Palladium/Graphene Oxide Nanocomposite for Hydrogen Gas Sensing Applications Based on Tapered Optical Fiber
Abstract
1. Introduction
2. Materials and Methods
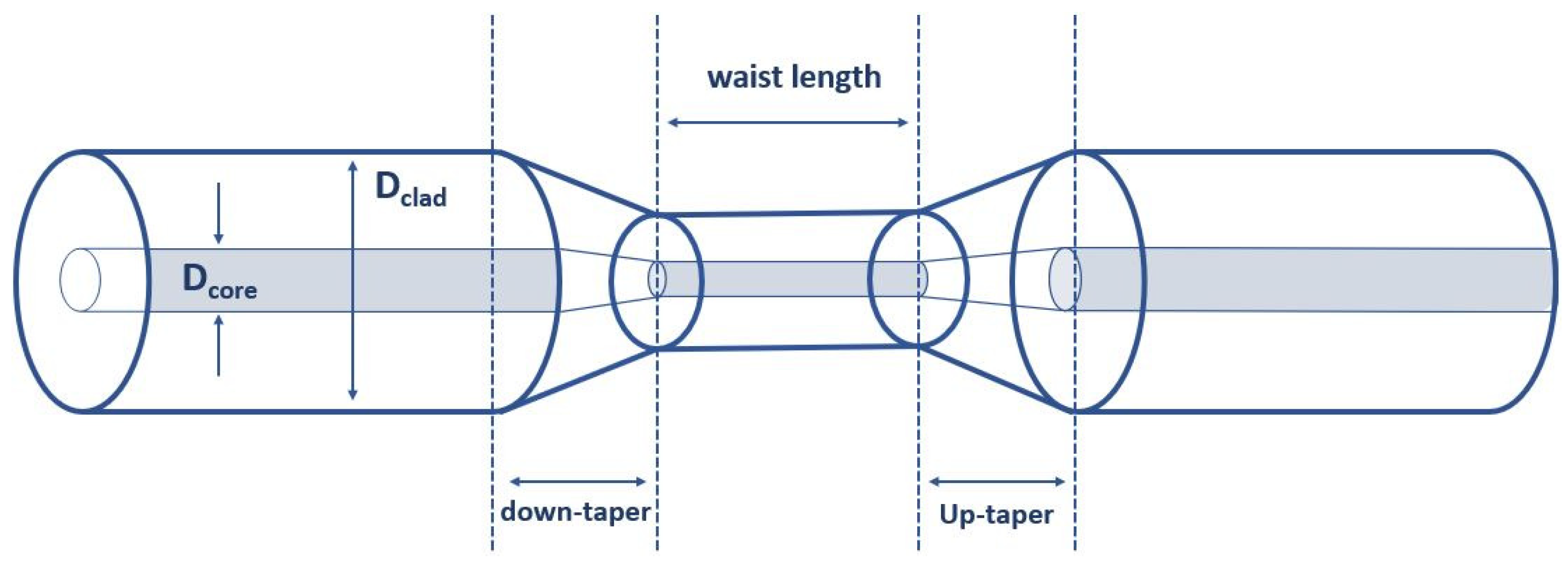
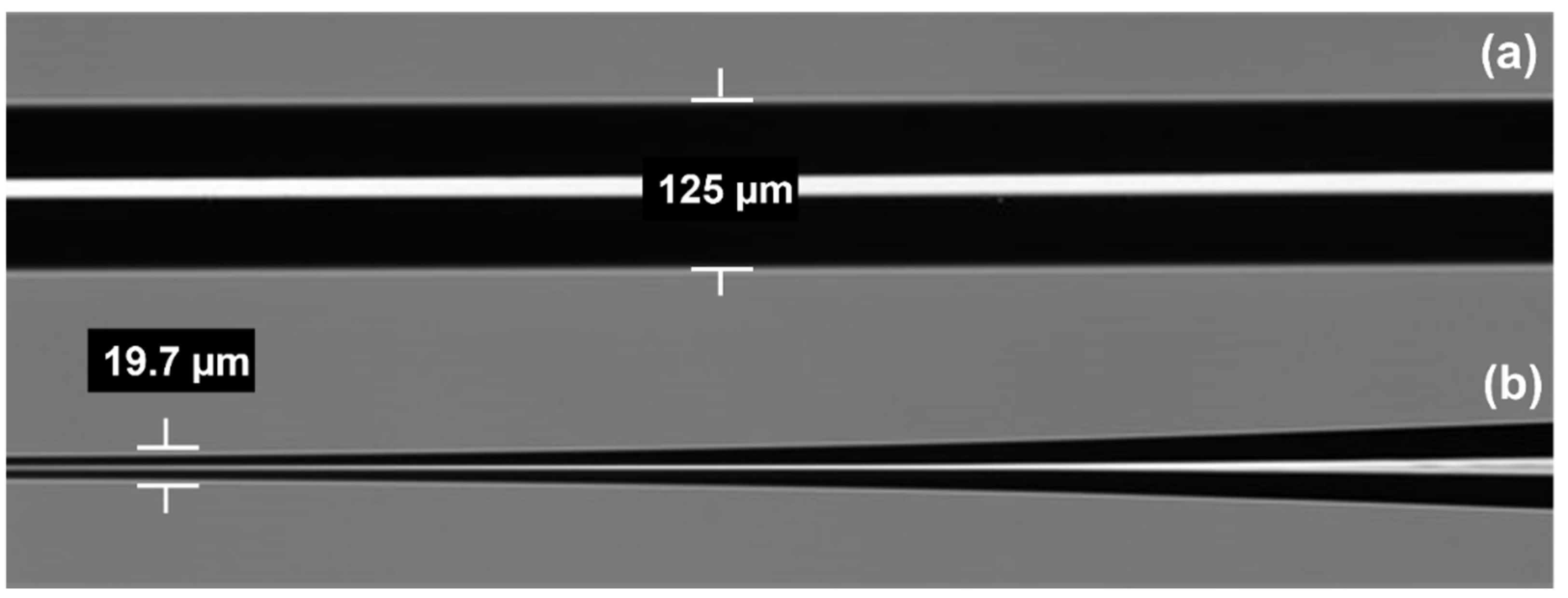
2.1. Design and Fabrication of Optical Fiber Transducers
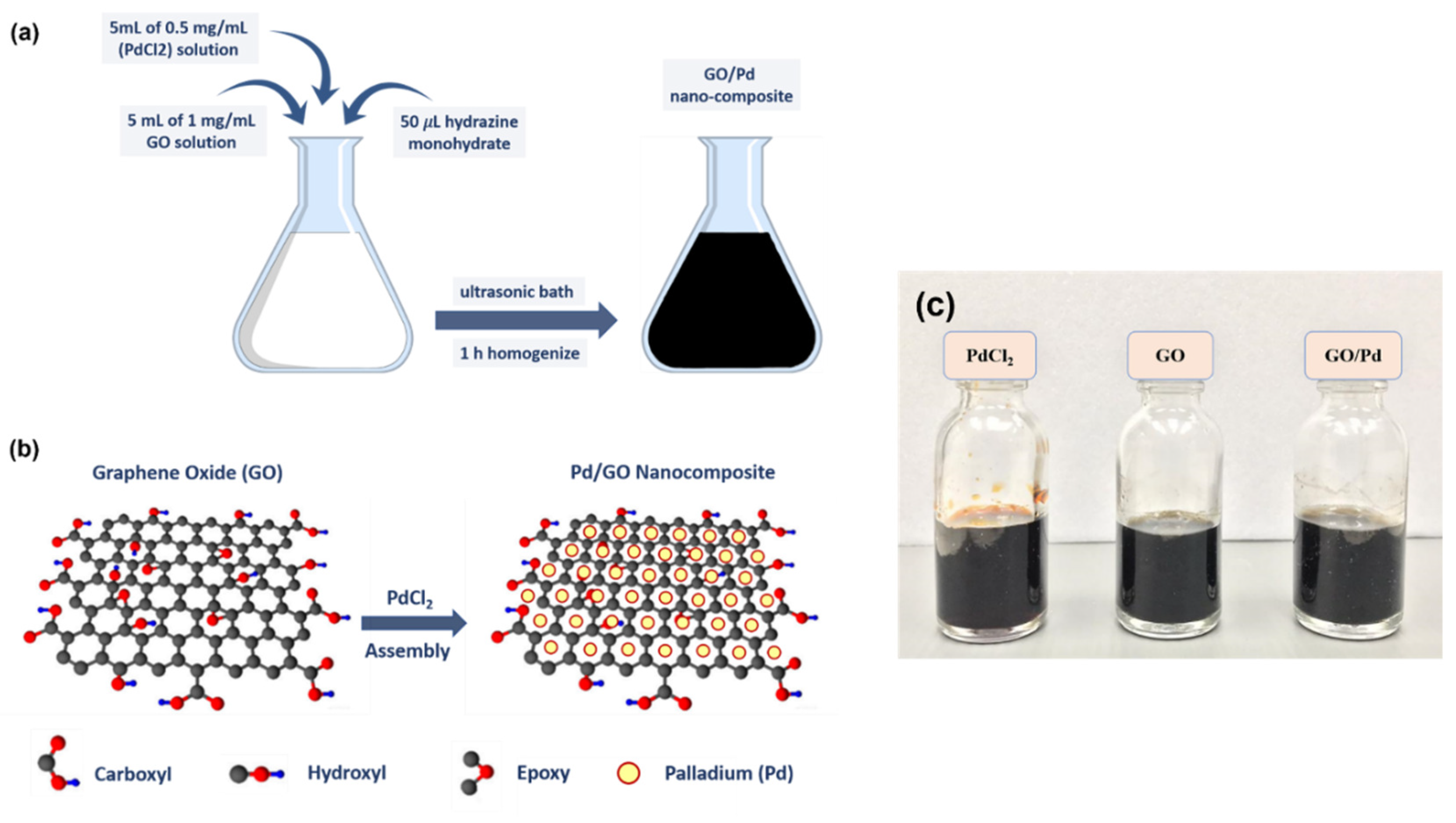
2.2. Synthesis and Deposition of Pd/GO Nanocomposite
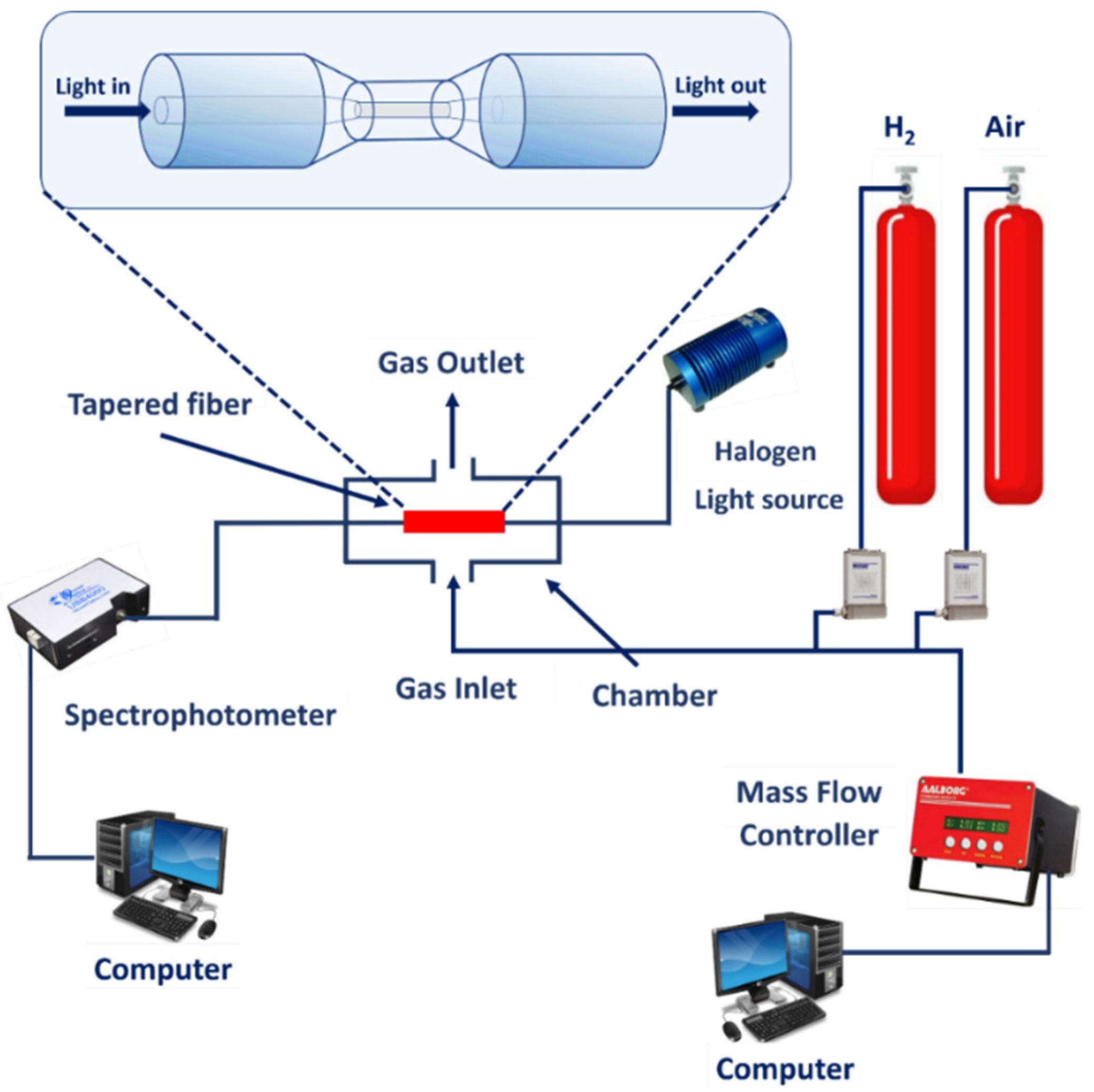
2.3. Optical Gas Testing Setup
3. Characterisation and Chemical Sensing Results
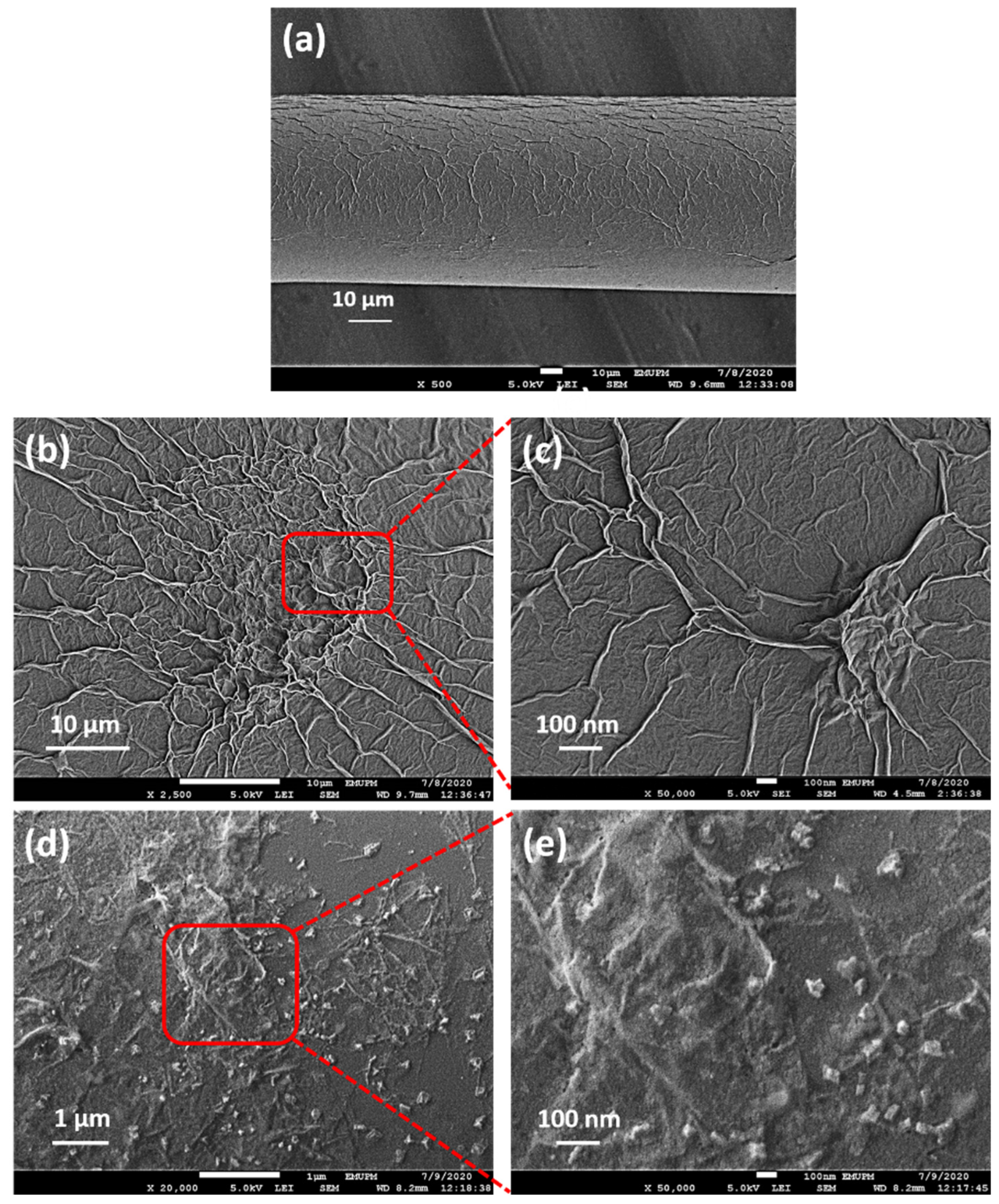
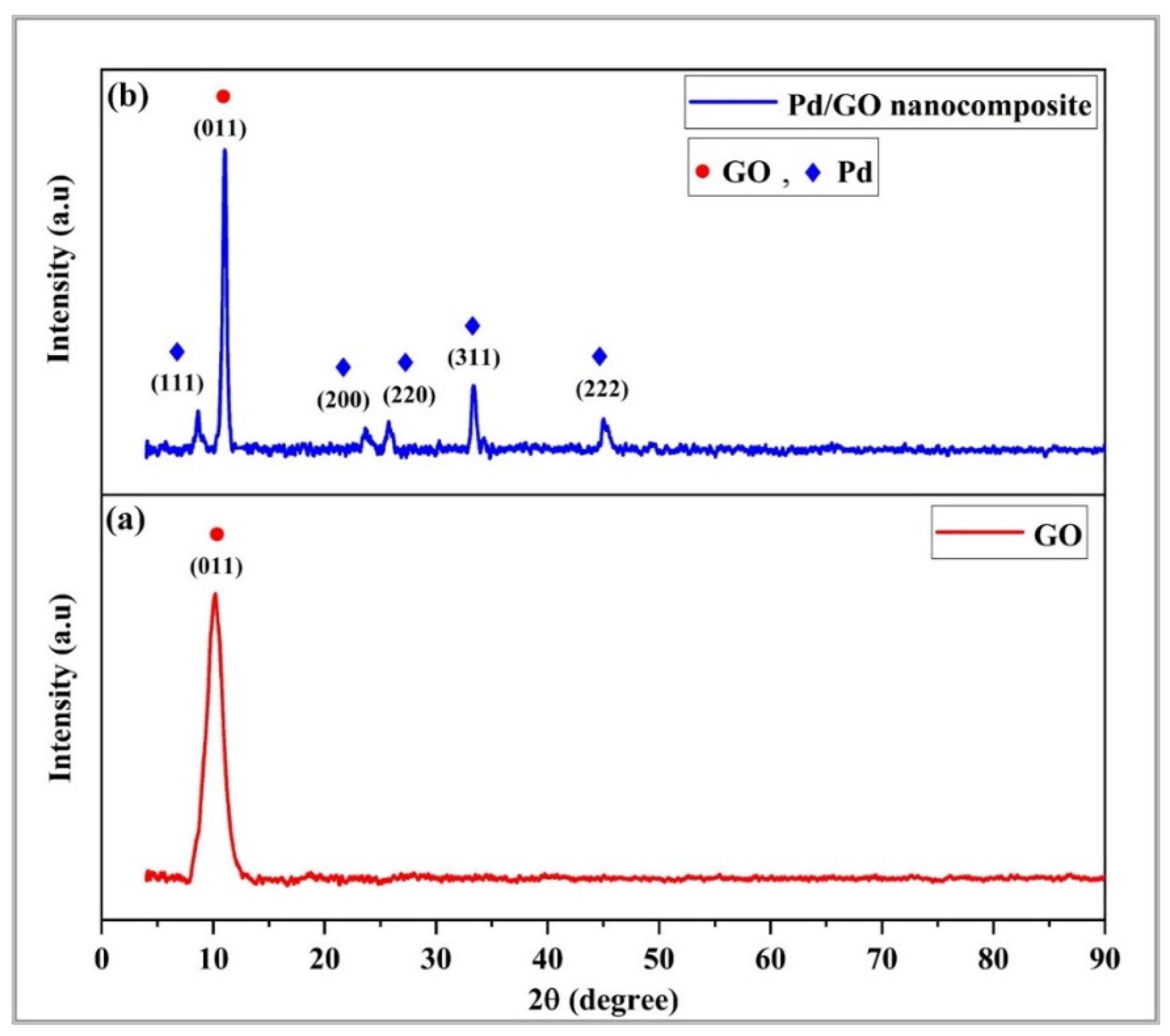
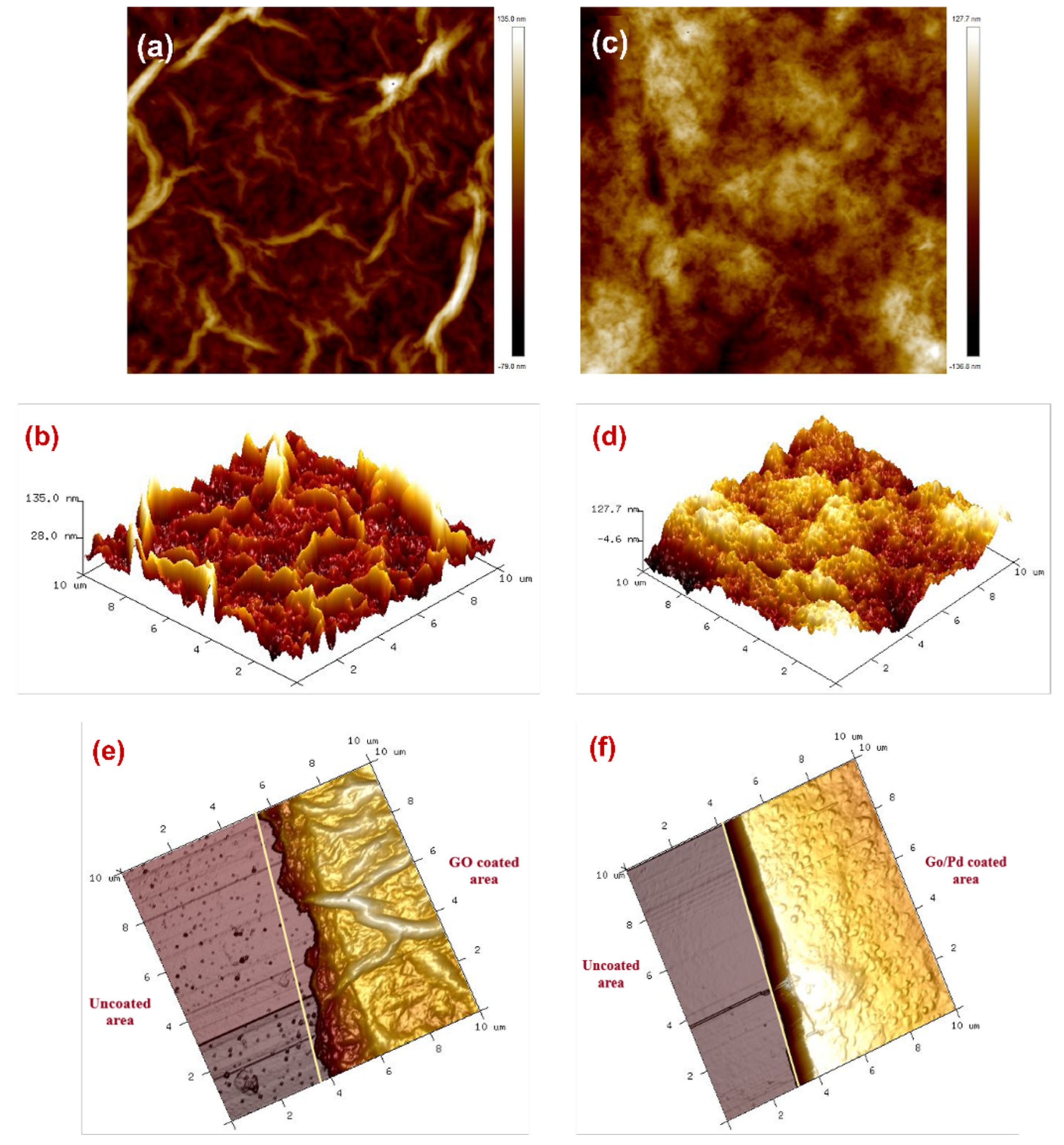
3.1. Micro-Characterizations of Pd/GO Nanocomposite
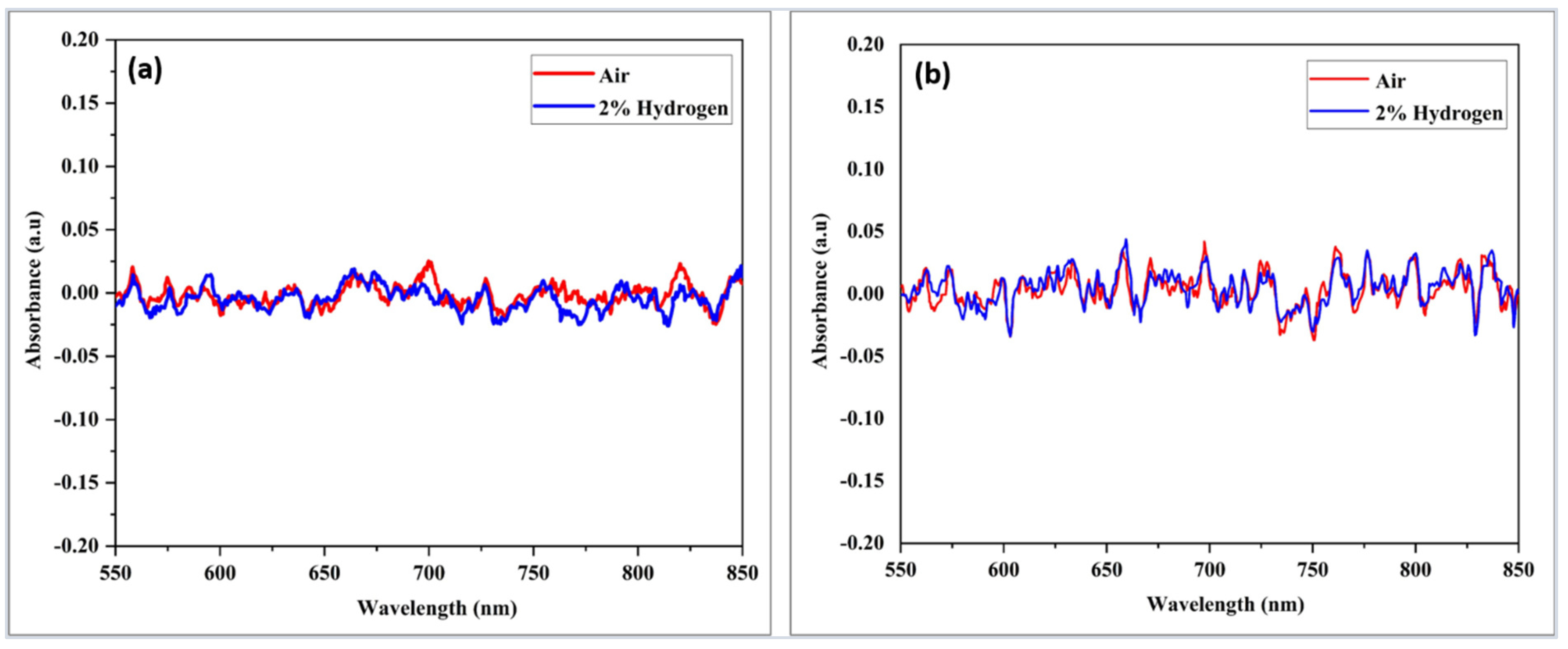
3.2. Hydrogen (H2) Response-Based Pd/GO Nanocomposite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dimitriou, P.; Kumar, M.; Tsujimura, T.; Suzuki, Y. Combustion and Emission Characteristics of a Hydrogen-Diesel Dual-Fuel Engine. Int. J. Hydrogen Energy 2018, 43, 13605–13617. [Google Scholar] [CrossRef]
- Sagar, S.M.V.; Agarwal, A.K. Knocking Behavior and Emission Characteristics of a Port Fuel Injected Hydrogen Enriched Compressed Natural Gas Fueled Spark Ignition Engine. Appl. Therm. Eng. 2018, 141, 42–50. [Google Scholar] [CrossRef]
- Singh, A.P.; Kumar, D.; Agarwal, A.K. Particulate Characteristics of Laser Ignited Hydrogen Enriched Compressed Natural Gas Engine. Int. J. Hydrogen Energy 2020, 45, 18021–18031. [Google Scholar] [CrossRef]
- Althubaiti, M.; Bernard, M.; Musilek, P. Fuzzy Logic Controller for Hybrid Renewable Energy System with Multiple Types of Storage. In Proceedings of the IEEE 30th Canadian Conference on Electrical and Computer Engineering, Windsor, ON, Canada, 30 April–3 May 2017. [Google Scholar] [CrossRef]
- Bychkov, A.L.; Korobeynikov, S.M.; Ryzhkina, A.Y. Determination of the Hydrogen Diffusion Coefficient in Transformer Oil. Tech. Phys. 2011, 56, 421–422. [Google Scholar] [CrossRef]
- Islam, M.M.; Lee, G.; Hettiwatte, S.N. A Review of Condition Monitoring Techniques and Diagnostic Tests for Lifetime Estimation of Power Transformers. Electr. Eng. 2018, 100, 581–605. [Google Scholar] [CrossRef]
