IL-17/Notch1/STAT3 Pathway Contributes to 5-Fluorouracil-Induced Intestinal Mucositis in Rats: Amelioration by Thymol Treatment
Abstract
1. Introduction
2. Results
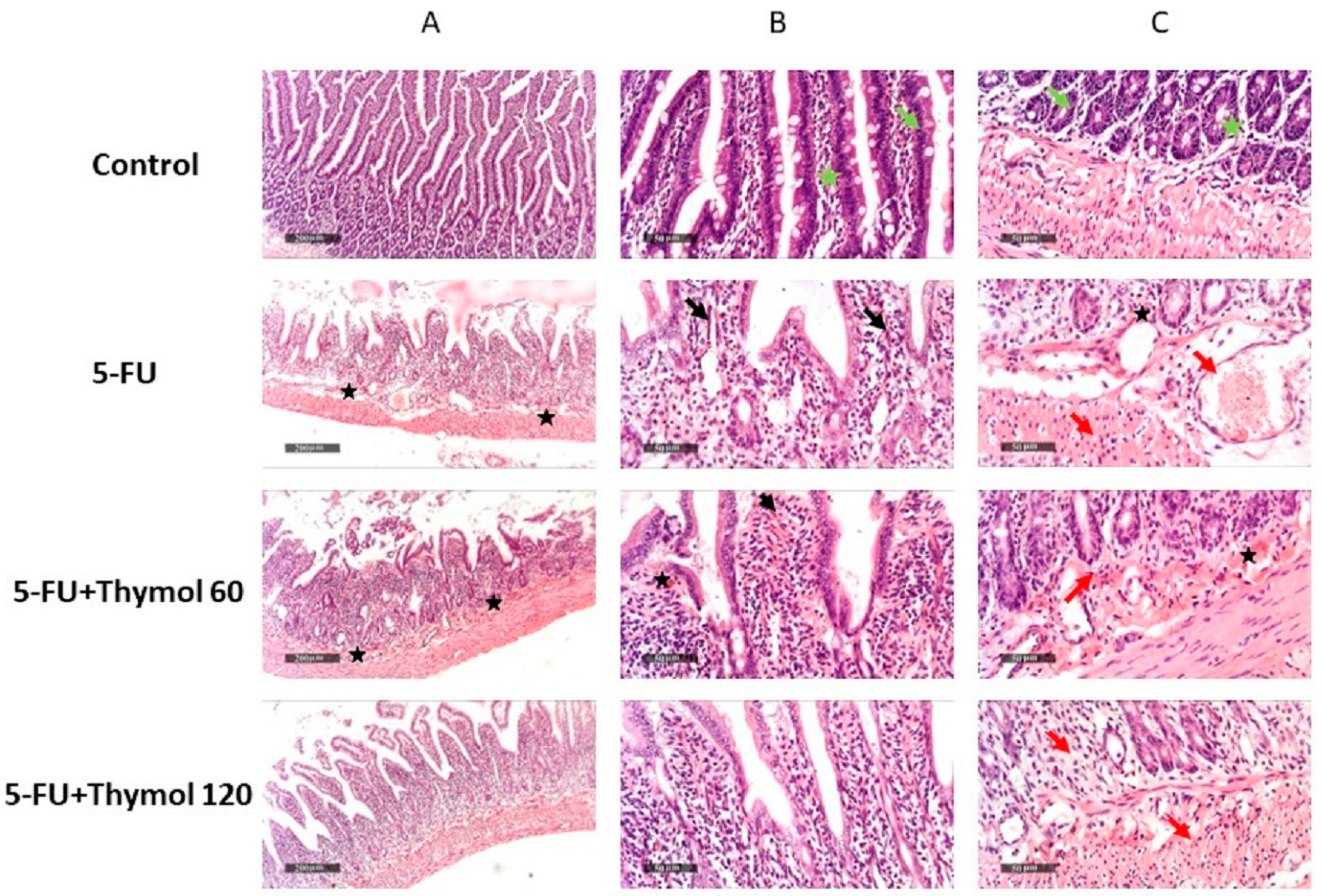
2.1. Impact of Thymol Administration on the Histopathological Features of Intestinal Samples of 5-FU-Intoxicated Rats
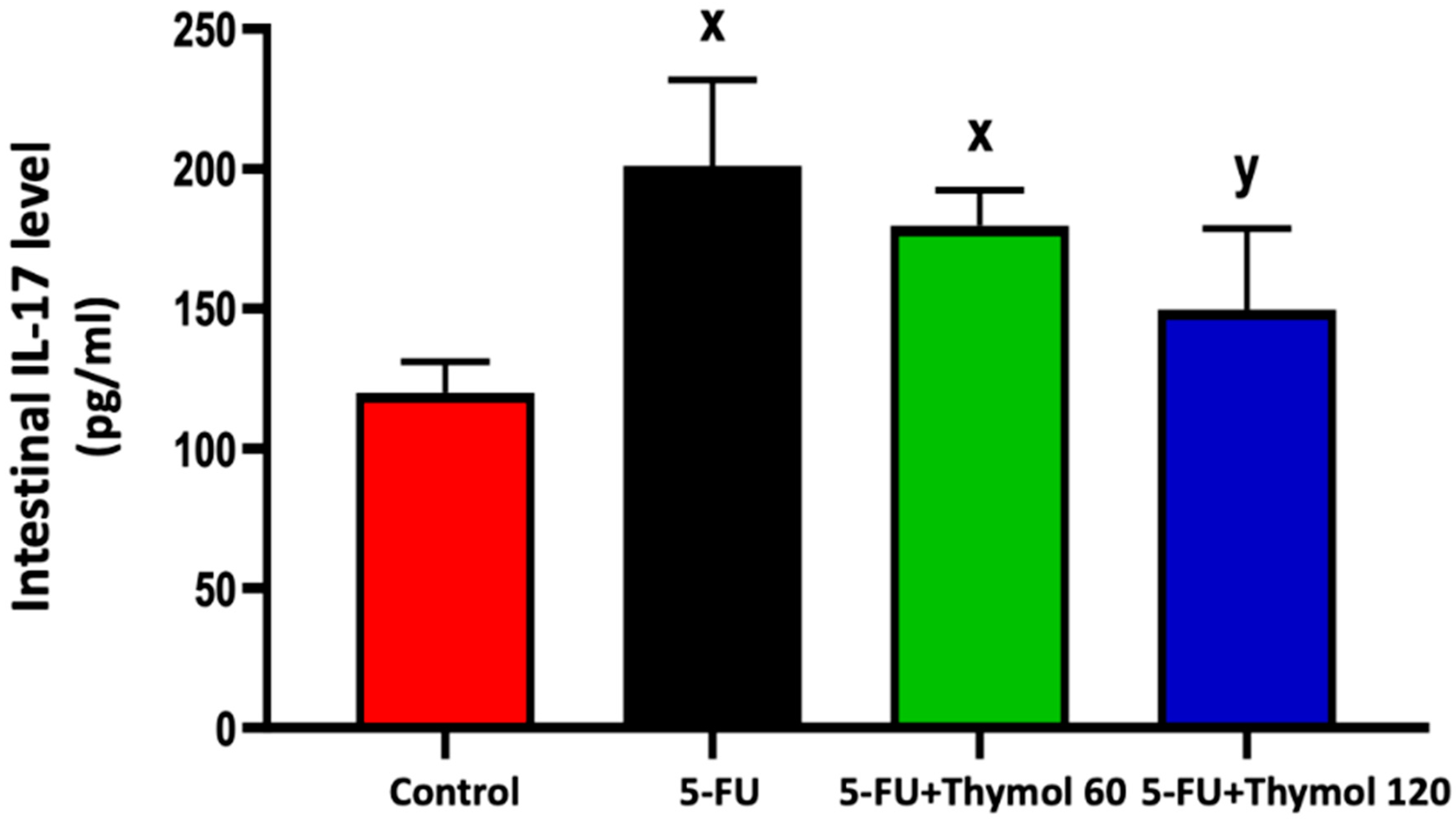
2.2. Thymol Administration Suppresses the Intestinal Expression of IL-17 in the 5-FU-Intoxicated Rats
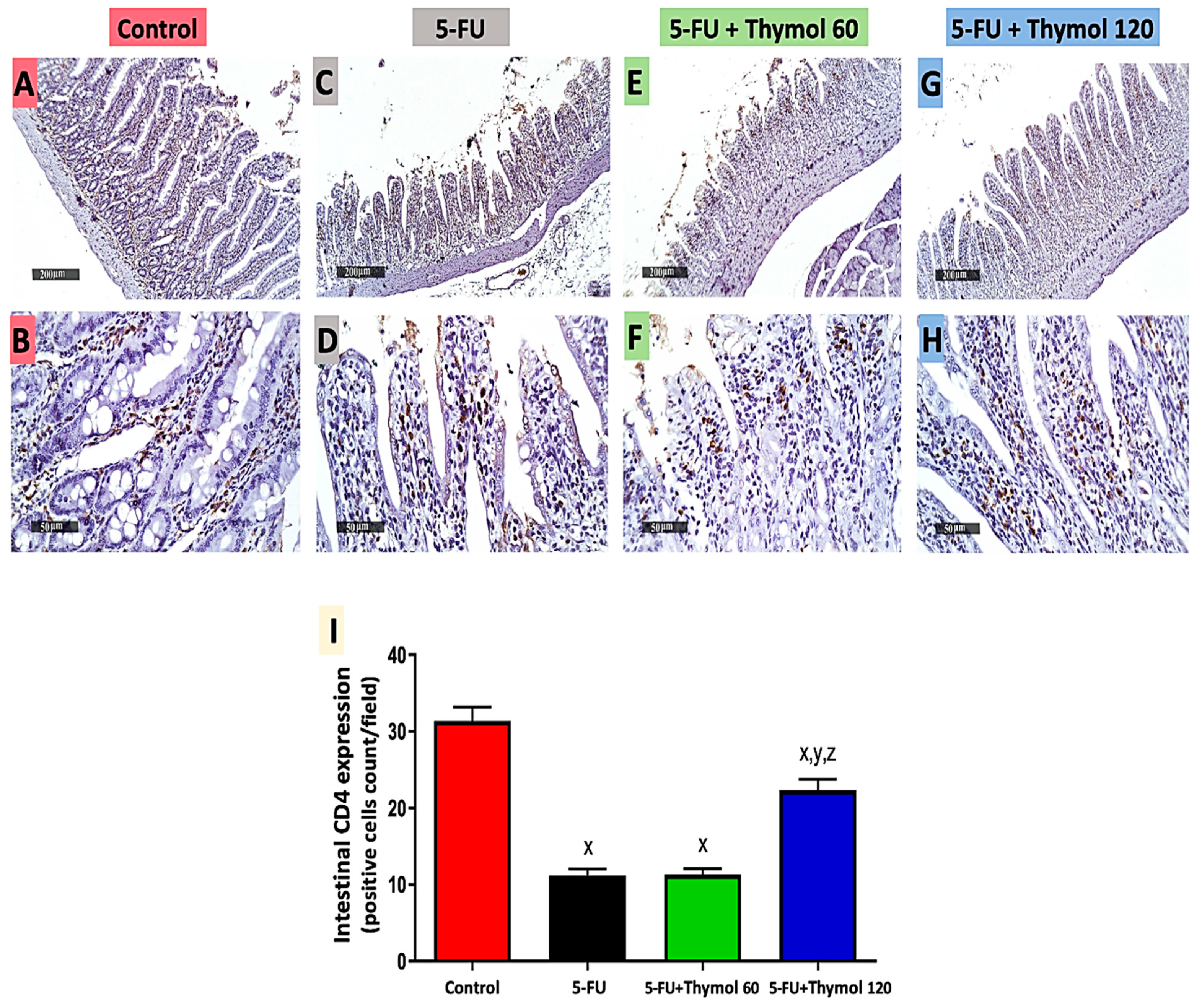
2.3. Thymol Administration Induced CD4 Intestinal Expression in the 5-FU-Intoxicated Rats
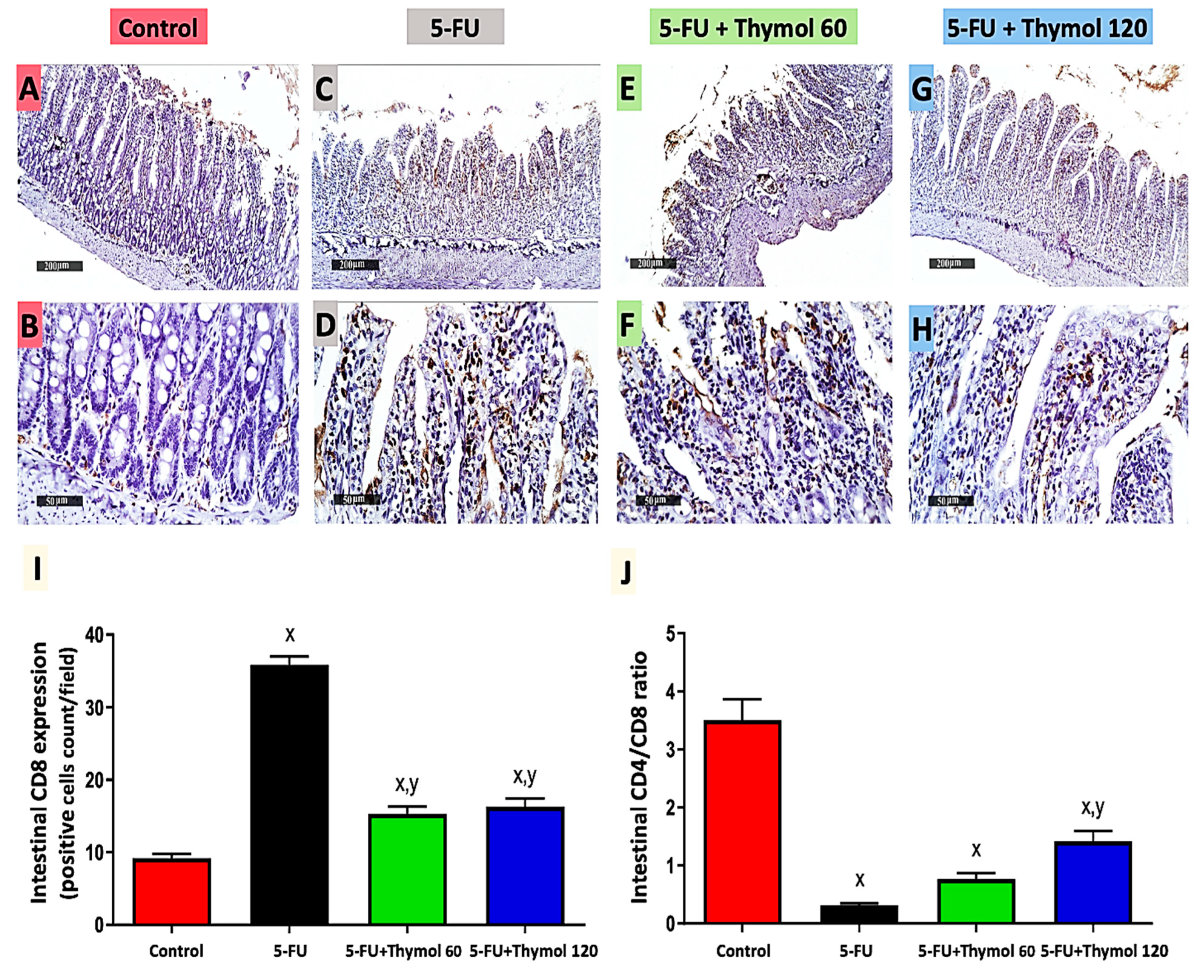
2.4. Impact of Thymol Administration on CD8 Intestinal Expression and CD4/CD8 Ratio in the 5-FU-Intoxicated Rats
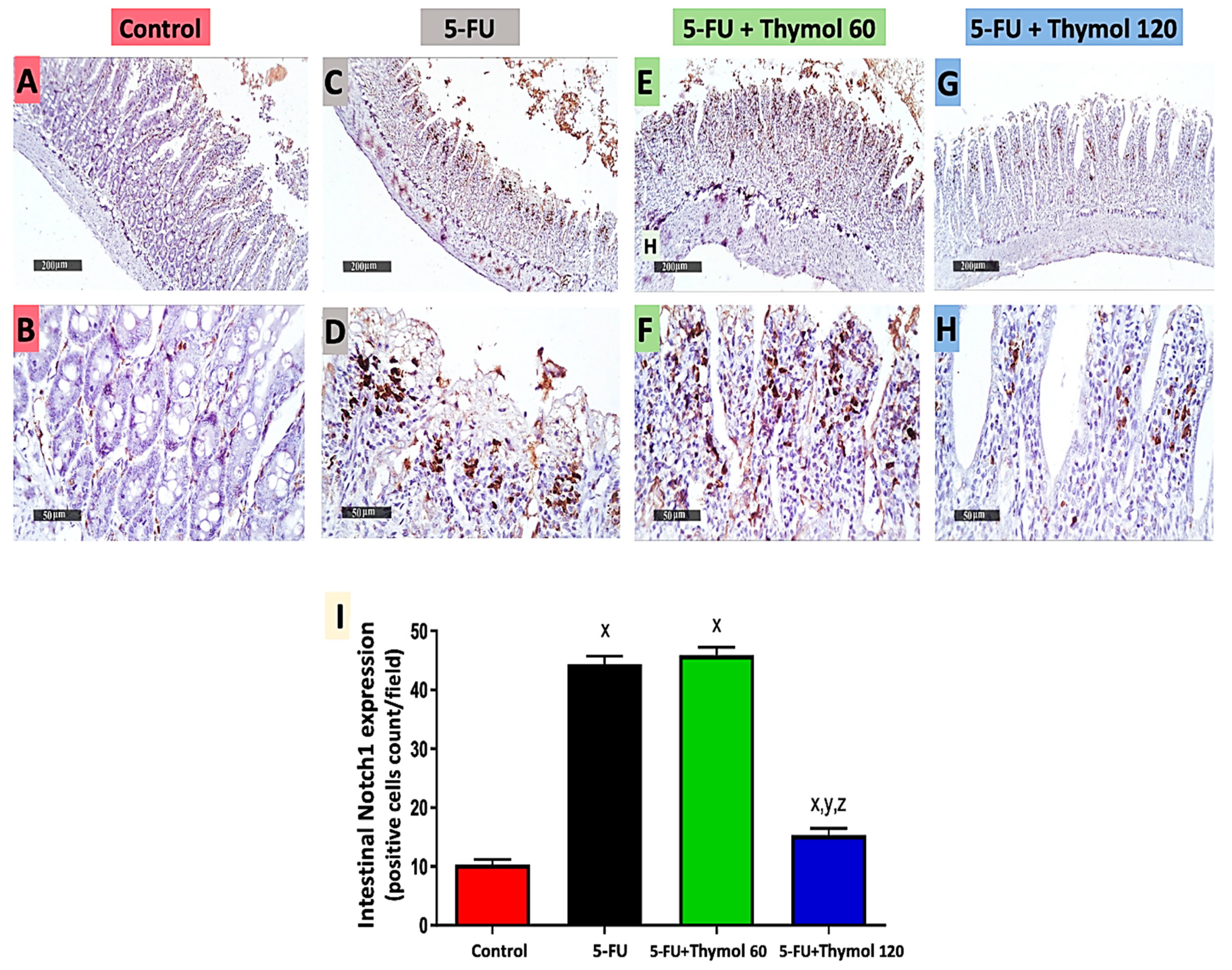
2.5. Impact of Thymol Administration on Notch1 Intestinal Expression in the 5-FU-Intoxicated Rats
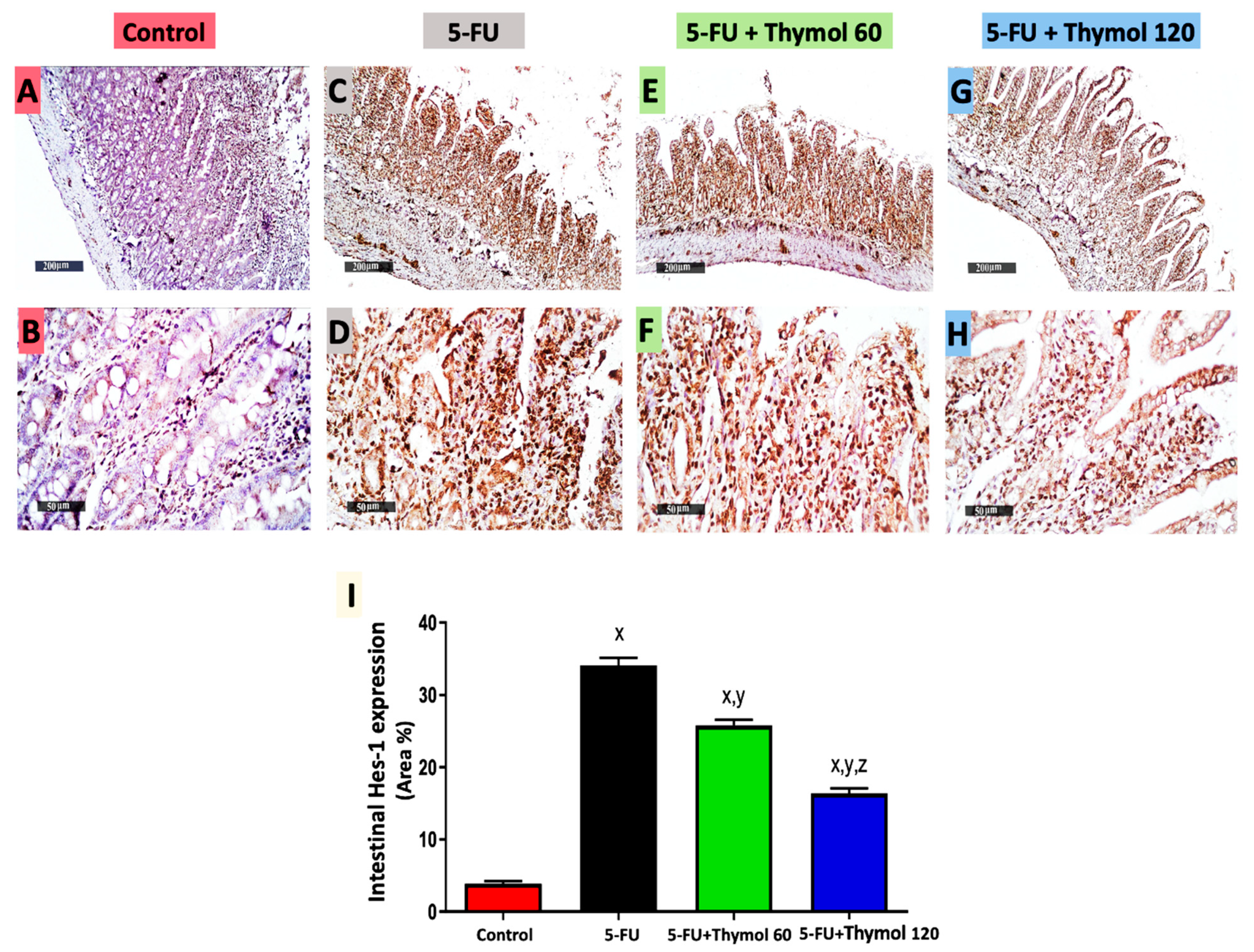
