Effect of Soybean Isoflavones on Proliferation and Related Gene Expression of Sow Mammary Gland Cells In Vitro
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell and Chemicals
2.2. Cell Culture and Treatment
2.3. Cell Morphology and Cell Viability
2.4. Immunocytochemistry
2.5. Functional Gene Expression of Mammary Epithelial Cells in Sows
2.6. Western Blotting
2.7. Statistical Analyses
3. Results
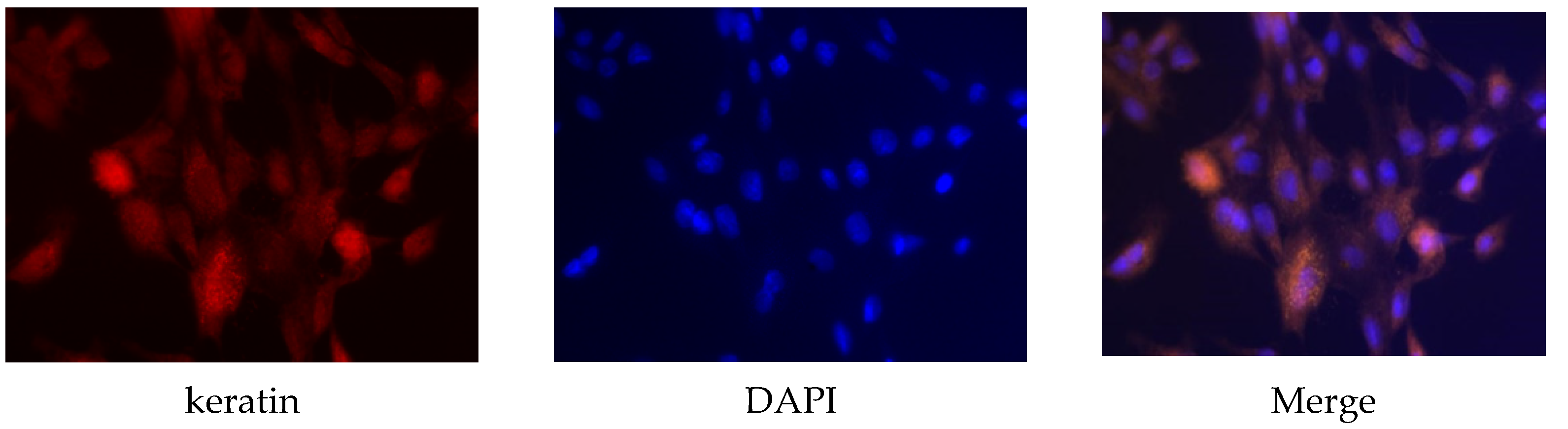
3.1. Identification of Sow Mammary Cell
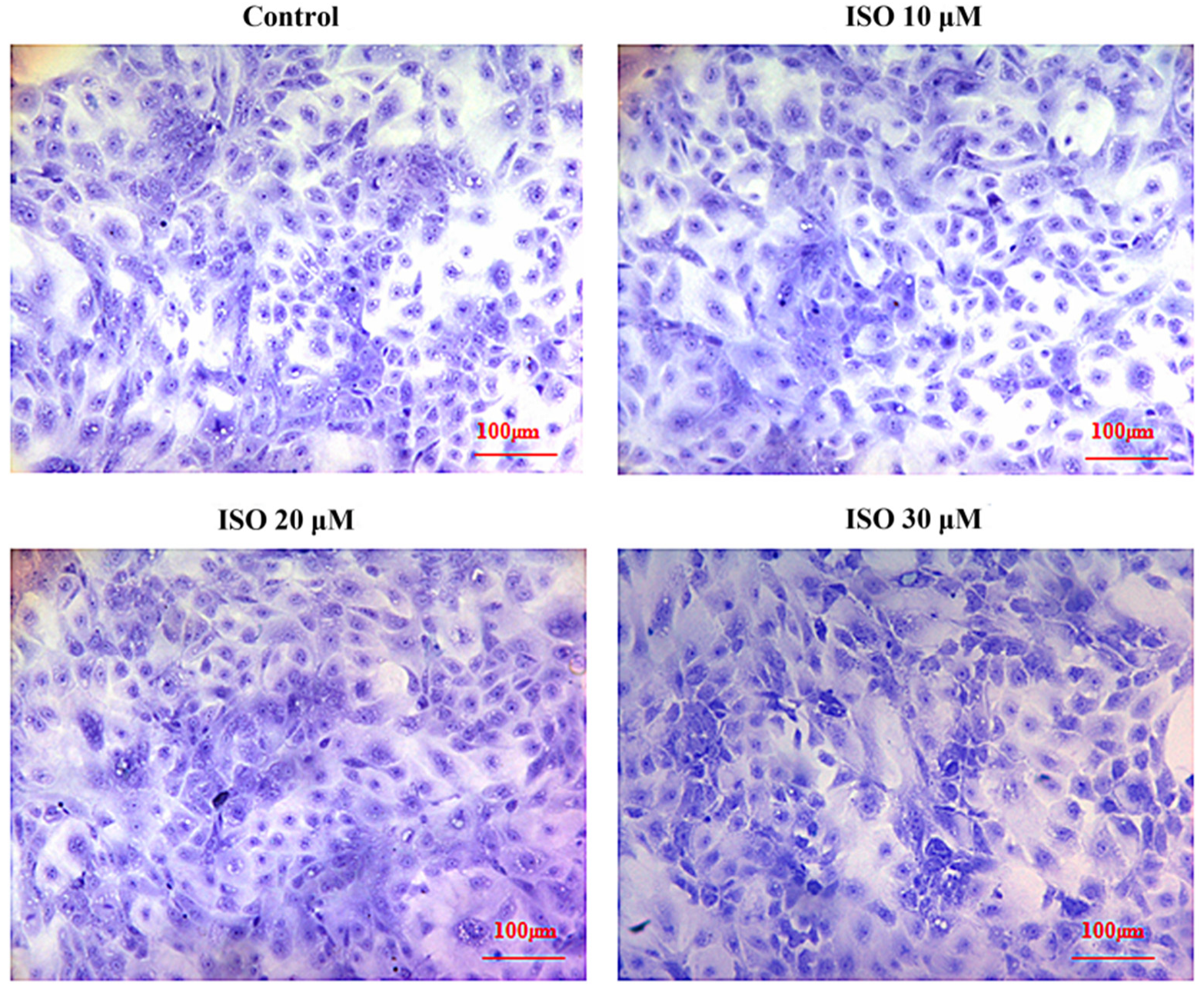
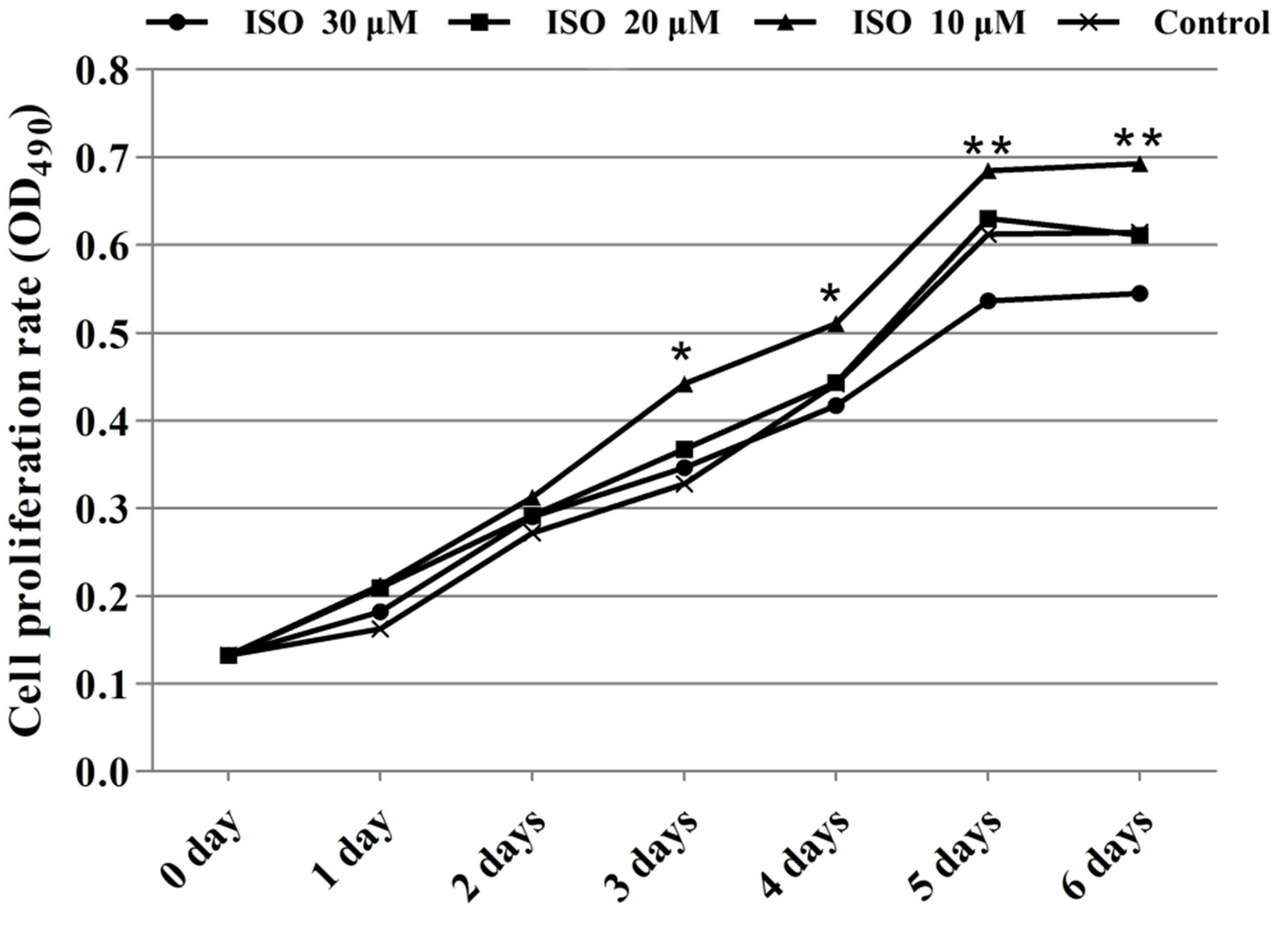
3.2. Effects of ISO on Proliferation of Sow Mammary Cells
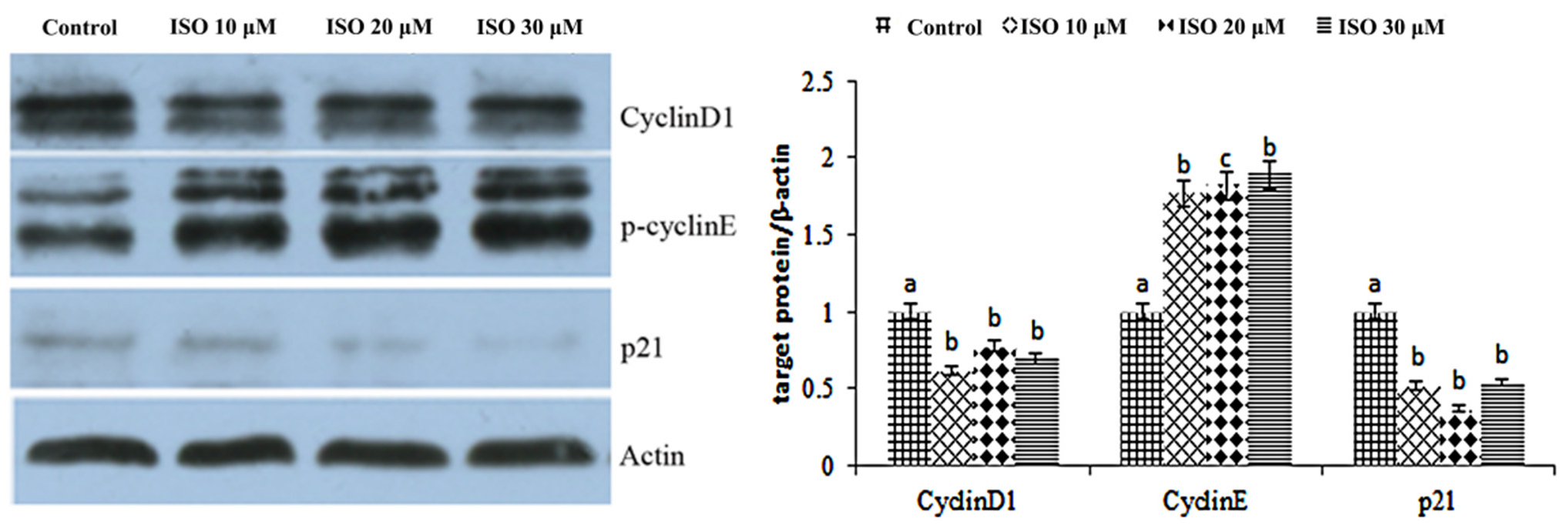
3.3. Identification of Key Proteins Related to Cell Proliferation
3.4. Functional Gene Expression in Mammary Gland Cell
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.J.; Gao, K.G.; Zheng, C.T.; Wu, Z.J.; Yang, X.F.; Wang, L.; Ma, X.Y.; Zhou, A.G.; Jiang, Z.J. Effect of dietary supplementation with glycitein during late pregnancy and lactation on antioxidative indices and performance of primiparous sows. J. Anim. Sci. 2015, 93, 2246–2254. [Google Scholar] [CrossRef] [PubMed]
- Vanden Hole, C.; Aerts, P.; Prims, S.; Ayuso, M.; Van Cruchten, S.; Van Ginneken, C. Does intrauterine crowding affect locomotor development? A comparative study of motor performance, neuromotor maturation and gait variability among piglets that differ in birth weight and vitality. PLoS ONE 2018, 13, e0195961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capuco, A.V.; Wood, D.L.; Baldwin, R.; Mcleod, K.; Paape, M.J. Mammary cell number, proliferation, and apoptosis during a bovine lactation: Relation to milk production and effect of bST. J. Dairy Sci. 2001, 84, 2177–2187. [Google Scholar] [CrossRef]