- Peixoto, G.; Pantoja-Filho, J.L.R.; Agnelli, J.A.B.; Barboza, M.; Zaiat, M. Hydrogen and Methane Production, Energy Recovery, and Organic Matter Removal from Effluents in a Two-Stage Fermentative Process. Appl. Biochem. Biotechnol. 2012, 168, 651–671. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S. A Review on Production, Storage of Hydrogen and Its Utilization as an Energy Resource. J. Ind. Eng. Chem. 2014, 20, 1148–1156. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen Sensors—A Review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Bevenot, X.; Trouillet, A.; Veillas, C.; Gagnaire, H.; Clement, M. Hydrogen Leak Detection Using an Optical Fibre Sensor for Aerospace Applications. Sens. Actuators B Chem. 2000, 67, 57–67. [Google Scholar] [CrossRef]
- Caumon, P.; Zulueta, M.L.-B.; Louyrette, J.; Albou, S.; Bourasseau, C.; Mansilla, C. Flexible Hydrogen Production Implementation in the French Power System: Expected Impacts at the French and European Levels. Energy 2015, 81, 556–562. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The Role of Hydrogen and Fuel Cells in the Global Energy System. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water Electrolysis Based on Renewable Energy for Hydrogen Production. Cuihua Xuebao/Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Vinoth Kanna, I.; Paturu, P. A Study of Hydrogen as an Alternative Fuel. Int. J. Ambient. Energy 2020, 41, 1433–1436. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Reaction Systems for Solar Hydrogen Production via Water Splitting with Particulate Semiconductor Photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen Production from Renewable and Sustainable Energy Resources: Promising Green Energy Carrier for Clean Development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Bunker, C.E.; Smith, M.J. Nanoparticles for Hydrogen Generation. J. Mater. Chem. 2011, 21, 12173–12180. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Palmisano, V.; Bader, M.A. Developments in Gas Sensor Technology for Hydrogen Safety. Int. J. Hydrogen Energy 2014, 39, 20474–20483. [Google Scholar] [CrossRef]
