Artificial Dim Light at Night during Pregnancy Can Affect Hormonal and Metabolic Rhythms in Rat Offspring
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Melatonin Assay
4.4. Corticosterone and Thyroid Hormones Assays
4.5. Vasopressin Assay
4.6. Plasma Glucose, Cholesterol, and Triacylglycerols Measurement
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Dibner, C.; Schibler, U.; Albrecht, U. The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Takahashi, J.S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 2019, 63, R93–R102. [Google Scholar] [CrossRef] [PubMed]
- Grubisic, M.; Haim, A.; Bhusal, P.; Dominoni, D.M.; Gabriel, K.M.A.; Jechow, A.; Kupprat, F.; Lerner, A.; Marchant, P.; Riley, W.; et al. Light pollution, circadian photoreception, and melatonin in vertebrates. Sustainability 2019, 11, 6400. [Google Scholar] [CrossRef]
- Kyba, C.C.M.; Kuester, T.; de Miguel, A.S.; Baugh, K.; Jechow, A.; Hölker, F.; Bennie, J.; Elvidge, C.D.; Gaston, K.J.; Guanter, L. Artificially lit surface of Earth at night increasing in radiance and extent. Sci. Adv. 2017, 3, e1701528. [Google Scholar] [CrossRef]
- Cho, Y.; Ryu, S.-H.; Lee, B.R.; Kim, K.H.; Lee, E.; Choi, J. Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol. Int. 2015, 32, 1294–1310. [Google Scholar] [CrossRef]
- Härmä, M.; Laitinen, J.T.; Partinen, M.; Suvanto, S. The effect of four-day round trip flights over 10 time zones on the circadian variation of salivary melatonin and Cortisol in airline flight attendants. Ergonomics 1994, 37, 1479–1489. [Google Scholar] [CrossRef]
- Lowden, A.; Åkerstedt, T. Eastward long distance flights, sleep and wake patterns in air crews in connection with a two-day layover. J. Sleep Res. 1999, 8, 15–24. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Laden, F.; Speizer, F.E.; Willett, W.C.; Hunter, D.J.; Kawachi, I.; Colditz, G.A. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J. Natl. Cancer Inst. 2001, 93, 1563–1568. [Google Scholar] [CrossRef]
- Papantoniou, K.; Devore, E.E.; Massa, J.; Strohmaier, S.; Vetter, C.; Yang, L.; Shi, Y.; Giovannucci, E.; Speizer, F.; Schernhammer, E.S. Rotating night shift work and colorectal cancer risk in the nurses’ health studies. Int. J. Cancer 2018, 143, 2709–2717. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Okamoto, N.; Tomioka, K.; Nezu, S.; Ikada, Y.; Kurumatani, N. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: A cross-sectional analysis of the HEIJO-KYO study. J. Clin. Endocrinol. Metab. 