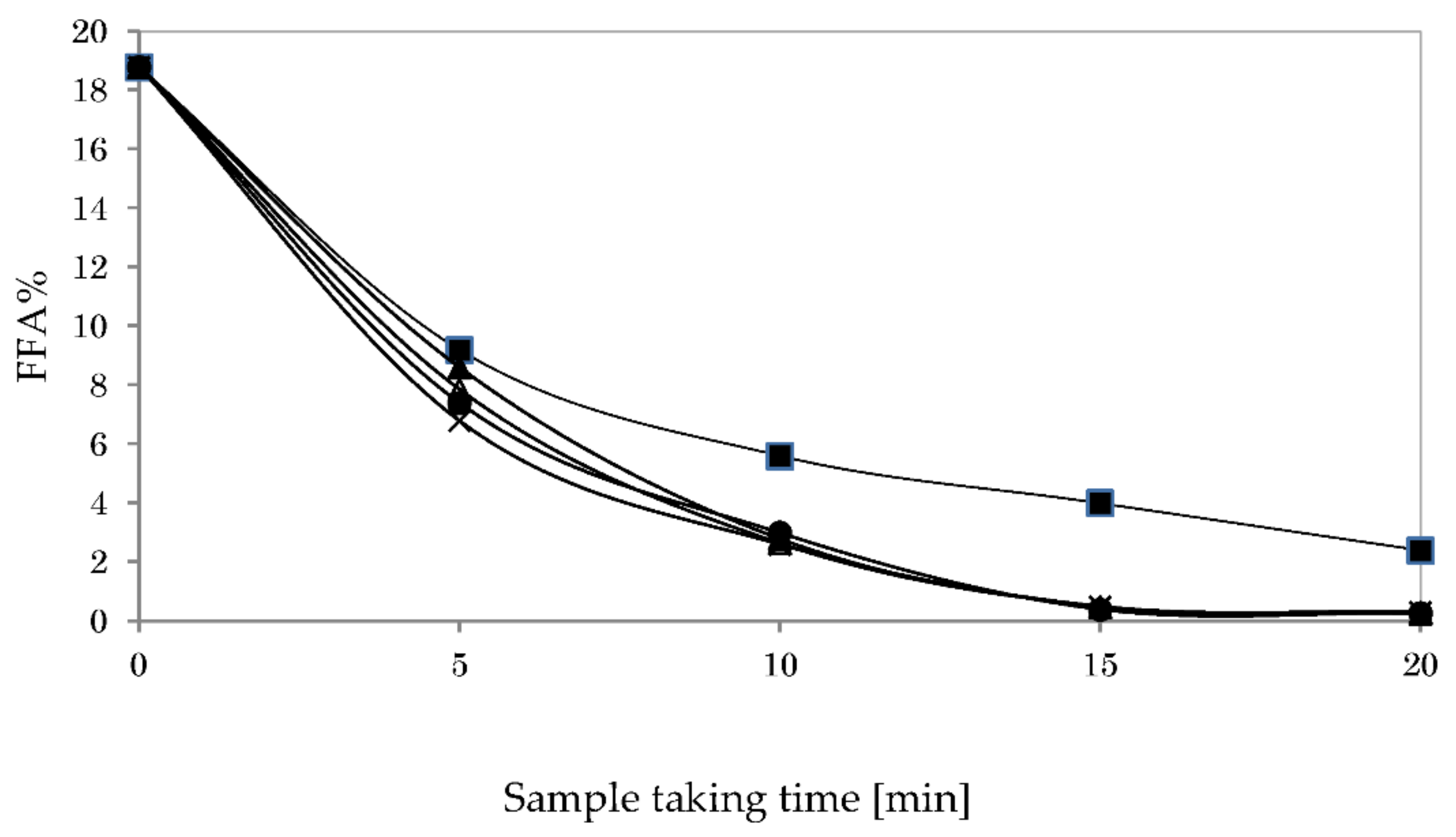
3.6. Acid Esterification and Glycerolysis
3.7. Practical Implications of the Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pitt, F.D.; Domingos, A.M.; Barros, A.C. Purification of residual glycerol recovered from biodiesel production. S. Afr. J. Chem. Eng. 2019, 29, 42–51. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Li, R.-J. Fuel properties of biodiesel produced from the crude fish oil from the soapstock of marine fish. Fuel Process. Technol. 2009, 90, 130–136. [Google Scholar] [CrossRef]
- Ogunkunle, O.; Ahmed, N. Overview of Biodiesel Combustion in Mitigating the Adverse Impacts of Engine Emissions on the Sustainable Human–Environment Scenario. Sustainability 2021, 13, 5465. [Google Scholar] [CrossRef]
- Thilakarathne, D.; Miyuranga, K.V.; Arachchige, U.S.P.R.; Weerasekara, N.A.; Jayasinghe, R.A. Production of biodiesel from waste cooking oil in laboratory scale: A review. Int. J. Sci. Eng. Sci. 2021, 5, 28–34. [Google Scholar]
- Bazooyar, B.; Ghorbani, A.; Shariati, A. Combustion performance and emissions of petrodiesel and biodiesels based on various vegetable oils in a semi industrial boiler. Fuel 2011, 90, 3078–3092. [Google Scholar] [CrossRef]
- Colombo, K.; Ender, L.; Santos, M.; Barros, A.C. Production of biodiesel from Soybean Oil and Methanol, catalyzed by calcium oxide in a recycle reactor. S. Afr. J. Chem. Eng. 2019, 28, 19–25. [Google Scholar] [CrossRef]
- Miyuranga, K.A.V.; Thilakarathne, D.; Arachchige, U.S.P.R.; Jayasinghe, R.A.; Weerasekara, N.A. Catalysts for Biodiesel Production: A Review. Asian J. Chem. 2021, 33, 1985–1999. [Google Scholar] [CrossRef]
- Nasir, N.F.; Mirus, M.F.; Ismail, M. Purification of crude glycerol from transesterification reaction of palm oil using direct method and multistep method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 243, 12015. [Google Scholar] [CrossRef]
- Arachchige, U.S.P.R.; Miyuranga, K.A.V.; Thilakarathne, D.; Jayasinghe, R.A.; Weerasekara, N.A. Biodiesel-Alkaline Transesterification Process for Methyl Ester Production. Nat. Environ. Pollut. Technol. 2021, 20, 1973–1980. [Google Scholar] [CrossRef]
- Tsoutsos, T.; Tournaki, S.; Gkouskos, Z.; Paraíba, O.; Giglio, F.; García, P.Q.; Braga, J.; Adrianos, H.; Filice, M. Quality Characteristics of Biodiesel Produced from Used Cooking Oil in Southern Europe. ChemEngineering 2019, 3, 19. [Google Scholar] [CrossRef]
- Sampath, P.R.A.; Samarakoon, S.P.A.G.L.; Ismail, F.M.; Gunawardena, S.H.P. Biodiesel production from high FFA Rubber Seed oil. In Proceedings of the 14th ERU Symposium, Faculty of Engineering, University of Moratuwa, Moratuwa, Sri Lanka, 2008. [Google Scholar]
- Mizik, T.; Gyarmati, G. Economic and Sustainability of Biodiesel Production—A Systematic Literature Review. Clean Technol. 2021, 3, 19–36. [Google Scholar] [CrossRef]