- Sazali, N. Emerging Technologies by Hydrogen: A Review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, H.; Qian, X.; Zhang, Y.; An, G.; Zhao, Y. Recent Advancements in Optical Fiber Hydrogen Sensors. Sens. Actuators B Chem. 2017, 244, 393–416. [Google Scholar] [CrossRef]
- Gu, H.; Wang, Z.; Hu, Y. Hydrogen Gas Sensors Based on Semiconductor Oxide Nanostructures. Sensors 2012, 12, 5517–5550. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Dai, J. Fiber Optic Hydrogen Sensors: A Review. Photonic Sens. 2014, 4, 300–324. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, L.; Wang, G.; Xiang, F.; Qin, Y.; Wang, M.; Yang, M. Optical Fiber Grating Hydrogen Sensors: A Review. Sensors 2017, 17, 577. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Rodrigues, P.; Hierro-Rodriguez, A.; Guerreiro, A.; Jorge, P.; Santos, J.L.; Araújo, J.P.; Teixeira, J.M. Hydrogen Optical Metamaterial Sensor Based on Pd Dendritic Nanostructures. ChemistrySelect 2016, 1, 3854–3860. [Google Scholar] [CrossRef]
- González-Sierra, N.E.; Gómez-Pavón, L.d.C.; Pérez-Sánchez, G.F.; Luis-Ramos, A.; Zaca-Morán, P.; Muñoz-Pacheco, J.M.; Chávez-Ramírez, F. Tapered Optical Fiber Functionalized with Palladium Nanoparticles by Drop Casting and Laser Radiation for H2 and Volatile Organic Compounds Sensing Purposes. Sensors 2017, 17, 2039. [Google Scholar] [CrossRef] [PubMed]
- Bavili, N.; Balkan, T.; Morova, B.; Eryürek, M.; Uysallı, Y.; Kaya, S.; Kiraz, A. Highly Sensitive Optical Sensor for Hydrogen Gas Based on a Polymer Microcylinder Ring Resonaator. Sens. Actuators B Chem. 2020, 310, 127806. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Q.L.; Zhang, Y.N. High-Sensitive Hydrogen Sensor Based on Photonic Crystal Fiber Model Interferometer. IEEE Trans. Instrum. Meas. 2017, 66, 2198–2203. [Google Scholar] [CrossRef]
- Alkhabet, M.M.; Girei, S.H.; Paiman, S.; Arsad, N.; Mahdi, M.A.; Yaacob, M.H. Highly Sensitive Hydrogen Sensor Based on Palladium-Coated Tapered Optical Fiber at Room Temperature. Eng. Proc. 2020, 2, 8. [Google Scholar]