2.6. Impact of Thymol Administration on Hes-1 Intestinal Expression in the 5-FU-Intoxicated Rats
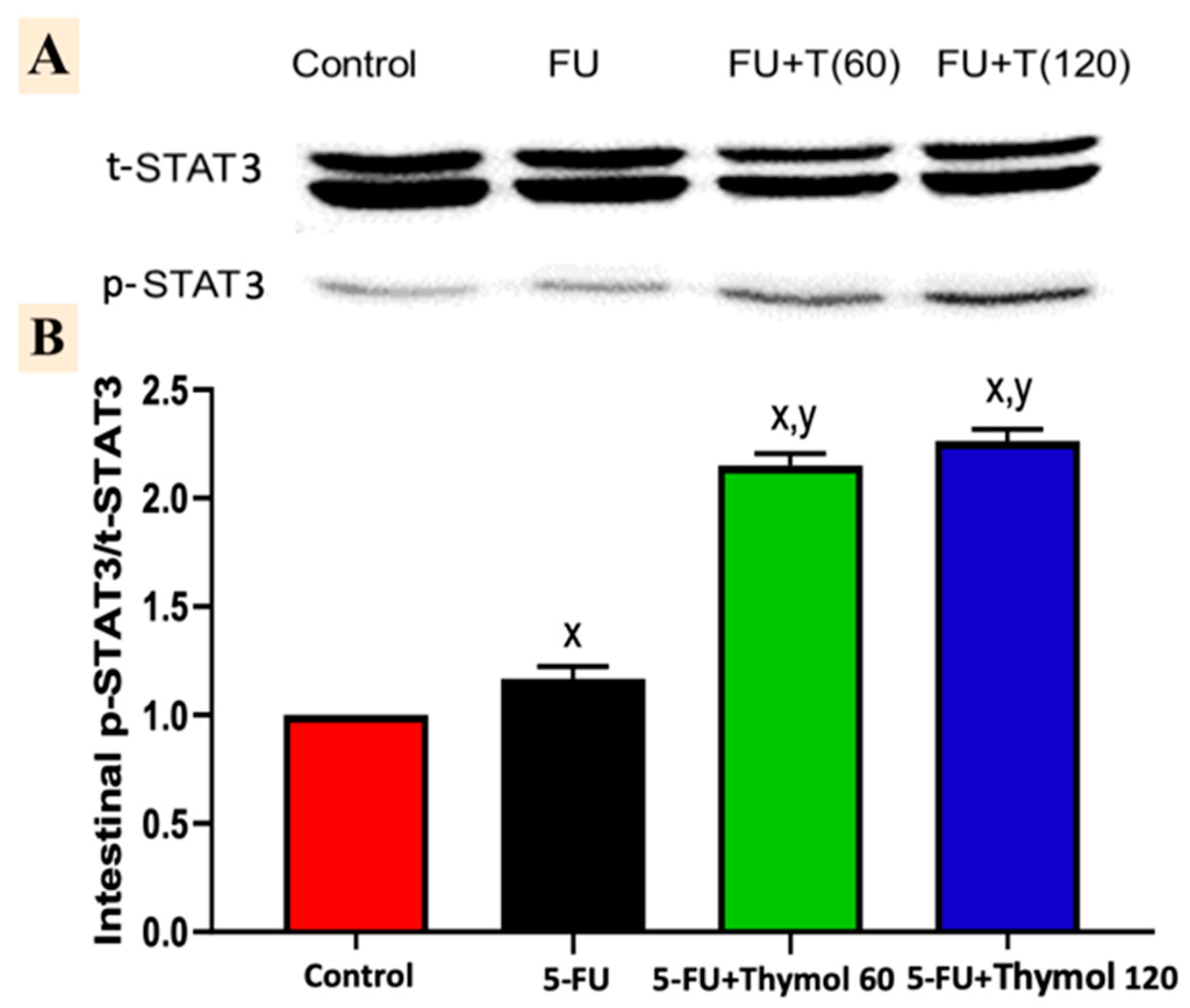
2.7. Impact of Thymol Administration on the p-STAT3/t-STAT3 Intestinal Ratio in the 5-FU-Intoxicated Rats
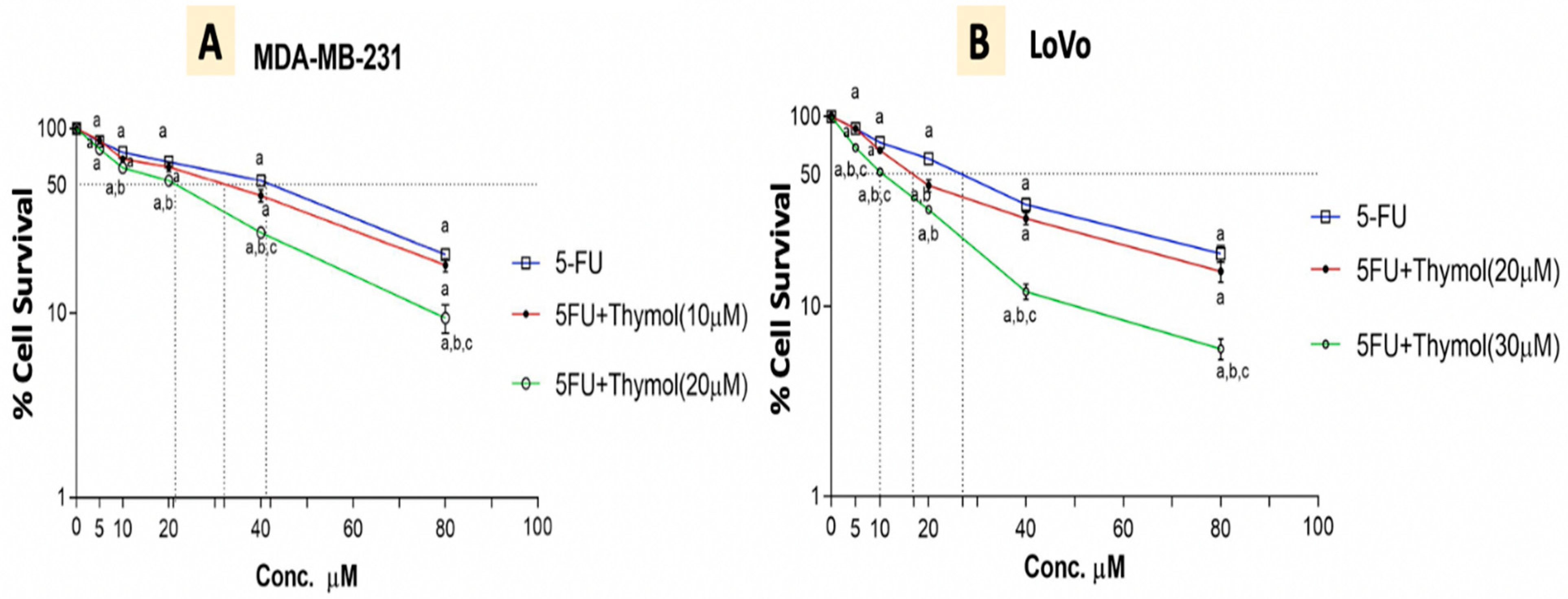
2.8. Thymol Augments the Cytotoxic Effect of 5-FU in Human Cancer Cells
3. Discussion
4. Materials and Methods
4.1. In Vivo Study
4.1.1. Experimental Animals and Design
4.1.2. Samples Collection
4.1.3. Histopathological Examination
4.1.4. ELISA Assessment of IL-17
4.1.5. Immunohistochemical Detection of CD4, CD8, Notch, and Hes-1, Expressions
4.1.6. Immunoblot Analysis of Phosphorylated-STAT3/STAT3
4.2. In Vitro Study
4.2.1. Cell Lines
4.2.2. Cytotoxicity WST-1 Assay
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliveira, M.M.B.; de Araújo, A.A.; Ribeiro, S.B.; de Sales Mota, P.C.M.; Marques, V.B.; da Silva Martins Rebouças, C.; Figueiredo, J.G.; Barra, P.B.; de Castro Brito, G.A.; de Carvalho Leitão, R.F. Losartan improves intestinal mucositis induced by 5-fluorouracil in mice. Sci. Rep. 2021, 11, 23241. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chun, H.J.; Choi, H.S.; Kim, E.S.; Keum, B.; Seo, Y.S.; Jeen, Y.T.; Lee, H.S.; Um, S.H.; Kim, C.D. Ursodeoxycholic acid attenuates 5-fluorouracil-induced mucositis in a rat model. Oncol. Lett. 2018, 16, 2585–2590. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.; Grant, G. Oral and intestinal mucositis—Causes and possible treatments. Aliment. Pharmacol. Ther. 2003, 18, 853–874. [Google Scholar] [CrossRef]
- De Koning, B.A.; van Dieren, J.M.; Lindenbergh-Kortleve, D.J.; Van Der Sluis, M.; Matsumoto, T.; Yamaguchi, K.; Einerhand, A.W.; Samsom, J.N.; Pieters, R.; Nieuwenhuis, E.E. Contributions of mucosal immune cells to methotrexate-induced mucositis. Int. Immunol. 2006, 18, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef]
- Gholijani, N.; Amirghofran, Z. Effects of thymol and carvacrol on T-helper cell subset cytokines and their main transcription factors in ovalbumin-immunized mice. J. Immunotoxicol. 2016, 13, 729–737. [Google Scholar] [CrossRef]
- Jovanovic, D.V.; Di Battista, J.A.; Martel-Pelletier, J.; Jolicoeur, F.C.; He, Y.; Zhang, M.; Mineau, F.; Pelletier, J.-P. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophages. J. Immunol. 1998, 160, 3513–3521. [Google Scholar]
- Tabas, I.; Lichtman, A.H. Monocyte-macrophages and T cells in atherosclerosis. Immunity 2017, 47, 621–634. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, J.; Li, C.; Liu, J.; Liu, H. Sulforaphane attenuates dextran sodium sulphate induced intestinal inflammation via IL-10/STAT3 signaling mediated macrophage phenotype switching. Food Sci. Hum. Wellness 2022, 11, 129–142. [Google Scholar] [CrossRef]
- Gu, C.; Wu, L.; Li, X. IL-17 family: Cytokines, receptors and signaling. Cytokine 2013, 64, 477–485. [Google Scholar] [CrossRef]