2013, 98, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Esaki, Y.; Kitajima, T.; Obayashi, K.; Saeki, K.; Fujita, K.; Iwata, N. Light exposure at night and sleep quality in bipolar disorder: The APPLE cohort study. J. Affect. Disord. 2019, 257, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Aubrecht, T.G.; Meléndez-Fernández, O.H.; Weil, Z.M.; Nelson, R.J. Dim light at night disrupts molecular circadian rhythms and increases body weight. J. Biol. Rhythm. 2013, 28, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Rumanova, V.S.; Okuliarova, M.; Foppen, E.; Kalsbeek, A.; Zeman, M. Exposure to dim light at night alters daily rhythms of glucose and lipid metabolism in rats. Front. Physiol. 2022, 13, 973461. [Google Scholar] [CrossRef]
- Stenvers, D.J.; van Dorp, R.; Foppen, E.; Mendoza, J.; Opperhuizen, A.-L.; Fliers, E.; Bisschop, P.H.; Meijer, J.H.; Kalsbeek, A.; Deboer, T. Dim light at night disturbs the daily sleep-wake cycle in the rat. Sci. Rep. 2016, 6, 35662. [Google Scholar] [CrossRef]
- Cissé, Y.M.; Russart, K.; Nelson, R.J. Exposure to dim light at night prior to conception attenuates offspring innate immune responses. PLoS ONE 2020, 15, e0231140. [Google Scholar] [CrossRef]
- Okuliarova, M.; Mazgutova, N.; Majzunova, M.; Rumanova, V.S.; Zeman, M. Dim Light at Night Impairs Daily Variation of Circulating Immune Cells and Renal Immune Homeostasis. Front. Immunol. 2021, 11, 614960. [Google Scholar] [CrossRef]
- Nehme, P.; Amaral, F.; Lowden, A.; Skene, D.; Cipolla-Neto, J.; Moreno, C. Reduced melatonin synthesis in pregnant night workers: Metabolic implications for offspring. Med. Hypotheses 2019, 132, 109353. [Google Scholar] [CrossRef]
- Bonzini, M.; Palmer, K.T.; Coggon, D.; Carugno, M.; Cromi, A.; Ferrario, M.M. Shift work and pregnancy outcomes: A systematic review with meta-analysis of currently available epidemiological studies. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 1429–1437. [Google Scholar] [CrossRef]
- Nováková, M.; Sládek, M.; Sumová, A. Exposure of Pregnant Rats to Restricted Feeding Schedule Synchronizes the SCN Clocks of Their Fetuses under Constant Light but Not under a Light-Dark Regime. J. Biol. Rhythm. 2010, 25, 350–360. [Google Scholar] [CrossRef]
- Seron-Ferre, M.; Valenzuela, G.J.; Torres-Farfan, C. Circadian clocks during embryonic and fetal development. Birth Defects Res. Part C Embryo Today Rev. 2007, 81, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Sutovska, H.; Babarikova, K.; Zeman, M.; Molcan, L. Prenatal Hypoxia Affects Foetal Cardiovascular Regulatory Mechanisms in a Sex- and Circadian-Dependent Manner: A Review. Int. J. Mol. Sci. 2022, 23, 2885. [Google Scholar] [CrossRef] [PubMed]