- Villatoro, J.; Luna-Moreno, D.; Monzón-Hernández, D. Optical Fiber Hydrogen Sensor for Concentrations below the Lower Explosive Limit. Sens. Actuators B Chem. 2005, 110, 23–27. [Google Scholar] [CrossRef]
- Toda, K.; Furue, R.; Hayami, S. Recent Progress in Applications of Graphene Oxide for Gas Sensing: A Review. Anal. Chim. Acta 2015, 878, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ping, D.; Dong, X. Recent Developments of Graphene Oxide-Based Membranes: A Review. Membranes 2017, 7, 52. [Google Scholar] [CrossRef]
- Wang, J.; Singh, B.; Park, J.H.; Rathi, S.; Lee, I.Y.; Maeng, S.; Joh, H.I.; Lee, C.H.; Kim, G.H. Dielectrophoresis of Graphene Oxide Nanostructures for Hydrogen Gas Sensor at Room Temperature. Sens. Actuators B Chem. 2014, 194, 296–302. [Google Scholar] [CrossRef]
- Rasch, F.; Postica, V.; Schütt, F.; Mishra, Y.K.; Nia, A.S.; Lohe, M.R.; Feng, X.; Adelung, R.; Lupan, O. Highly Selective and Ultra-Low Power Consumption Metal Oxide Based Hydrogen Gas Sensor Employing Graphene Oxide as Molecular Sieve. Sens. Actuators B Chem. 2020, 320, 128363. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.X.; Yan, Q.Q.; Xu, S.L.; Chu, S.Q.; Chen, P.; Lin, Y.; Liang, H.W. A Sulfur-Tethering Synthesis Strategy toward High-Loading Atomically Dispersed Noble Metal Catalysts. Sci. Adv. 2019, 5, eaax6322. [Google Scholar] [CrossRef]
- Fu, L.; Yu, A. Electroanalysis of Dopamine Using Reduced Graphene Oxide-Palladium Nanocomposites. Nanosci. Nanotechnol. Lett. 2015, 7, 147–151. [Google Scholar] [CrossRef]
- Chunxia, Y.; Hui, D.; Wei, D.; Chaowei, X. Weakly-Coupled Multicore Optical Fiber Taper-Based High-Temperature Sensor. Sens. Actuators A Phys. 2018, 280, 139–144. [Google Scholar] [CrossRef]
- Korposh, S.; James, S.W.; Lee, S.W.; Tatam, R.P. Tapered Optical Fibre Sensors: Current Trends and Future Perspectives. Sensors 2019, 19, 2294. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Zhang, M.; Farrell, G.; Wang, R.; Lewis, E.; Wang, P. Highly Sensitive Strain Sensor Based on Composite Interference Established within S-Tapered Multimode Fiber Structure. Opt. Express 2018, 26, 33982. [Google Scholar] [CrossRef] [PubMed]