- Felizardo, P.; Machado, J.; Vergueiro, D.; Correia, M.J.N.; Gomes, J.P.; Bordado, J. Study on the glycerolysis reaction of high free fatty acid oils for use as biodiesel feedstock. Fuel Process. Technol. 2011, 92, 1225–1229. [Google Scholar] [CrossRef]
- Tizvar, R.; McLean, D.D.; Kates, M.; Dubé, M.A. Optimal Separation of Glycerol and Methyl Oleate via Liquid−Liquid Extraction. J. Chem. Eng. Data 2009, 54, 1541–1550. [Google Scholar] [CrossRef]
- ASTM. Standard Specification for Biodiesel Fuel (B100) Blend Stock for Distillate Fuels, Designation D-6751-02; American Society for Testing and Materials: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Santibáñez, C.; Varnero, M.T.; Bustamante, M. Residual Glycerol from Biodiesel Manufacturing, Waste or Potential Source of Bioenergy: A Review. Chil. J. Agric. Res. 2011, 71, 469–475. [Google Scholar] [CrossRef]
- Brunschwig, C.; Moussavou, W.; Blin, J. Use of bioethanol for biodiesel production. Prog. Energy Combust. Sci. 2012, 38, 283–301. [Google Scholar] [CrossRef]
- Pott, R.W.; Howe, C.J.; Dennis, J.S. The purification of crude glycerol derived from biodiesel manufacture and its use as a substrate by Rhodopseudomonas palustris to produce hydrogen. Bioresour. Technol. 2014, 152, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Andrade, I.; Avella-Moreno, E.; Sierra-Cantor, J.F.; Guerrero-Fajardo, C.A.; Sodre, J.R. Purification of glycerol from biodiesel production by sequential extraction monitored by 1H NMR. Fuel Process. Technol. 2015, 132, 99–104. [Google Scholar] [CrossRef]
- Ardi, M.; Aroua, M.; Hashim, N.A. Progress, prospect and challenges in glycerol purification process: A review. Renew. Sustain. Energy Rev. 2015, 42, 1164–1173. [Google Scholar] [CrossRef]
- Oliveira, M.; Ramos, A.; Monteiro, E.; Rouboa, A. Improvement of the Crude Glycerol Purification Process Derived from Biodiesel Production Waste Sources through Computational Modeling. Sustainability 2022, 14, 1747. [Google Scholar] [CrossRef]
- Oro, C.E.D.; Bonato, M.; Oliveira, J.V.; Tres, M.V.; Mignoni, M.L.; Dallago, R.M. A new approach for salts removal from crude glycerin coming from industrial biodiesel production unit. J. Environ. Chem. Eng. 2019, 7, 102883. [Google Scholar] [CrossRef]
- Manosak, R.; Limpattayanate, S.; Hunsom, M. Sequential-refining of crude glycerol derived from waste used-oil methyl ester plant via a combined process of chemical and adsorption. Fuel Process. Technol. 2011, 92, 92–99. [Google Scholar] [CrossRef]
- Abdul Raman, A.A.; Tan, H.W.; Buthiyappan, A. Two-Step Purification of Glycerol as a Value Added by Product from the Biodiesel Production Process. Front. Chem. 2019, 7, 774. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, G.; Iulianelli, A.; Sanna, A.; Basile, A. Glycerol Production and Transformation: A Critical Review with Particular Emphasis on Glycerol Reforming Reaction for Producing Hydrogen in Conventional and Membrane Reactors. Membranes 2017, 7, 17. [Google Scholar] [CrossRef]