- Gilani, G.S.; Farmer, C.; Dyck, M.; Robertson, P.; Dahiya, J.; Sepehr, E.; Fan, L.; Nicolidakis, H.; Curran, I.; Cooke, G.M. Distribution of isoflavones in samples of serum, liver and mammary glands of rats or pigs fed dietary isoflavones. Ann. Nutr. Metab. 2011, 58, 171–180. [Google Scholar] [PubMed]
- Li, Y.P.; Jiang, X.R.; Wei, Z.X.; Cai, L.; Yin, J.D.; Li, X.L. Effects of soybean isoflavones on the growth performance, intestinal morphology and antioxidative properties in pigs. Animals 2020, 14, 2262–2270. [Google Scholar] [CrossRef]
- Andres, A.; Donovan, S.M.; Kuhlenschmidt, M.S. Soy isoflavones and virus infections. J. Nutr. Biochem. 2009, 20, 563–569. [Google Scholar] [CrossRef]
- Rahman Mazumder, M.A.; Hongsprabhas, P. Genistein as antioxidant and antibrowning agents in in vivo and in vitro: A review. Biomed. Pharmacother. 2016, 82, 379–392. [Google Scholar] [CrossRef]
- Smith, B.N.; Dilger, R.N. Immunomodulatory potential of dietary soybean-derived isoflavones and saponins in pigs. J. Anim. Sci. 2018, 96, 1288–1304. [Google Scholar] [CrossRef]
- Wang, T.T.; Sathyamoorthy, N.; Phang, J.M. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis 1996, 17, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Li, D.S.; Dang, D.X.; Xu, S.Y.; Tian, Y.M.; Wu, D.; Su, Y.H. Soy isoflavones supplementation improves reproductive performance and serum antioxidant status of sows and the growth performance of their offspring. J. Anim. Physiol. Anim. Nutr. 2022, 6, 1168–1276. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Santell, R.C.; Haslam, S.Z.; Helferich, W.G. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998, 58, 3833–3838. [Google Scholar] [PubMed]