- Omidvar, A.; Jaleh, B.; Nasrollahzadeh, M. Preparation of the GO/Pd Nanocomposite and Its Application for the Degradation of Organic Dyes in Water. J. Colloid Interface Sci. 2017, 496, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.-T.T.; Chung, G.-S.S. Characteristics of Resistivity-Type Hydrogen Sensing Based on Palladium-Graphene Nanocomposites. Int. J. Hydrogen Energy 2014, 39, 620–629. [Google Scholar] [CrossRef]
- Coelho, L.; de Almeida, J.M.M.M.; Santos, J.L.; Viegas, D. Fiber Optic Hydrogen Sensor Based on an Etched Bragg Grating Coated with Palladium. Appl. Opt. 2015, 54, 10342. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, J.; Larsen, B.; Stark, C.; Breitkreutz, A.; Lin, Z.Y.; Breitkreutz, B.J.; Ding, Y.; Colwill, K.; Pasculescu, A.; et al. ProHits: Integrated Software for Mass Spectrometry-Based Interaction Proteomics; Nature Publishing Group: Rockville Pike Bethesda, MD, USA, 2010; Volume 28. [Google Scholar]
- Shamsi, S.; Alagan, A.A.; Sarchio, S.N.E.; Md Yasin, F. Synthesis, Characterization, and Toxicity Assessment of Pluronic F127-Functionalized Graphene Oxide on the Embryonic Development of Zebrafish (Danio Rerio). Int. J. Nanomed. 2020, 15, 8311–8329. [Google Scholar] [CrossRef]
- Chamoli, P.; Das, M.K.; Kar, K.K. Structural, Optical and Electronic Characteristics of N-Doped Graphene Nanosheets Synthesized Using Urea as Reducing Agent and Nitrogen Precursor. Mater. Res. Express 2017, 4, 015012. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Y.; Zhang, Y.; Niu, Z.; Li, X. Amino-Functionalized Graphene Oxide for Cr (VI), Cu (II), Pb (II) and Cd (II) Removal from Industrial Wastewater. Open Chem. 2020, 18, 97–107. [Google Scholar] [CrossRef]
- Sun, K.G.; Hur, S.H. Highly Sensitive Non-Enzymatic Glucose Sensor Based on Pt Nanoparticle Decorated Graphene Oxide Hydrogel. Sens. Actuators B Chem. 2015, 210, 618–623. [Google Scholar]
- Aziz, A.; Lim, H.N.; Girei, S.H.; Yaacob, M.H.; Mahdi, M.A.; Huang, N.M.; Pandikumar, A. Silver/Graphene Nanocomposite-Modified Optical Fiber Sensor Platform for Ethanol Detection in Water Medium. Sens. Actuators B Chem. 2015, 206, 119–125. [Google Scholar]