- Supardan, M.D.; Adisalamun; Meldasari, Y.; Annisa, Y.; Mahlinda. Glycerolysis for Lowering Free FattyAcid of Waste Cooking Oil. In Proceedings of the 8th International Conference of Chemical Engineering on Science and Applications (ChESA), Banda Aceh, Indonesia, 9–11 September 2015. [Google Scholar]
- Suriaini, N.; Arpi, N.; Syamsuddin, Y.; Supardan, M.D. Use of Crude Glycerol for Glycerolysis of Free Fatty Acids in Crude Palm Oil. Int. J. Technol. 2021, 12, 760. [Google Scholar] [CrossRef]
- Kombe, G.G.; Temu, A.K.; Rajabu, H.M.; Mrema, G.D.; Kansedo, J.; Lee, K.T. Pre-Treatment of High Free Fatty Acids Oils by Chemical Re-Esterification for Biodiesel Production—A Review. Adv. Chem. Eng. Sci. 2013, 03, 242–247. [Google Scholar] [CrossRef]
- Fregolente, P.B.L.; Fregolente, L.V.; Pinto, G.M.F.; Batistella, B.C.; Wolf-Maciel, M.R.; Filho, R.M. Monoglycerides and Diglycerides Synthesis in a Solvent-Free System by Lipase-Catalyzed Glycerolysis. Appl. Biochem. Biotechnol. 2008, 146, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Elgharbawy, A.S.; Sadik, W.A.; Sadek, O.M.; Kasaby, M.A. Glycerolysis treatment to enhance biodiesel production from low-quality feedstocks. Fuel 2020, 284, 118970. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Maestro-Madurga, B.; Pesquera-Rodríguez, A.; Ramírez-López, C.; Lorenzo-Ibarreta, L.; Torrecilla-Soria, J.; Villarán-Velasco, M.C. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate by transesterification: Catalyst screening and reaction optimization. Appl. Catal. A Gen. 2009, 366, 315–324. [Google Scholar] [CrossRef]
- Sonntag, N.O.V. Glycerolysis of fats and methyl esters—Status, review and critique. J. Am. Oil Chem. Soc. 1982, 59, 795A–802A. [Google Scholar] [CrossRef]
- Noureddini, H.; Harkey, D.W.; Gutsman, M.R. A continuous process for the glycerolysis of soybean oil. J. Am. Oil Chem. Soc. 2004, 81, 203–207. [Google Scholar] [CrossRef]
- Gole, V.L.; Gogate, P.R. Intensification of glycerolysis reaction of higher free fatty acid containing sustainable feedstock using microwave irradiation. Fuel Process. Technol. 2014, 118, 110–116. [Google Scholar] [CrossRef]
- Cai, Z.-Z.; Wang, Y.; Teng, Y.-L.; Chong, K.-M.; Wang, J.-W.; Zhang, J.-W.; Yang, D.-P. A two-step biodiesel production process from waste cooking oil via recycling crude glycerol esterification catalyzed by alkali catalyst. Fuel Process. Technol. 2015, 137, 186–193. [Google Scholar] [CrossRef]
- Jansri, S. Preparation of Vegetable Oil as Biodiesel Feedstock Via Re-Esterification: A Suitable Catalyst. Energy Procedia 2015, 79, 143–148. [Google Scholar] [CrossRef]
- Binhayeeding, N.; Klomklao, S.; Sangkharak, K. Utilization of Waste Glycerol from Biodiesel Process as a Substrate for Mono-, Di-, and Triacylglycerol Production. Energy Procedia 2017, 138, 895–900. [Google Scholar] [CrossRef]
- Kombe, G.G.; Temu, A.K.; Rajabu, H.M.; Mrema, G.D.; Lee, K.T. Low Temperature Glycerolysis as a High FFA Pre-Treatment Method for Biodiesel Production. Adv. Chem. Eng. Sci. 2013, 3, 248–254. [Google Scholar] [CrossRef]