- Murrill, W.B.; Brown, N.M.; Zhang, J.X.; Manzolillo, P.A.; Barnes, S.; Lamartiniere, C.A. Prepubertal genistein exposure suppresses mammary cancer and enhances gland differentiation in rats. Carcinogenesis 1996, 17, 1451–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.Y.; Zhu, W.Z.; Shi, H.D.; Hewett, J.E.; Ruhlen, R.L.; MacDonald, R.S.; Rottinghaus, G.E.; Chen, Y.C.; Sauter, E.R. Soy isoflavones have an antiestrogenic effect and alter mammary promoter hypermethylation in healthy premenopausal women. Nutr. Cancer 2009, 61, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Mahn, K.; Borrás, C.; Knoc, G.A.; Taylor, P.; Khan, I.Y.; Sugden, D.; Poston, L.; Ward, J.P.T.; Sharpe, R.M.; Viña, J.; et al. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005, 19, 1755–1757. [Google Scholar] [CrossRef]
- Joy, S.; Siow, R.C.M.; Rowlands, D.J.; Marko, B.; Wyatt, A.W.; Aaronson, P.I.; Coen, C.W.; Kallo, I.; Jacob, R.; Mann, G.E. The isoflavone Equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cells. J. Biol. Chem. 2006, 281, 27335–27345. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Xia, R.M.; Chen, J.B.; Gao, J.; Luo, X.Y.; Ke, C.L.; Ren, C.H.; Li, J.Y.; Mi, Y.J. Inhibition of esophageal-carcinoma cell proliferation by genistein via suppression of JAK1/2-STAT3 and AKT/MDM2/p53 signaling pathways. Aging 2020, 12, 6240–6259. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Y.; Jiang, Z.Y.; Zhang, J.; Hu, Y.J.; Gao, K.G.; Wang, L.; Yang, X.F. Isoflavone Ameliorates H2O2 Induced Injury by Activating the Antioxidant System of Sow Mammary Gland Cell. Nat. Sci. 2015, 7, 571–580. [Google Scholar]
- Tian, Z.M.; Ma, X.Y.; Deng, D.; Cui, Y.Y.; Chen, W.D. Influence of Nitrogen Levels on Nutrient Transporters and Regulators of Protein Synthesis in Small Intestinal Enterocytes of Piglets. J. Agric. Food Chem. 2019, 67, 2782–2793. [Google Scholar] [CrossRef]