- Mohammadi, M.M.; Kumar, A.; Liu, J.; Liu, Y.; Thundat, T.; Swihart, M.T. Hydrogen Sensing at Room Temperature Using Flame-Synthesized Palladium-Decorated Crumpled Reduced Graphene Oxide Nanocomposites. ACS Sens. 2020, 5, 2344–2350. [Google Scholar] [CrossRef]
- Tong, X.; Wang, H.; Wang, G.; Wan, L.; Ren, Z.; Bai, J.; Bai, J. Controllable Synthesis of Graphene Sheets with Different Numbers of Layers and Effect of the Number of Graphene Layers on the Specific Capacity of Anode Material in Lithium-Ion Batteries. J. Solid State Chem. 2011, 184, 982–989. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis Route of Reduced Graphene Oxide via Thermal Reduction of Chemically Exfoliated Graphene Oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Escalona-Villalpando, R.A.; Gurrola, M.P.; Trejo, G.; Guerra-Balcázar, M.; Ledesma-García, J.; Arriaga, L.G. Electrodeposition of Gold on Oxidized and Reduced Graphite Surfaces and Its Influence on Glucose Oxidation. J. Electroanal. Chem. 2018, 816, 92–98. [Google Scholar] [CrossRef]
- Rout, D.R.; Senapati, P.; Sutar, H.; Sau, D.C.; Murmu, R. Graphene Oxide (GO) Supported Palladium (Pd) Nanocomposites for Enhanced Hydrogenation. Graphene 2019, 8, 33–51. [Google Scholar] [CrossRef]
- Gao, J.; Bao, F.; Zhu, Q.; Tan, Z.; Chen, T.; Cai, H.; Zhao, C.; Cheng, Q.; Yang, Y.; Ma, R. Attaching Hexylbenzene and Poly (9, 9-Dihexylfluorene) to Brominated Graphene via Suzuki Coupling Reaction. Polym. Chem. 2013, 4, 1672–1679. [Google Scholar] [CrossRef]
- Corso, A.J.; Tessarolo, E.; Guidolin, M.; Della Gaspera, E.; Martucci, A.; Angiola, M.; Donazzan, A.; Pelizzo, M.G. Room-Temperature Optical Detection of Hydrogen Gas Using Palladium Nano-Islands. Int. J. Hydrogen Energy 2018, 43, 5783–5792. [Google Scholar] [CrossRef]
- Arora, K.; Srivastava, S.; Solanki, P.R.; Puri, N.K. Electrochemical Hydrogen Gas Sensing Employing Palladium Oxide/Reduced Graphene Oxide (PdO-RGO) Nanocomposites. IEEE Sens. J. 2019, 19, 8262–8271. [Google Scholar] [CrossRef]
- Yaacob, M.H.; Breedon, M.; Kalantar-Zadeh, K.; Wlodarski, W. Absorption Spectral Response of Nanotextured WO3 Thin Films with Pt Catalyst towards H2. Sens. Actuators B Chem. 2009, 137, 115–120. [Google Scholar] [CrossRef]