- Roussel, L.; Houle, F.; Chan, C.; Yao, Y.; Bérubé, J.; Olivenstein, R.; Martin, J.G.; Huot, J.; Hamid, Q.; Ferri, L. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J. Immunol. 2010, 184, 4531–4537. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Takahashi, D.; Ebisawa, M.; Kakiguchi, K.; Yonemura, S.; Jinnohara, T.; Kanaya, T.; Fujimura, Y.; Ohmae, M.; Hase, K. Epithelial cell-intrinsic Notch signaling plays an essential role in the maintenance of gut immune homeostasis. J. Immunol. 2012, 188, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, P.F.; Gjølberg, T.T.; Krüger, S.; Haraldsen, G.; Andersen, J.T.; Sundlisæter, E. Targeting the Notch Signaling Pathway in Chronic Inflammatory Diseases. Front. Immunol. 2021, 12, 668207. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, C.-J.; Martin, B.N.; Bulek, K.; Kang, Z.; Zhao, J.; Bian, G.; Carman, J.A.; Gao, J.; Dongre, A. IL-17 induced NOTCH1 activation in oligodendrocyte progenitor cells enhances proliferation and inflammatory gene expression. Nat. Commun. 2017, 8, 15508. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, S.; Zhuang, Y.; Zhang, H.; Bai, J.; Hou, Q. Interleukin-17 stimulates STAT3-mediated endothelial cell activation for neutrophil recruitment. Cell. Physiol. Biochem. 2015, 36, 2340–2356. [Google Scholar] [CrossRef]
- Sougiannis, A.T.; VanderVeen, B.N.; Davis, J.M.; Fan, D.; Murphy, E.A. Understanding chemotherapy-induced intestinal mucositis and strategies to improve gut resilience. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G712–G719. [Google Scholar] [CrossRef]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef]
- Escobar, A.; Perez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Hassan, H.F.H.; Mansour, A.M.; Salama, S.A.; El-Sayed, E.-S.M. The chemopreventive effect of thymol against dimethylhydrazine and/or high fat diet-induced colon cancer in rats: Relevance to NF-κB. Life Sci. 2021, 274, 119335. [Google Scholar] [CrossRef]