- Spichiger, C.; Torres-Farfan, C.; Galdames, H.A.; Mendez, N.; Vazquez, P.A.; Richter, H.G. Gestation under chronic constant light leads to extensive gene expression changes in the fetal rat liver. Physiol. Genom. 2015, 47, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Mendez, N.; Abarzua-Catalan, L.; Vilches, N.; Galdames, H.A.; Spichiger, C.; Richter, H.; Valenzuela, G.J.; Seron-Ferre, M.; Torres-Farfán, C. Timed Maternal Melatonin Treatment Reverses Circadian Disruption of the Fetal Adrenal Clock Imposed by Exposure to Constant Light. PLoS ONE 2012, 7, e42713. [Google Scholar] [CrossRef]
- Sládek, M.; Sumová, A.; Kováčiková, Z.; Bendová, Z.; Laurinová, K.; Illnerová, H. Insight into molecular core clock mechanism of embryonic and early postnatal rat suprachiasmatic nucleus. Proc. Natl. Acad. Sci. USA 2004, 101, 6231–6236. [Google Scholar] [CrossRef]
- Weinert, D. Ontogenetic Development of the Mammalian Circadian System. Chronobiol. Int. 2005, 22, 179–205. [Google Scholar] [CrossRef]
- Jenni, O.G.; LeBourgeois, M.K. Understanding sleep–wake behavior and sleep disorders in children: The value of a model. Curr. Opin. Psychiatry 2006, 19, 282–287. [Google Scholar] [CrossRef]
- McGraw, K.; Hoffmann, R.; Harker, C.; Herman, J.H. The Development of Circadian Rhythms in a Human Infant. Sleep 1999, 22, 303–310. [Google Scholar] [CrossRef]
- Sumova, A.; Sladek, M.; Polidarova, L.; Novakova, M.; Houdek, P. Circadian system from conception till adulthood. Prog. Brain Res. 2012, 199, 83–103. [Google Scholar] [CrossRef]
- Roa, S.L.R.; Martinez, E.Z.; Martins, C.S.; Antonini, S.; de Castro, M.; Moreira, A.C. Postnatal Ontogeny of the Circadian Expression of the Adrenal Clock Genes and Corticosterone Rhythm in Male Rats. Endocrinology 2017, 158, 1339–1346. [Google Scholar] [CrossRef]
- Ohta, H.; Mitchell, A.C.; McMahon, D.G. Constant Light Disrupts the Developing Mouse Biological Clock. Pediatr. Res. 2006, 60, 304–308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sumová, A.; Čečmanová, V. Mystery of rhythmic signal emergence within the suprachiasmatic nuclei. Eur. J. Neurosci. 2020, 51, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Houdek, P.; Polidarová, L.; Nováková, M.; Matějů, K.; Kubík, Š.; Sumová, A. Melatonin administered during the fetal stage affects circadian clock in the suprachiasmatic nucleus but not in the liver. Dev. Neurobiol. 2015, 75, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Jáč, M.; Kiss, A.; Sumová, A.; Illnerová, H.; Ježová, D. Daily profiles of arginine vasopressin mRNA in the suprachiasmatic, supraoptic and paraventricular nuclei of the rat hypothalamus under various photoperiods. Brain Res. 2000, 887, 472–476. [Google Scholar] [CrossRef]