- Phan, D.T.; Chung, G.S. A Novel Pd Nanocube-Graphene Hybrid for Hydrogen Detection. Sens. Actuators B Chem. 2014, 199, 354–360. [Google Scholar] [CrossRef]
- Basu, S.; Basu, P.K. Nanocrystalline Metal Oxides for Methane Sensors: Role of Noble Metals. J. Sens. 2009, 2009, 1–20. [Google Scholar] [CrossRef]
- Renganathan, B.; Sastikumar, D.; Srinivasan, R.; Ganesan, A.R. Nanocrystalline Samarium Oxide Coated Fiber Optic Gas Sensor. Mater. Sci. Eng. B 2014, 186, 122–127. [Google Scholar] [CrossRef]
- Yahya, N.A.M.; Hamid, M.R.Y.; Ibrahim, S.A.; Ong, B.H.; Rahman, N.A.; Zain, A.R.M.; Mahdi, M.A.; Yaacob, M.H. H2 Sensor Based on Tapered Optical Fiber Coated with MnO2 Nanostructures. Sens. Actuators B Chem. 2017, 246, 421–427. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, L.; Sun, L.; Li, J.; Ran, Y.; Guan, B.-O. Highly Sensitive Fiber Taper Interferometric Hydrogen Sensors. IEEE Photonics J. 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Dai, J.; Yang, M.; Yu, X.; Lu, H. Optical Hydrogen Sensor Based on Etched Fiber Bragg Grating Sputtered with Pd/Ag Composite Film. Opt. Fiber Technol. 2013, 19, 26–30. [Google Scholar] [CrossRef]
- Cretu, V.; Postica, V.; Stoianov, D.; Trofim, V.; Sontea, V.; Lupan, O. Hydrogen Gas Sensor Based on Nanograined Pd/α-MoO3 Belts. IFMBE Proc. 2016, 55, 361–364. [Google Scholar] [CrossRef]
- Chung, M.G.; Kim, D.-H.; Seo, D.K.; Kim, T.; Im, H.U.; Lee, H.M.; Yoo, J.-B.; Hong, S.-H.; Kang, T.J.; Kim, Y.H. Flexible Hydrogen Sensors Using Graphene with Palladium Nanoparticle Decoration. Sens. Actuators B Chem. 2012, 169, 387–392. [Google Scholar] [CrossRef]
- Lange, U.; Hirsch, T.; Mirsky, V.M.; Wolfbeis, O.S. Hydrogen Sensor Based on a Graphene-Palladium Nanocomposite. Electrochim. Acta 2011, 56, 3707–3712. [Google Scholar] [CrossRef]




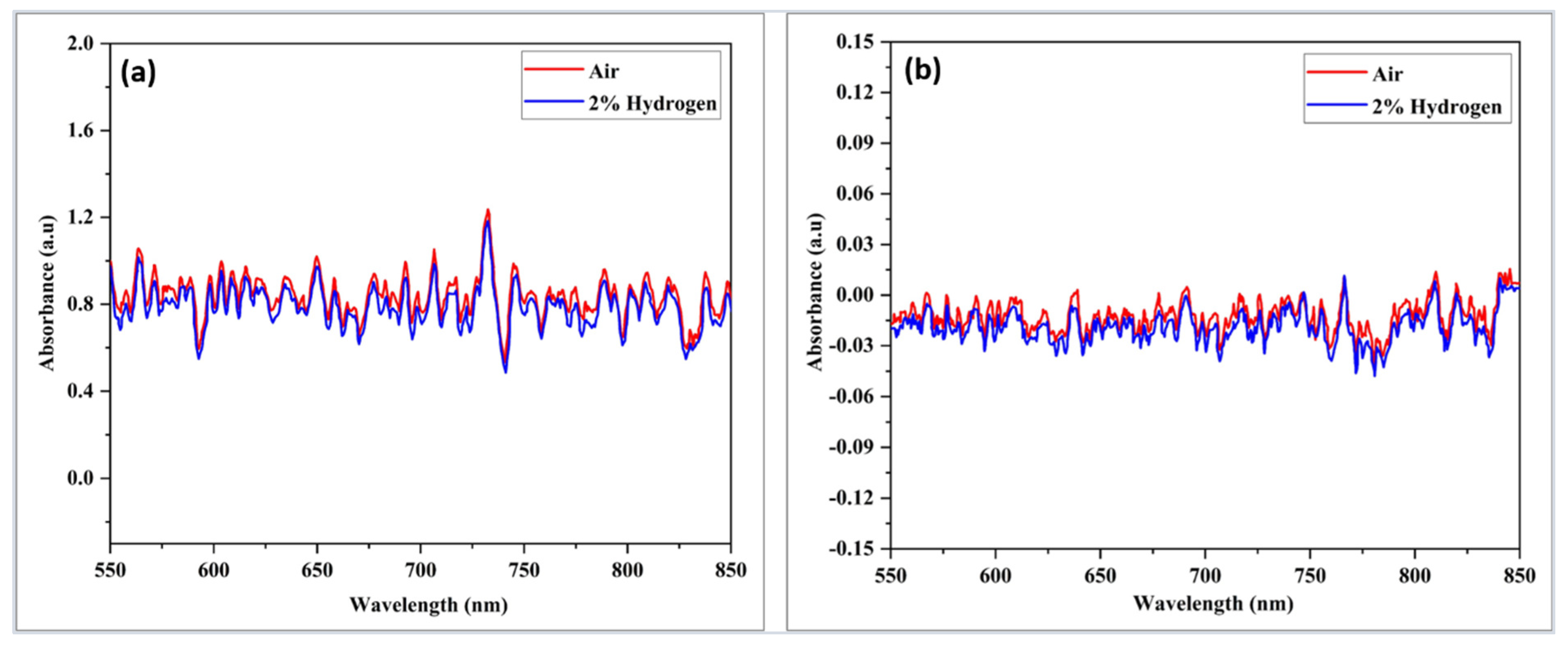
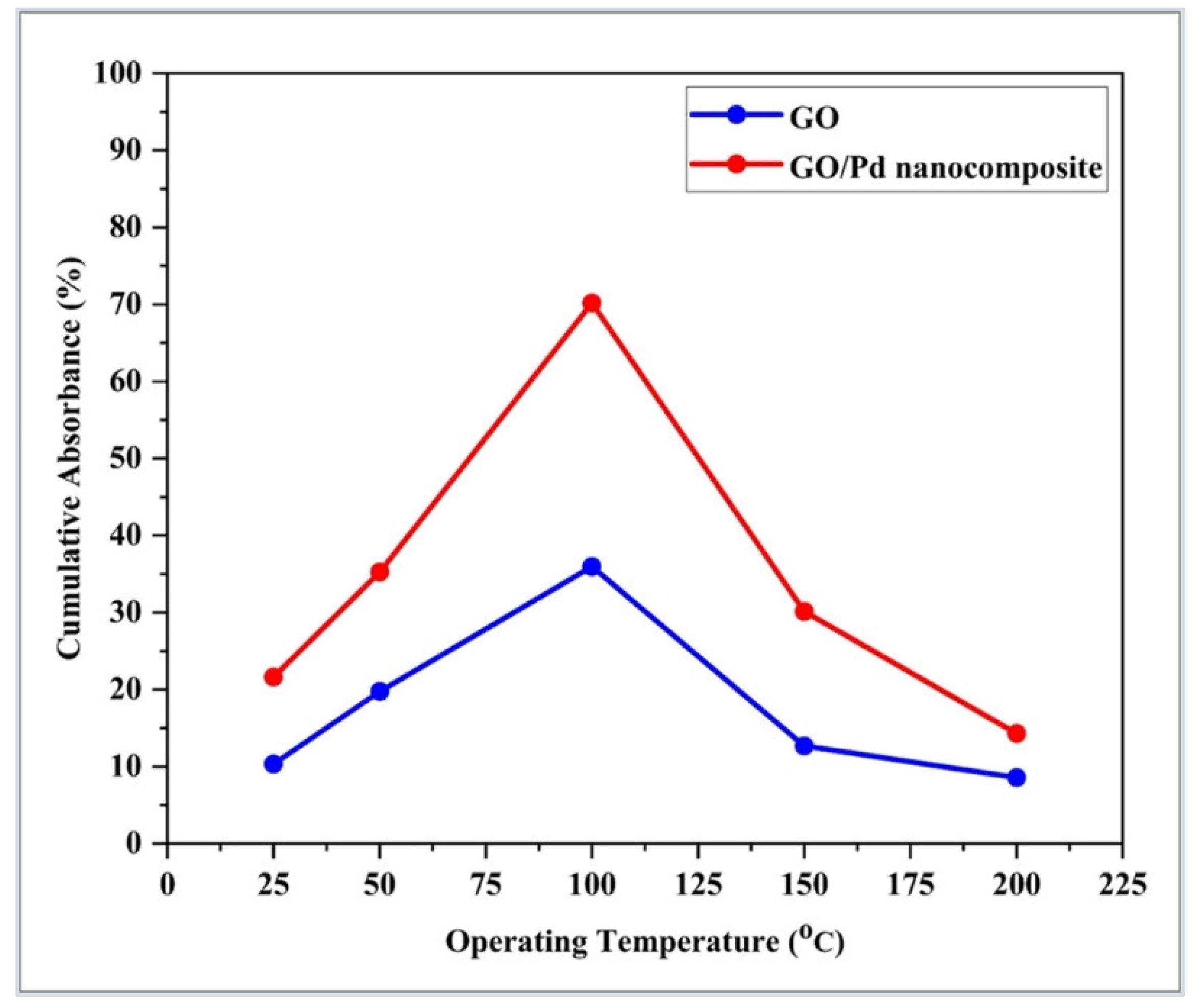
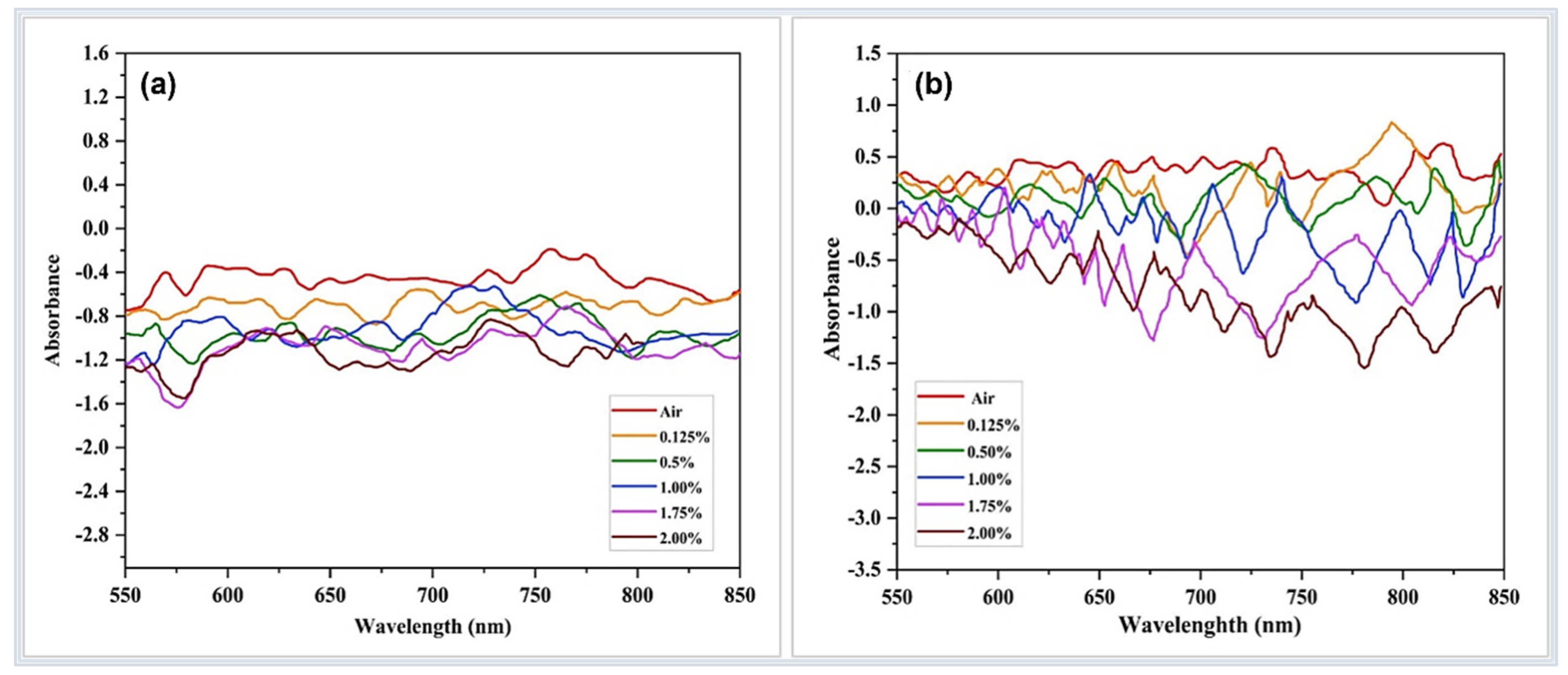
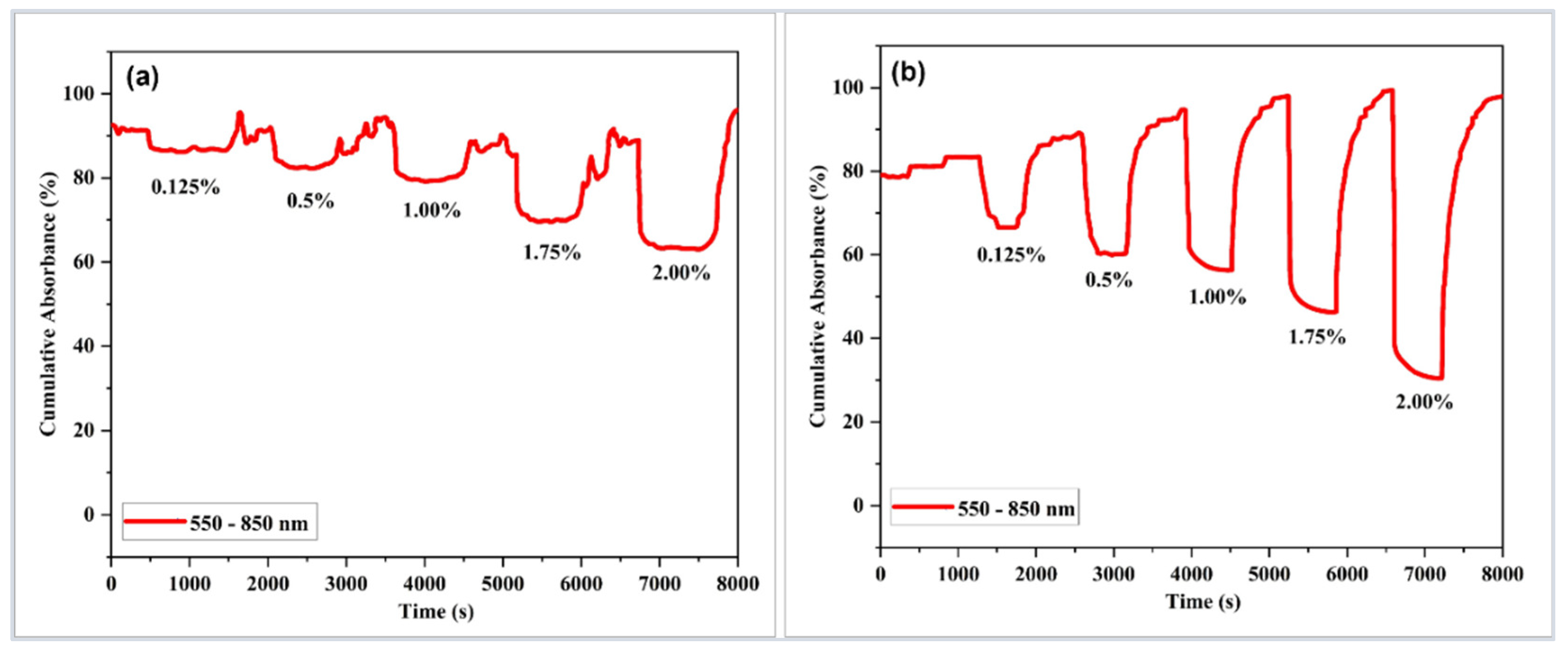
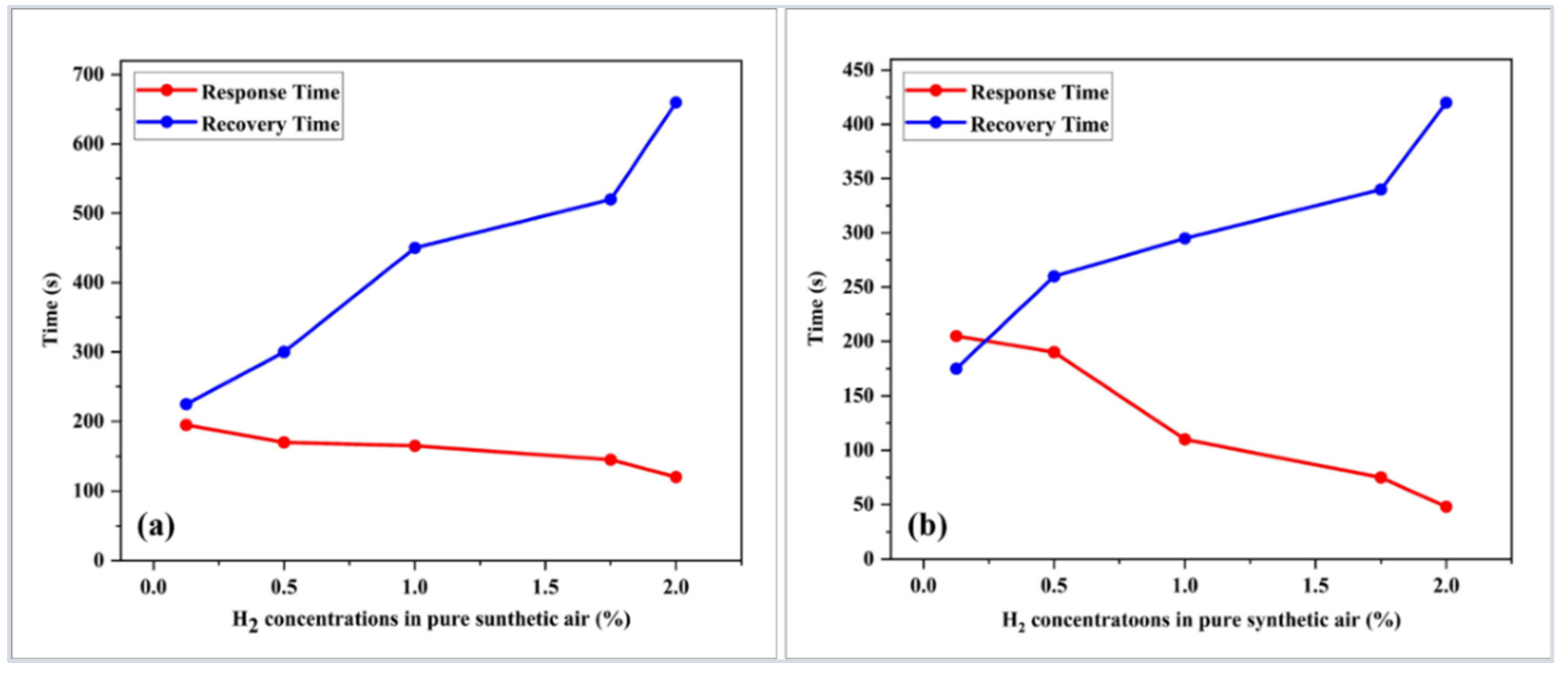
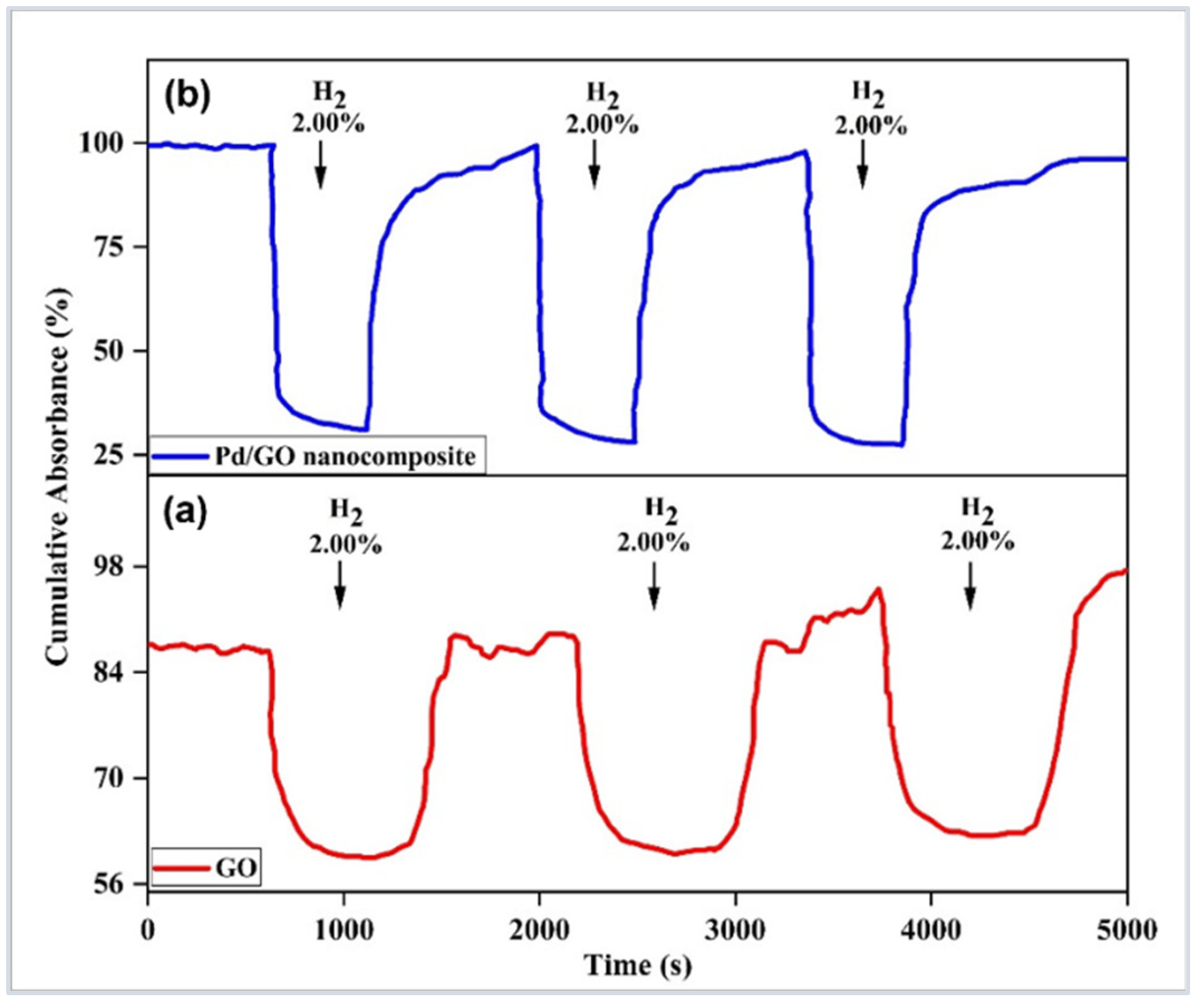