- Sotoca, A.M.; Ratman, D.; vander Saa, P.; Strom, A.; Gustafsson, J.A.; Vervoort, J.; Rietjiens, I.M.; Murk, A.J. Phytoestrogen-mediated inhibition of proliferation of the human T47D breast cancer cells depends on the ERalpha/ERbeta ratio. J. Steroid Biochem. Mol. Biol. 2008, 112, 171–178. [Google Scholar] [CrossRef]
- Liu, H.; Du, J.; Hu, C.; Qi, H.; Wang, X.; Wang, S.; Liu, Q.; Li, Z. Delayed activation of extracellular-signal-regulated kinase 1/2 is involved in genistein- and equol-induced cell proliferation and estrogenreceptor-alpha-mediated transcription in MCF-7 breast cancer cells. J. Nutr. Biochem. 2010, 21, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Gaete, L.; Tchernitchin, A.N.; Bustamante, R.; Villena, J.; Lemus, I.; Gidekel, M.; Cabrera, G.; Astorga, P. Daidzein–Estrogen Interaction in the Rat Uterus and Its Effect on Human Breast Cancer Cell Growth. J. Med. Food 2012, 15, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Kumi-Diaka, J.; Sanderson, N.A.; Hall, A. The mediating role of caspase-3 protease in the intracellular mechanism of genistein-induced apoptosis in human prostatic carcinoma cell lines, DU145 and LNCaP. Biol. Cell 2000, 92, 595–604. [Google Scholar] [CrossRef]
- Bartek, J.; Lukas, J.; Bartkova, J. Perspective defects in cell cycle control and cancer. J. Pathol. 1999, 187, 95–99. [Google Scholar] [CrossRef]
- Hu, X.; Moscinski, L.C. Cdc2: A monopotent or pluripotent CDK. Cell Proliferat. 2011, 44, 205–211. [Google Scholar] [CrossRef]
- Neumeister, P.; Pixley, F.J.; Xiong, Y.; Xie, H.F.; Wu, K.M.; Ashton, A.; Cammer, M.; Chan, A.; Symons, M.; Stanley, E.R.; et al. Cyclin D1 governs adhesion and motility of macrophases. Mol. Cell Biol. 2003, 14, 2005–2015. [Google Scholar] [CrossRef] [Green Version]