- Okuliarova, M.; Dzirbikova, Z.; Rumanova, V.S.; Foppen, E.; Kalsbeek, A.; Zeman, M. Disrupted Circadian Control of Hormonal Rhythms and Anticipatory Thirst by Dim Light at Night. Neuroendocrinology 2022, 112, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.A.; Kennaway, D. Melatonin in rat milk and the likelihood of its role in postnatal maternal entrainment of rhythms. Am. J. Physiol. Integr. Comp. Physiol. 2002, 282, R797–R804. [Google Scholar] [CrossRef][Green Version]
- Molcan, L.; Sutovska, H.; Okuliarova, M.; Senko, T.; Krskova, L.; Zeman, M. Dim light at night attenuates circadian rhythms in the cardiovascular system and suppresses melatonin in rats. Life Sci. 2019, 231, 116568. [Google Scholar] [CrossRef]
- Delorme, T.C.; Srikanta, S.B.; Fisk, A.S.; Cloutier, M.; Sato, M.; Pothecary, C.A.; Merz, C.; Foster, R.G.; Brown, S.A.; Peirson, S.N.; et al. Chronic Exposure to Dim Light at Night or Irregular Lighting Conditions Impact Circadian Behavior, Motor Coordination, and Neuronal Morphology. Front. Neurosci. 2022, 16, 855154. [Google Scholar] [CrossRef]
- Illnerová, H.; Buresová, M.; Presl, J. Melatonin rhythm in human milk. J. Clin. Endocrinol. Metab. 1993, 77, 838–841. [Google Scholar] [CrossRef][Green Version]
- Mendez, N.; Halabi, D.; Spichiger, C.; Salazar, E.R.; Vergara, K.; Alonso-Vasquez, P.; Carmona, P.; Sarmiento, J.M.; Richter, H.G.; Seron-Ferre, M.; et al. Gestational Chronodisruption Impairs Circadian Physiology in Rat Male Offspring, Increasing the Risk of Chronic Disease. Endocrinology 2016, 157, 4654–4668. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tamura, H.; Kashida, S.; Takayama, H.; Yamagata, Y.; Karube, A.; Sugino, N.; Kato, H. Changes of serum melatonin level and its relationship to feto-placental unit during pregnancy. J. Pineal Res. 2001, 30, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Nakamura, Y.; Terron, M.P.; Flores-Alvarado, L.J.; Manchester, L.C.; Tan, D.-X.; Sugino, N.; Reiter, R.J. Melatonin and pregnancy in the human. Reprod. Toxicol. 2008, 25, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.C.; Mannion, J. Entrainment of hamster pup circadian rhythms by prenatal melatonin injections to the mother. Am. J. Physiol. Integr. Comp. Physiol. 1988, 255, R439–R448. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, S.; Zygouropoulos, N.; Zahiu, C.; Zagrean, A. Role of melatonin in embryo fetal development. J. Med. Life 2014, 7, 488–492. [Google Scholar] [PubMed]