- Kombe, G.G. Re-esterification of high free fatty acid oils for biodiesel production. Biofuels 2015, 6, 31–36. [Google Scholar] [CrossRef]
- Supardan, M.D.; Lubis, Y.M.; Annisa, Y.; Mustapha, W.A.W. Effect of Co-solvent Addition on Glycerolysis of Waste Cooking Oil. Pertanika J. Sci. Technol. 2017, 25, 1203–1210. [Google Scholar]
- Wang, Y.; Ma, S.; Wang, L.; Tang, S.; Riley, W.W.; Reaney, M.J.T. Solid superacid catalyzed glycerol esterification of free fatty acids in waste cooking oil for biodiesel production. Eur. J. Lipid Sci. Technol. 2011, 114, 315–324. [Google Scholar] [CrossRef]
- Miyuranga, K.A.V.; Arachchige, U.S.P.R.; Thilakarathne, D.; Jayasinghe, R.A.; Weerasekara, N.A. Effects of Physico-Chemical Properties of the Blended Diesel and Waste Cooking Oil Biodiesel. Asian J. Chem. 2022, 34, 319–323. [Google Scholar] [CrossRef]
- Encinar, J.M.; Sánchez, N.; Martínez, G.; García, L. Study of biodiesel production from animal fats with high free fatty acid content. Bioresour. Technol. 2011, 102, 10907–10914. [Google Scholar] [CrossRef] [PubMed]
- Kombe, G.G.; Temu, A.K.; Rajabu, H.M.; Mrema, G.D. High Free Fatty Acid (FFA) Feedstock PreTreatment Method for Biodiesel Production. In Proceedings of the 2nd International Conference on Advance in Engineering and Technology, Noida, India, 20–11 December 2011; pp. 176–182. [Google Scholar]
- Elkady, M.F.; Zaatout, A.; Balbaa, O. Production of Biodiesel from Waste Vegetable Oil via KM Micromixer. J. Chem. 2015, 2015, 630168. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S.; Piao, X. Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 2008, 87, 216–221. [Google Scholar] [CrossRef]
- Van Gerpen, J.; Shanks, B.; Pruszko, R.; Clements, D.; Knothe, G. Biodiesel Production Technology: August 2002—January 2004; (No. NREL/SR-510-36244); National Renewable Energy Lab: Golden, CO, USA, 2004. [Google Scholar] [CrossRef]
- Rodrigues, A.; Bordado, J.C.; dos Santos, R.G. Upgrading the Glycerol from Biodiesel Production as a Source of Energy Carriers and Chemicals—A Technological Review for Three Chemical Pathways. Energies 2017, 10, 1817. [Google Scholar] [CrossRef]
- Degfie, T.A.; Mamo, T.T.; Mekonnen, Y.S. Optimized Biodiesel Production from Waste Cooking Oil (WCO) using Calcium Oxide (CaO) Nano-catalyst. Sci. Rep. 2019, 9, 18982. [Google Scholar] [CrossRef]
- Knothe, G.; Krahl, J.; Van Gerpen, J. (Eds.) The Biodiesel Handbook, 2nd ed.; AOCS Press: Urbana, IL, USA, 2005. [Google Scholar]
- Kongjao, S.; Damronglerd, S.; Hunsom, M. Purification of crude glycerol derived from waste used-oil methyl ester plant. Korean J. Chem. Eng. 2010, 27, 944–949. [Google Scholar] [CrossRef]
- Kafuku, G.; Mbarawa, M. Biodiesel production from Croton megalocarpus oil and its process optimization. Fuel 2010, 89, 2556–2560. [Google Scholar] [CrossRef]