- Sheer, C.J.; Robert, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Gen. Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markopoulos, G.S.; Roupakia, E.; Tokamani, M.; Vartholomatos, G.; Tzavaras, T.; Hatziapostolou, M.; Fackelmayer, F.O.; Sandaltzopoulos, R.; Polytarchou, C.; Kolettas, E. Senescence-associated microRNAs target cell cycle regulatory genes in normal human lung fibroblasts. Exp. Gerontol. 2017, 96, 110–122. [Google Scholar] [CrossRef]
- Siwicky, M.D.; Petrik, J.J.; Moorehead, R.A. The function of IGF-1R in NNK-mediated lung tunorigenesis. Lung Cancer 2011, 71, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Clapper, J.; Taylor, A. Components of the porcine anterior pituitary insulin-like growth factor system throughout the estrous cycle. Domest. Anim. Endocrinol. 2011, 40, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Park, H.J.; Yang, S.G.; Kim, M.J.; Kim, I.S.; Jegal, H.G.; Wee, G.; Yang, H.Y.; Park, J.J.; Choo, Y.K.; et al. Exogenous Ganglioside GT1b Enhances Porcine Oocyte Maturation, Including the Cumulus Cell Expansion and Activation of EGFR and ERK1/2 Signaling. Reprod. Sci. 2020, 27, 278–289. [Google Scholar] [CrossRef]
- Conti, M.; Hsieh, M.; Park, J.Y.; Su, Y.Q. Role of the epidermal growth factor network in ovarian follicles. Mol. Endocrinol. 2006, 20, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.G.; Mercado-Uribe, I.; Yang, G.; Bast, R.C., Jr.; Amin, H.M.; Lai, R.; Liu, J.S. The role of constitutively active signal transducer and activator of transcription 3 in ovarian tumorigenesis and prognosis. Cancer 2006, 107, 2730–2740. [Google Scholar] [CrossRef] [PubMed]
- Kumisada, K.; Negoro, S.; Tone, E.; Funamoto, M.; Osugi, T.; Yamada, S.; Okabe, M.; Kishimoto, T.; Yamauchi-Takihara, K. Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy. Proc. Natl. Acad. Sci. USA 2000, 97, 315. [Google Scholar] [CrossRef] [Green Version]
- Darnell, J.E., Jr. Reflections on STAT3, STAT5 and STAT6 as fat STATs. Proc. Natl. Acad. Sci. USA 1996, 93, 6221–6224. [Google Scholar] [CrossRef] [Green Version]