- Bates, K.; Herzog, E.D. Maternal-Fetal Circadian Communication During Pregnancy. Front. Endocrinol. 2020, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Yoshikawa, T.; Biscoe, E.W.; Numano, R.; Gallaspy, L.M.; Soulsby, S.; Papadimas, E.; Pezuk, P.; Doyle, S.E.; Tei, H.; et al. Ontogeny of Circadian Organization in the Rat. J. Biol. Rhythm. 2009, 24, 55–63. [Google Scholar] [CrossRef]
- Ošťádalová, I.; Babický, A. Periodization of the Early Postnatal Development in the Rat With Particular Attention to the Weaning Period. Physiol. Res. 2012, 61, S1–S7. [Google Scholar] [CrossRef]
- Gizowski, C.; Zaelzer, C.; Bourque, C.W. Clock-driven vasopressin neurotransmission mediates anticipatory thirst prior to sleep. Nature 2016, 537, 685–688. [Google Scholar] [CrossRef]
- Bankir, L.; Bichet, D.G.; Morgenthaler, N.G. Vasopressin: Physiology, assessment and osmosensation. J. Intern. Med. 2017, 282, 284–297. [Google Scholar] [CrossRef]
- Daniel, S.S.; Stark, R.I.; Husain, M.K.; Sanocka, U.M.; James, L.S. Excretion of Vasopressin in the Hypoxic Lamb: Comparison between Fetus and Newborn. Pediatr. Res. 1984, 18, 227–231. [Google Scholar] [CrossRef]
- Yoshimura, M.; Conway-Campbell, B.; Ueta, Y. Arginine vasopressin: Direct and indirect action on metabolism. Peptides 2021, 142, 170555. [Google Scholar] [CrossRef] [PubMed]
- LaFranchi, S.H. Thyroid Function in Preterm/Low Birth Weight Infants: Impact on Diagnosis and Management of Thyroid Dysfunction. Front. Endocrinol. 2021, 12, 666207. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.; El-Gareib, A.; El-Bakry, A.; El-Tawab, S.A.; Ahmed, R. Thyroid hormones states and brain development interactions. Int. J. Dev. Neurosci. 2008, 26, 147–209. [Google Scholar] [CrossRef] [PubMed]
- De Escobar, G.M.; Obregón, M.J.; del Rey, F.E. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Prac. Res. Clin. Endocrinol. Metab. 2004, 18, 225–248. [Google Scholar] [CrossRef]
- Fisher, D.A.; Klein, A.H. Thyroid Development and Disorders of Thyroid Function in the Newborn. N. Engl. J. Med. 1981, 304, 702–712. [Google Scholar] [CrossRef]
- Howdeshell, K.L. A model of the development of the brain as a construct of the thyroid system. Environ. Health Perspect. 2002, 110, 337–348. [Google Scholar] [CrossRef]
- Russell, W.; Harrison, R.; Smith, N.; Darzy, K.; Shalet, S.; Weetman, A.P.; Ross, R.J. Free Triiodothyronine Has a Distinct Circadian Rhythm That Is Delayed but Parallels Thyrotropin Levels. J. Clin. Endocrinol. Metab. 2008, 93, 2300–2306. [Google Scholar] [CrossRef]