- Miyuranga, K.A.V.; Balasuriya, B.M.C.M.; Arachchige, U.S.P.R.; Jayasinghe, R.A.; Weerasekara, N.A. A Comparative Analysis of Impact of Hexane, Diethyl Ether, Toluene and Acetone on Biodiesel Transesterification Process. Asian J. Chem. 2022, 34, 2545–2550. [Google Scholar] [CrossRef]
- Miyuranga, K.A.V.; Balasuriya, B.M.C.M.; Thilakarathna, D.; Arachchige, U.S.P.R.; Weerasekara, N.A.; Jayasinghe, R.A. Production of Biodiesel Using Acetone as a CoSolvent. Int. J. Eng. Sci. 2022, 6, 52–56. [Google Scholar]
- Hasheminejad, M.; Tabatabaei, M.; Mansourpanah, Y.; Far, M.K.; Javani, A. Upstream and downstream strategies to economize biodiesel production. Bioresour. Technol. 2011, 102, 461–468. [Google Scholar] [CrossRef]
- Singhabhandhu, A.; Tezuka, T. A perspective on incorporation of glycerin purification process in biodiesel plants using waste cooking oil as feedstock. Energy 2010, 35, 2493–2504. [Google Scholar] [CrossRef]
- Pachauri, N.; He, B. Value-added utilization of crude glycerol from biodiesel production: A survey of current research activities. In Proceedings of the ASABE Annual International Meeting, Portland, Oregon, 9–12 July 2006; p. 16. [Google Scholar]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della Pina, C. Recent advances in the conversion of bioglycerol into value-added products. Eur. J. Lipid Sci. Technol. 2009, 111, 788–799. [Google Scholar] [CrossRef]
- Yang, F.; A Hanna, M.; Sun, R. Value-added uses for crude glycerol--a byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.; Abdullah, A.Z. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Leoneti, A.B.; Aragão-Leoneti, V.; de Oliveira, S.V.W.B. Glycerol as a by-product of biodiesel production in Brazil: Alternatives for the use of unrefined glycerol. Renew. Energy 2012, 45, 138–145. [Google Scholar] [CrossRef]
- Vasudevan, P.T.; Briggs, M. Biodiesel production—Current state of the art and challenges. J. Ind. Microbiol. Biotechnol. 2008, 35, 421–430. [Google Scholar] [CrossRef]



| Catalyst | Temperature | Oil-To-Glycerol Ratio | Time | Speed | FFA Conversion | Reference |
|---|---|---|---|---|---|---|
| zinc acetate 0.6 wt.% | 200 °C | 1:2 molar ratio | 240 min | 350 rpm | 9.1 to 0.6 wt.%. | [35] |
| ZnO 0.6 wt.% | 150 °C | 50 wt.% | 180 min | 600 rpm | 20 to 1.5 wt.% | [36] |
| Lipase 0.5 wt.% | 40 °C | 1:6 molar ratio | 1440 min | 250 rpm | [37] | |
| NaOH 1.75 wt.% | 65 °C | 224 wt.% | 73 min | 800 rpm | 4.6 to 0.07 wt.% | [38] |
| NaOH 1.75 wt.% | 56 °C | 234 wt.% | 85 min | 800 rpm | 6.50 to 0.06 wt.% | [39] |
| NaOH 1.3 wt.% | 65 °C | 1:2 molar ratio | 20 min | 600 rpm | 6.1 to 0.5 wt.% | [26] |
| NaOH 0.875 wt.% | 65 °C | 1:1 molar ratio | 150 min | 600 rpm | 5.7 to 0.45 wt% | [40] |
| SO42−/ZrO2–Al2O3 0.3 wt.% | 200 °C | 1:4 molar ratio | 240 min | 200 rpm | 44.2 to 0.66 wt% | [41] |
| metallic zinc 0.1 wt.% | 220 °C | 1:10 molar ratio | 120 min | 300 rpm | 50 to 5 wt.% | [13] |
| Property | Test Method | Crude Glycerol | Methanol-Free Glycerol |