- Bromberg, J. Stat proteins and oncogenesis. J. Clin. Investig. 2002, 109, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A.; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in Postmenopausal, hormoneReceptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef] [Green Version]
- Bissler, J.J.; Kingswood, J.C.; Radzikowska, E.; Zonnenberg, B.A.; Frost, M.; Belousova, E.; Sauter, M.; Nonomura, N.; Brakemeier, S.; de Vries, P.J.; et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complexor sporadic Lymphangioleiomyomatosis(EXIST-2): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2013, 381, 817–824.37. [Google Scholar] [CrossRef]
- Backwell, F.R.; Bequette, B.J.; Wilson, D.; Metcalf, J.A.; Franklin, M.F.; Beever, D.E.; Lobley, G.E.; MacRae, J.C. Evidence for the utilization of PePtides for milk Protein synthesis in the lactating dairy goat in vivo. Am. J. Physiol. 1996, 271, 955–960. [Google Scholar] [CrossRef]
- Lara, M.A.C.; Gama, L.T.; Bufar, a.G.; Sereno, J.R.B.; Celegato, E.M.L.; de Abreu, U.P. Genetic polymorphisms at the κ-casein locus in Pantaneiro cattle. Arch. Zootec. 2002, 51, 99–105. [Google Scholar]
- Feuermann, Y.; Mabjeesh, S.J.; Shamay, A. Leptin affects prolactin action on milk protein and fat synthesis in the bovine mammary gland. Dairy Sci. 2004, 87, 2941–2946. [Google Scholar] [CrossRef]
- Denver, R.J.; Bonett, R.M.; Boores, G.C. Evolution of leptin structure and function. Neuroendocrinology 2011, 94, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Akers, R.M.; Jiang, H. Growth hormone can induce expression of four major milk protein genes in transfected MAC-T cells. J. Dairy Sci. 2008, 91, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.J.; Haslam, S.Z.; Conrad, S.E. Estrogen and progesterone are critical regulators of Stat5a expression in the mouse mammary gland. Endocrinology 2008, 149, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, A.A. Regulation of Protein Synthesis in the Mammary Gland. Master’s Thesis, Massey University, Palmerston North, New Zealand, 2007. [Google Scholar]