- Philippe, J.; Dibner, C. Thyroid Circadian Timing. J. Biol. Rhythm. 2015, 30, 76–83. [Google Scholar] [CrossRef]
- Campos-Barros, A.; Musa, A.; Flechner, A.; Hessenius, C.; Gaio, U.; Meinhold, H.; Baumgartner, A. Evidence for Circadian Variations of Thyroid Hormone Concentrations and Type II 5′-Iodothyronine Deiodinase Activity in the Rat Central Nervous System. J. Neurochem. 2002, 68, 795–803. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Fliers, E.; Franke, A.N.; Wortel, J.; Buijs, R.M. Functional Connections between the Suprachiasmatic Nucleus and the Thyroid Gland as Revealed by Lesioning and Viral Tracing Techniques in the Rat. Endocrinology 2000, 141, 3832–3841. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Buijs, R.M.; Van Schaik, R.; Kaptein, E.; Visser, T.J.; Doulabi, B.Z.; Fliers, E. Daily Variations in Type II Iodothyronine Deiodinase Activity in the Rat Brain as Controlled by the Biological Clock. Endocrinology 2005, 146, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.M. Physiological and Molecular Basis of Thyroid Hormone Action. Physiol. Rev. 2001, 81, 1097–1142. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Zeman, M.; Molcan, L.; Herichova, I.; Okuliarova, M. Endocrine and cardiovascular rhythms differentially adapt to chronic phase-delay shifts in rats. Chronobiol. Int. 2016, 33, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Molcan, L. Time Distributed Data Analysis by Cosinor. Online Application. Available online: https://cosinor.online/app/cosinor.php (accessed on 28 August 2022).
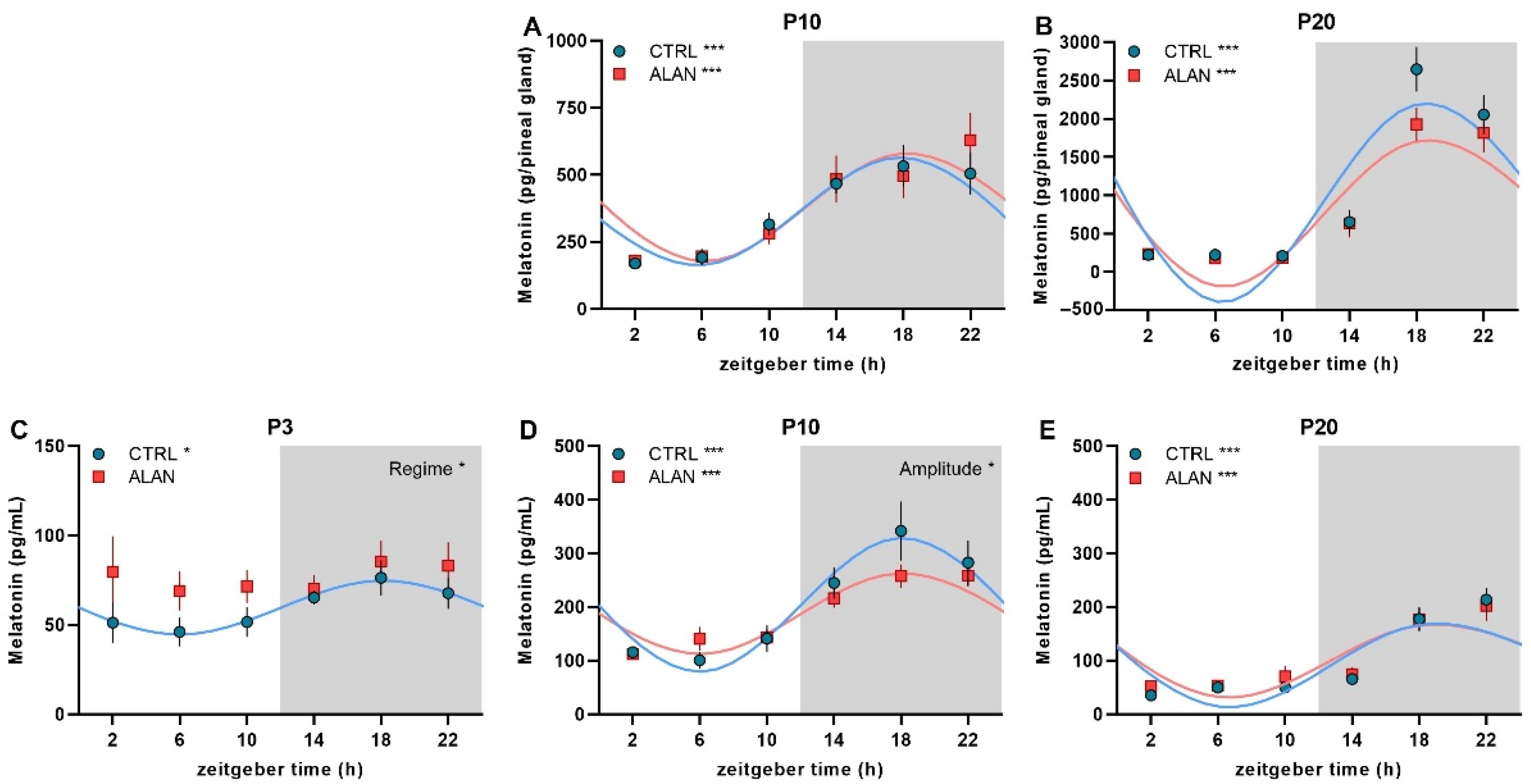
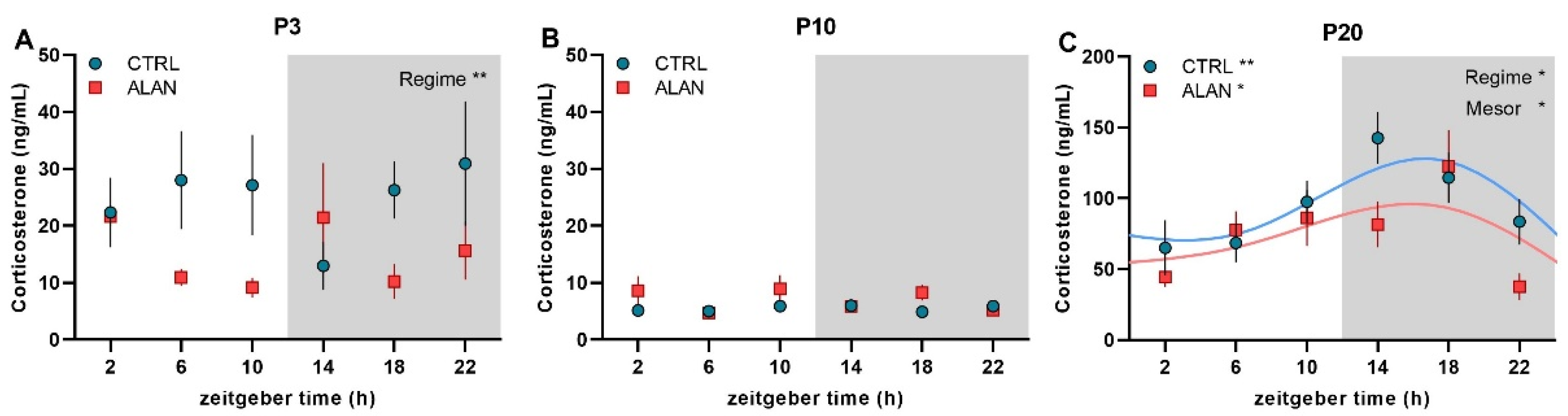
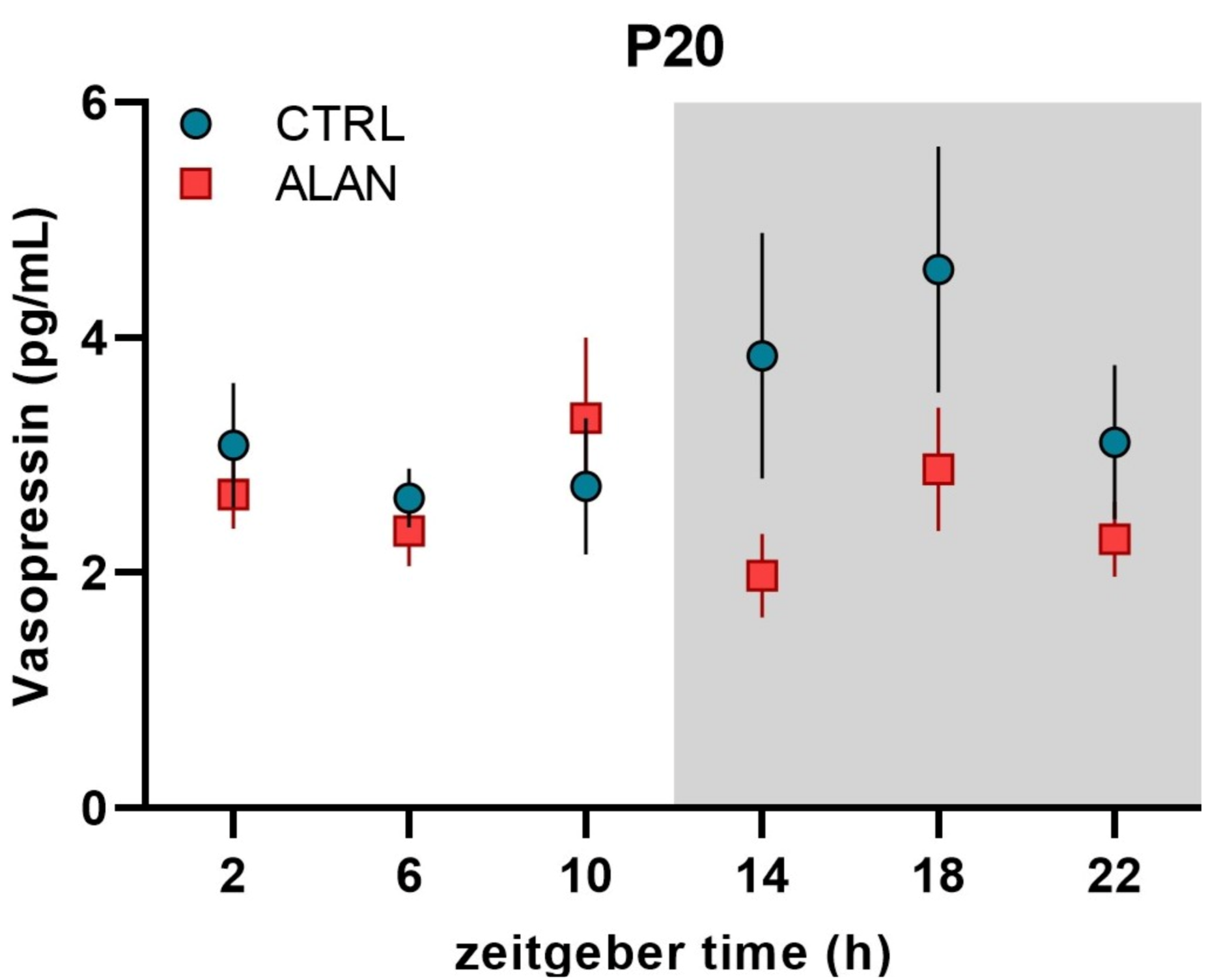
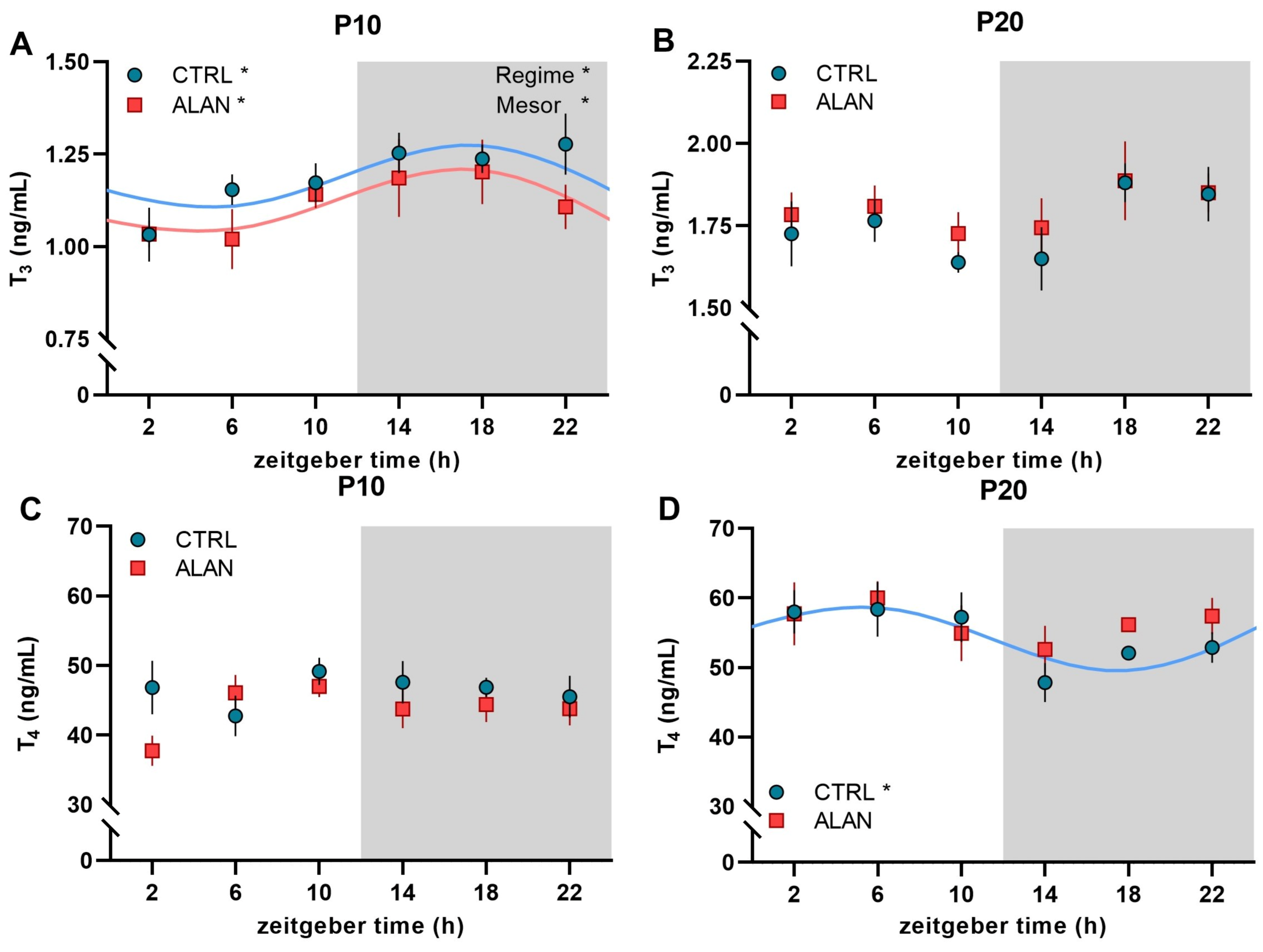

| Mesor | Amplitude | Acrophase hh:mm (ZT) | R2 | F-Test | p-Value | |
|---|---|---|---|---|---|---|
| P10 Pineal MEL (pg/gland) | ||||||
| CTRL | 358.6 ± 48.6 | 203.1 ± 134.9 | 17:30 ± 1:17 | 0.55 | F(2,28) = 17.27 | 0.000 |
| ALAN | 383.5 ± 62.9 | 203.5 ± 116.7 | 18:28 ± 1:42 | 0.41 | F(2,30) = 10.57 | 0.000 |
| P20 Pineal MEL (pg/gland) | ||||||
| CTRL | 972.4 ± 207.8 | 1349.6 ± 1047.2 | 19:05 ± 0:48 | 0.73 | F(2,29) = 38.43 | 0.000 |
| ALAN | 825.9 ± 164.7 | 1010.9 ± 778.3 | 19:19 ± 0:52 | 0.71 | F(2,30) = 36.28 | 0.000 |
| P3 Plasma MEL (pg/mL) | ||||||
| CTRL | 59.7 ± 6.9 | 15.2 ± 5.5 | 18:08 ± 2:27 | 0.23 | F(2,32) = 4.78 | 0.015 |
| ALAN | - | - | - | 0.04 | F(2,32) = 0.69 | 0.507 |
| P10 Plasma MEL (pg/mL) | ||||||
| CTRL | 204.3 ± 26.1 | 125.5 ± 88.6 | 18:06 ± 1:07 | 0.57 | F(2,33) = 22.21 | 0.000 |