|---|---|---|---|
| Glycerol (wt.%) | ASTM D 6584 | 16.25 | 23.28 |
| Moisture (wt.%) | ASTM D 4377-00 (2011) | 2.18 | 2.16 |
| Methanol (wt.%) | Simple distillation | 8 | 0 |
| Ash (wt.%) | ASTM D 482-19 | 5.982 | 6.260 |
| Total salt (w/v%) | AOAC 965. 01 | 5.2 | 5.2 |
| MONG content (wt.%) 1 | ISO 2464 | 75.588 | 68.300 |
| pH at 25 °C | pH meter | 10 | 9.4 |
| Density (at 25 °C g/mL) | ASTM D891-95 (2004) | 1.036 | 1.152 |
| Color | Naked eye observation | Dark brown | Deep dark brown |
| Property | Test Method | Purified Glycerol in This Study | Commercially Available Glycerol |
|---|---|---|---|
| Glycerol (wt.%) | ASTM D 6584 | 94.0 | 99.9 |
| Moisture (wt.%) | ASTM D 4377-00 (2011) | 0.20 | 0.01 |
| Methanol (wt.%) | Simple distillation | 0 | 0 |
| Ash (wt.%) | ASTM D 482-19 | <0.001 | 0.000 |
| Total salt (w/v%) | AOAC 965. 01 | 2.1 | 0.0 |
| MONG content (wt.%) 1 | ISO 2464 | 5.800 | 0.000 |
| pH at 25 °C | pH meter | 6.99 | 6.98 |
| Density (at 25 °C g/mL) | ASTM D891-95 (2004) | 1.26 | 1.27 |
| Color | Naked eye observation | Colorless | Colorless |
| Item | CAS Number | Unit Price | Quantity | Crude Glycerol Purification | Commercially Available Glycerine |
|---|---|---|---|---|---|
| H3PO4 (90%) | 7664-38-2 | $1.04/L | 5 L | $5.2 | |
| NaOH (99%) | 1310-73-2 | $0.74/Kg | 2 L | $1.48 | |
| Silica beads (99%) | 7631-86-9 | $1.04/Kg | 2 Kg | $2.08 | |
| Activated carbon (99%) | 7440-44-0 | $2.58/Kg | 0.05 Kg | $0.129 | |
| Electricity | $0.069/kWh | 2 kWh | $0.138 | ||
| Operational cost | $0.69/h | 4 h | $2.76 | ||
| Commercial glycerol (99.6%) | 56-81-8 | $19.68/L | 1 L | $19.68 | |
| Total cost | 1 L | $11.787 | $19.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyuranga, K.A.V.; Arachchige, U.S.P.R.; Jayasinghe, R.A.; Samarakoon, G. Purification of Residual Glycerol from Biodiesel Production as a Value-Added Raw Material for Glycerolysis of Free Fatty Acids in Waste Cooking Oil. Energies 2022, 15, 8856. https://doi.org/10.3390/en15238856
Miyuranga KAV, Arachchige USPR, Jayasinghe RA, Samarakoon G. Purification of Residual Glycerol from Biodiesel Production as a Value-Added Raw Material for Glycerolysis of Free Fatty Acids in Waste Cooking Oil. Energies. 2022; 15(23):8856. https://doi.org/10.3390/en15238856
Chicago/Turabian StyleMiyuranga, K. A. Viraj, Udara S. P. R. Arachchige, Randika A. Jayasinghe, and Gamunu Samarakoon. 2022. "Purification of Residual Glycerol from Biodiesel Production as a Value-Added Raw Material for Glycerolysis of Free Fatty Acids in Waste Cooking Oil" Energies 15, no. 23: 8856. https://doi.org/10.3390/en15238856
APA StyleMiyuranga, K. A. V., Arachchige, U. S. P. R., Jayasinghe, R. A., & Samarakoon, G. (2022). Purification of Residual Glycerol from Biodiesel Production as a Value-Added Raw Material for Glycerolysis of Free Fatty Acids in Waste Cooking Oil. Energies, 15(23), 8856. https://doi.org/10.3390/en15238856