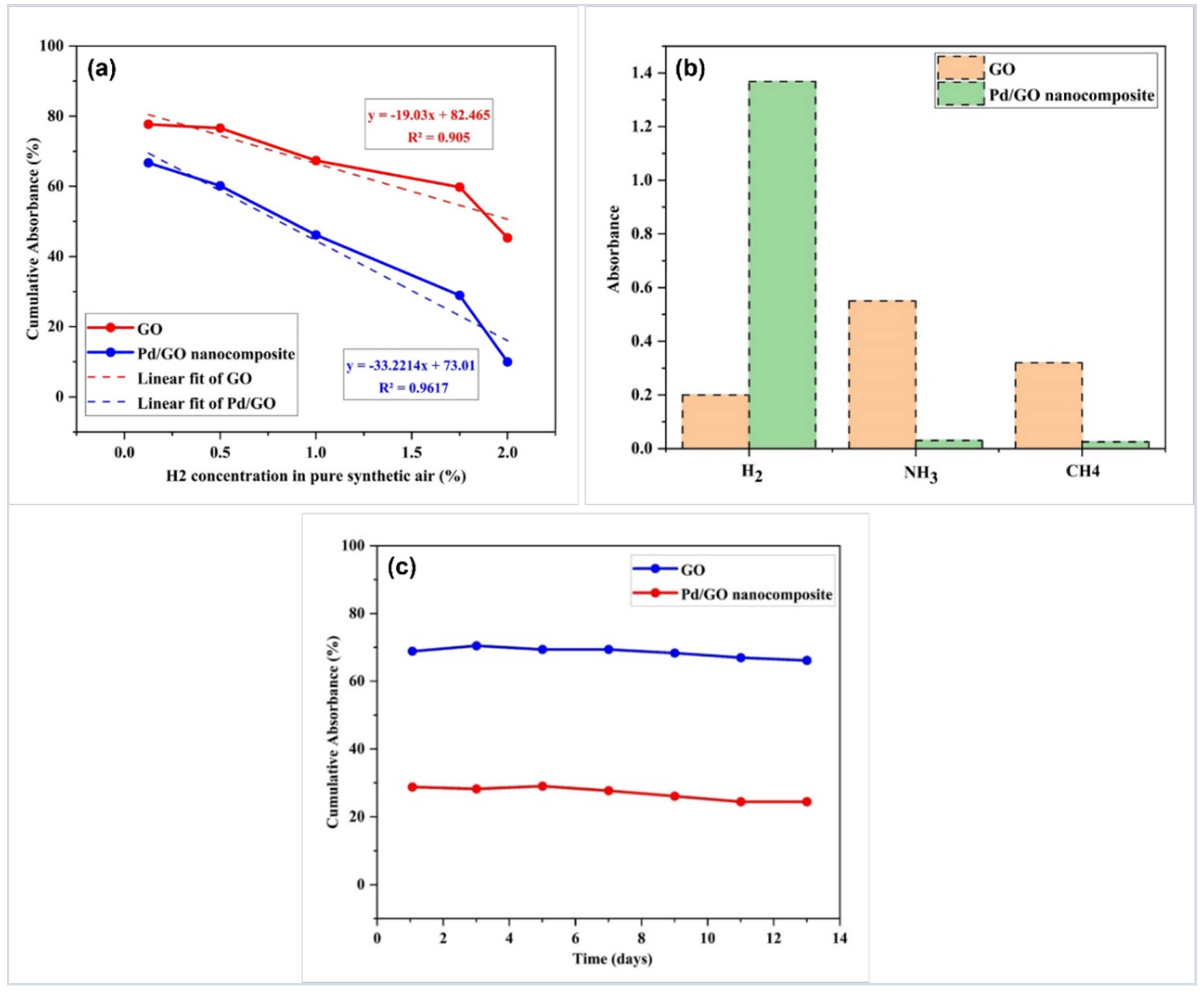
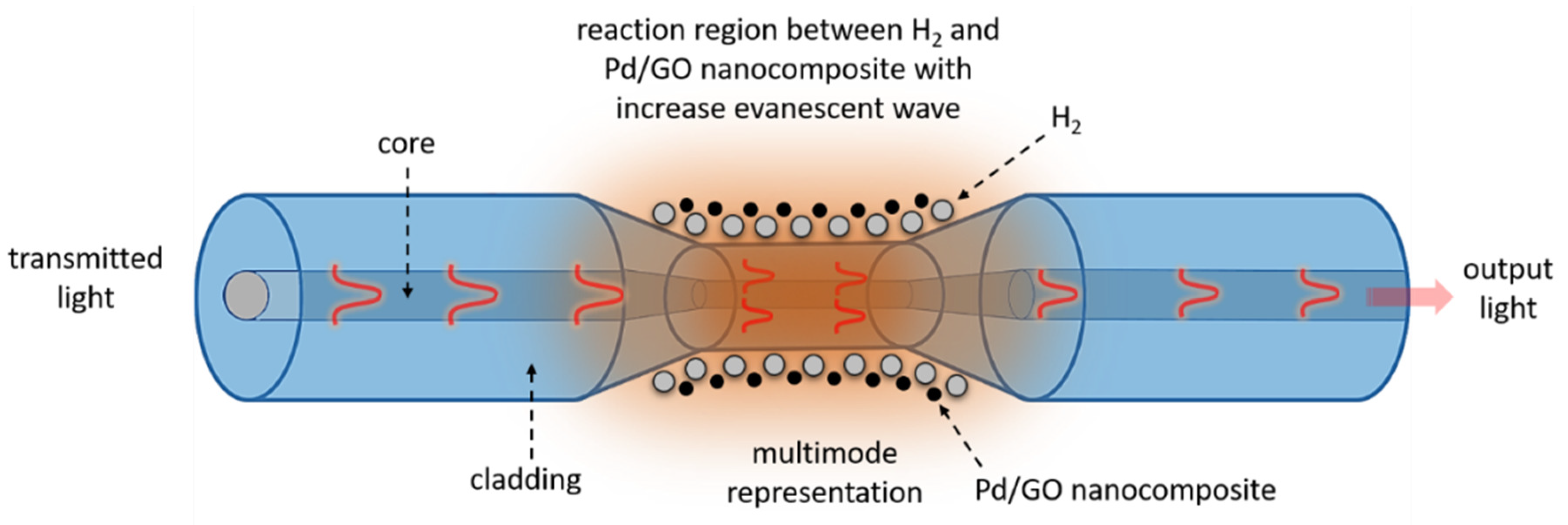
| Sensor-Based | Sensor Structure | Operating Temperature °C | Sensitivity (Abs/%) | Response Time (s) | Ref. |
|---|---|---|---|---|---|
| Pd NPs | Quartz substrate | RT | 15.40 | 50 | [29] |
| GO | PMMA fiber | RT | 2.33 | 90 | [33] |
| Pd/MnO2 | Tapered MMF | 3.609 | 240 | [61] | |
| Pd NPs | Mach-Zehnder interferometer | RT | 2.58 | 30 | [62] |
| Pd NPs | Tapered MMF | RT | 4.285 | 100 | [30] |
| Pd/Ag | Etched FBG | RT | 2.112 | 280 | [63] |
| Pd/α-MoO3 | Substrate with Pd electrode | 200 | 1.46 | 230 | [64] |
| GO | Tapered MMF | RT | 19.03 | 120 | This work |
| Pd/GO nanocomposite | Tapered MMF | RT | 33.221 | 48 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhabet, M.M.; Yaseen, Z.M.; Eldirderi, M.M.A.; Khedher, K.M.; Jawad, A.H.; Girei, S.H.; Salih, H.K.; Paiman, S.; Arsad, N.; Mahdi, M.A.; et al. Palladium/Graphene Oxide Nanocomposite for Hydrogen Gas Sensing Applications Based on Tapered Optical Fiber. Materials 2022, 15, 8167. https://doi.org/10.3390/ma15228167
Alkhabet MM, Yaseen ZM, Eldirderi MMA, Khedher KM, Jawad AH, Girei SH, Salih HK, Paiman S, Arsad N, Mahdi MA, et al. Palladium/Graphene Oxide Nanocomposite for Hydrogen Gas Sensing Applications Based on Tapered Optical Fiber. Materials. 2022; 15(22):8167. https://doi.org/10.3390/ma15228167
Chicago/Turabian StyleAlkhabet, Mohammed Majeed, Zaher Mundher Yaseen, Moutaz Mustafa A. Eldirderi, Khaled Mohamed Khedher, Ali H. Jawad, Saad Hayatu Girei, Husam Khalaf Salih, Suriati Paiman, Norhana Arsad, Mohd Adzir Mahdi, and et al. 2022. "Palladium/Graphene Oxide Nanocomposite for Hydrogen Gas Sensing Applications Based on Tapered Optical Fiber" Materials 15, no. 22: 8167. https://doi.org/10.3390/ma15228167
APA StyleAlkhabet, M. M., Yaseen, Z. M., Eldirderi, M. M. A., Khedher, K. M., Jawad, A. H., Girei, S. H., Salih, H. K., Paiman, S., Arsad, N., Mahdi, M. A., & Yaacob, M. H. (2022). Palladium/Graphene Oxide Nanocomposite for Hydrogen Gas Sensing Applications Based on Tapered Optical Fiber. Materials, 15(22), 8167. https://doi.org/10.3390/ma15228167