| ALAN | 187.9 ± 16.1 | 75.5 ± 52.7 * | 18:09 ± 1:09 | 0.56 | F(2,33) = 21.05 | 0.000 |
| P20 Plasma MEL (pg/mL) | ||||||
| CTRL | 97.8 ± 17.0 | 85.6 ± 61.4 | 19:46 ± 1:03 | 0.60 | F(2,32) = 24.04 | 0.000 |
| ALAN | 104.8 ± 17.2 | 74.1 ± 49.8 | 19:41 ± 1:15 | 0.52 | F(2,33) = 17.78 | 0.000 |
| P3 Plasma CORT (ng/mL) | ||||||
| CTRL | - | - | - | 0.02 | F(2,32) = 0.32 | 0.728 |
| ALAN | - | - | - | 0.03 | F(2,32) = 0.42 | 0.664 |
| P10 Plasma CORT (ng/mL) | ||||||
| CTRL | - | - | - | 0.01 | F(2,33) = 0.23 | 0.792 |
| ALAN | - | - | - | 0.00 | F(2,33) = 0.05 | 0.949 |
| P20 Plasma CORT (ng/mL) | ||||||
| CTRL | 95.3 ± 13.1 | 37.0 ± 18.5 | 14:57 ± 1:54 | 0.32 | F(2,33) = 7.69 | 0.002 |
| ALAN | 74.6 ± 14.5 * | 28.5 ± 8.1 | 13:58 ± 2:46 | 0.19 | F(2,32) = 3.77 | 0.034 |
| P20 Plasma VP (pg/mL) | ||||||
| CTRL | - | - | - | 0.11 | F(2,33) = 2.05 | 0.145 |
| ALAN | - | - | - | 0.01 | F(2,33) = 0.16 | 0.852 |
| Mesor | Amplitude | Acrophase hh:mm (ZT) | R2 | F-Test | p-Value | |
|---|---|---|---|---|---|---|
| P10 Plasma T3 (ng/mL) | ||||||
| CTRL | 1.18 ± 0.05 | 0.14 ± 0.03 | 16:22 ± 2:58 | 0.19 | F(2,30) = 3.43 | 0.045 |
| ALAN | 1.12 ± 0.05 * | 0.15 ± 0.04 | 15:42 ± 2:49 | 0.21 | F(2,27) = 3.55 | 0.045 |
| P20 Plasma T3 (ng/mL) | ||||||
| CTRL | - | - | - | 0.15 | F(2,32) = 2.8 | 0.076 |
| ALAN | - | - | - | 0.06 | F(2,32) = 0.96 | 0.393 |
| P10 Plasma T4 (ng/mL) | ||||||
| CTRL | - | - | - | 0.03 | F(2,32) = 0.47 | 0.627 |
| ALAN | - | - | - | 0.13 | F(2,30) = 2.16 | 0.133 |
| P20 Plasma T4 (ng/mL) | ||||||
| CTRL | 54.4 ± 2.3 | 6.2 ± 2.05 | 4:39 ± 2:29 | 0.21 | F(2,32) = 4.28 | 0.022 |
| ALAN | - | - | - | 0.07 | F(2,32) = 1.22 | 0.310 |
| Mesor | Amplitude | Acrophase hh:mm (ZT) | R2 | F-Test | p-Value | |
|---|---|---|---|---|---|---|
| P3 Plasma GLU (mmol/L) | ||||||
| CTRL | 4.48 ± 0.18 | 0.66 ± 0.41 | 16:02 ± 1:28 | 0.46 | F(2,31) = 13.29 | 0.000 |
| ALAN | - | - | - | 0.13 | F(2,29) = 2.1 | 0.141 |
| P10 Plasma GLU (mmol/L) | ||||||
| CTRL | - | - | - | 0.08 | F(2,32) = 1.48 | 0.242 |
| ALAN | - | - | - | 0.09 | F(2,29) = 1.52 | 0.236 |
| P20 Plasma GLU (mmol/L) | ||||||
| CTRL | - | - | - | 0.03 | F(2,33) = 0.43 | 0.656 |
| ALAN | - | - | - | 0.01 | F(2,33) = 0.21 | 0.814 |
| P3 Plasma CHOL (mmol/L) | ||||||
| CTRL | 0.8 ± 0.09 | 0.25 ± 0.13 | 17:40 ± 1:57 | 0.34 | F(2,29) = 7.54 | 0.002 |
| ALAN | - | - | - | 0.08 | F(2,29) = 1.26 | 0.300 |
| P10 Plasma CHOL (mmol/L) | ||||||
| CTRL | 1.48 ± 0.16 | 0.34 ± 0.11 | 19:47 ± 2:30 | 0.21 | F(2,32) = 4.33 | 0.022 |
| ALAN | 1.60 ± 0.13 | 0.41 ± 0.23 | 19:50 ± 1:45 | 0.40 | F(2,29) = 9.72 | 0.001 |