- Akers, R.M. Major advances associated with hormone and growth factor regulation of mammary growth and lactation in dairy cows. J. Dairy Sci. 2006, 89, 1222–1234. [Google Scholar] [CrossRef]


| Gene Name | Forward Primer (5′-3′) | Reverse Primer (3′-5′) | Accession Numbers | Product Length (bp) |
|---|---|---|---|---|
| β-actin | CACGCCATCCTgCGTCTGGA | AGCACCGTGTTGGCGTAGAG | DQ452569.1 | 270 |
| IGFR | CTCCAAGCCTAAGCAAAATGAT | TGCGTGGTGAAGACTCCGTC | NM_001291858.2 | 259 |
| EGFR | GGATAGGGATTGGCGAGTTT | GCATAGCACAGGTTTCGGTTT | NM_214007.1 | 400 |
| STAT3 | TGGGTGGAGAAGGACATCA | TAGACCAGCGGAGACACAAG | HM462247.1 | 149 |
| AKT | CCTGAAGAAGGAGGTCATCG | TCGTGGGTCTGGAAGGAGTA | NM_001159776.1 | 123 |
| β-casein | CTTGATCGCCATGAAGCTC | GAGCAGAGGCAGAGAAAGGAC | EU213063.1 | 472 |
| k-casein | GACGCTGGACTTCCTTCGAGATC | CAGAAAAGACACAGTCCAAGGCG | X51977.1 | 196 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Cui, Y.; Tian, Z.; Yu, M. Effect of Soybean Isoflavones on Proliferation and Related Gene Expression of Sow Mammary Gland Cells In Vitro. Animals 2022, 12, 3241. https://doi.org/10.3390/ani12233241
Ma X, Cui Y, Tian Z, Yu M. Effect of Soybean Isoflavones on Proliferation and Related Gene Expression of Sow Mammary Gland Cells In Vitro. Animals. 2022; 12(23):3241. https://doi.org/10.3390/ani12233241
Chicago/Turabian StyleMa, Xinyan, Yiyan Cui, Zhimei Tian, and Miao Yu. 2022. "Effect of Soybean Isoflavones on Proliferation and Related Gene Expression of Sow Mammary Gland Cells In Vitro" Animals 12, no. 23: 3241. https://doi.org/10.3390/ani12233241