| P20 Plasma CHOL (mmol/L) | ||||||
| CTRL | - | - | - | 0.08 | F(2,33) = 1.41 | 0.258 |
| ALAN | - | - | - | 0.08 | F(2,33) = 1.4 | 0.261 |
| P3 Plasma TAG (mmol/L) | ||||||
| CTRL | - | - | - | 0.10 | F(2,29) = 1.57 | 0.226 |
| ALAN | - | - | - | 0.04 | F(2,28) = 0.54 | 0.590 |
| P10 Plasma TAG (mmol/L) | ||||||
| CTRL | 0.94 ± 0.1 | 0.29 ± 0.14 | 17:27 ± 1:54 | 0.31 | F(2,32) = 7.33 | 0.002 |
| ALAN | - | - | - | 0.11 | F(2,29) = 1.8 | 0.183 |
| P20 Plasma TAG (mmol/L) | ||||||
| CTRL | - | - | - | 0.14 | F(2,33) = 2.78 | 0.077 |
| ALAN | - | - | - | 0.02 | F(2,33) = 0.35 | 0.710 |
| CTRL | ALAN | p-Value | |
|---|---|---|---|
| Pregnant females (n) | 9 | 11 | |
| Weight of dams at G0 (mean ± SEM) | 220.89 ± 3.83 g | 228.82 ± 4.73 g | 0.223 |
| Weight of dams at G20 (mean ± SEM) | 354.44 ± 7.31 g | 365.64 ± 11.63 g | 0.450 |
| Weight gain between G0 and G20 (mean ± SEM) | 60.63 ± 3.07% | 59.57 ± 2.86% | 0.802 |
| Total number of pups (n) | 108 | 132 | |
| Litter size/dam (mean ± SEM) | 12 ± 1.12 | 12 ± 0.77 | 0.849 |
| Weight of pup at P1 (mean ± SEM) | 6.59 ± 0.27 g | 6.71 ± 0.22 g | 0.331 |
| Number of pups in P3 (Male/Female) (n) | 18/18 | 22/13 | |
| Number of pups in P10 (Male/Female) (n) | 14/22 | 23/13 | |
| Number of pups in P20 (Male/Female) (n) | 20/16 | 17/19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzirbíková, Z.; Stebelová, K.; Kováčová, K.; Okuliarová, M.; Olexová, L.; Zeman, M. Artificial Dim Light at Night during Pregnancy Can Affect Hormonal and Metabolic Rhythms in Rat Offspring. Int. J. Mol. Sci. 2022, 23, 14544. https://doi.org/10.3390/ijms232314544
Dzirbíková Z, Stebelová K, Kováčová K, Okuliarová M, Olexová L, Zeman M. Artificial Dim Light at Night during Pregnancy Can Affect Hormonal and Metabolic Rhythms in Rat Offspring. International Journal of Molecular Sciences. 2022; 23(23):14544. https://doi.org/10.3390/ijms232314544
Chicago/Turabian StyleDzirbíková, Zuzana, Katarína Stebelová, Katarína Kováčová, Monika Okuliarová, Lucia Olexová, and Michal Zeman. 2022. "Artificial Dim Light at Night during Pregnancy Can Affect Hormonal and Metabolic Rhythms in Rat Offspring" International Journal of Molecular Sciences 23, no. 23: 14544. https://doi.org/10.3390/ijms232314544
APA StyleDzirbíková, Z., Stebelová, K., Kováčová, K., Okuliarová, M., Olexová, L., & Zeman, M. (2022). Artificial Dim Light at Night during Pregnancy Can Affect Hormonal and Metabolic Rhythms in Rat Offspring. International Journal of Molecular Sciences, 23(23), 14544. https://doi.org/10.3390/ijms232314544