- Al-Khrashi, L.A.; Badr, A.M.; AL-Amin, M.A.; Mahran, Y.F. Thymol ameliorates 5-fluorouracil-induced intestinal mucositis: Evidence of down-regulatory effect on TGF-β/MAPK pathways through NF-κB. J. Biochem. Mol. Toxicol. 2021, 36, e22932. [Google Scholar] [CrossRef]
- Al Serwi, R.H.; Darwish, S.F.; Mahran, Y.F. Growth hormone modulates the inflammatory and apoptotic pathways incorporated in fluorouracil-induced oral mucositis in rats. Egypt. Dent. J. 2020, 66, 327–336. [Google Scholar] [CrossRef][Green Version]
- Mi, H.; Dong, Y.; Zhang, B.; Wang, H.; Peter, C.C.; Gao, P.; Fu, H.; Gao, Y. Bifidobacterium infantis ameliorates chemotherapy-induced intestinal mucositis via regulating T cell immunity in colorectal cancer rats. Cell. Physiol. Biochem. 2017, 42, 2330–2341. [Google Scholar] [CrossRef] [PubMed]
- Fujino, S.; Andoh, A.; Bamba, S.; Ogawa, A.; Hata, K.; Araki, Y.; Bamba, T.; Fujiyama, Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003, 52, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, R.; Tsuchiya, K.; Nemoto, Y.; Akiyama, J.; Nakamura, T.; Kanai, T.; Watanabe, M. Requirement of Notch activation during regeneration of the intestinal epithelia. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G23–G35. [Google Scholar] [CrossRef]
- Kamakura, S.; Oishi, K.; Yoshimatsu, T.; Nakafuku, M.; Masuyama, N.; Gotoh, Y. Hes binding to STAT3 mediates crosstalk between Notch and JAK–STAT signalling. Nat. Cell Biol. 2004, 6, 547–554. [Google Scholar] [CrossRef]
- Hsu, K.-W.; Hsieh, R.-H.; Huang, K.-H.; Fen-Yau Li, A.; Chi, C.-W.; Wang, T.-Y.; Tseng, M.-J.; Wu, K.-J.; Yeh, T.-S. Activation of the Notch1/STAT3/Twist signaling axis promotes gastric cancer progression. Carcinogenesis 2012, 33, 1459–1467. [Google Scholar] [CrossRef]
- Rébé, C.; Végran, F.; Berger, H.; Ghiringhelli, F. STAT3 activation: A key factor in tumor immunoescape. Jak-stat 2013, 2, e23010. [Google Scholar] [CrossRef]
- Wang, L.; Song, B.; Hu, Y.; Chen, J.; Zhang, S.; Chen, D.; Wang, J. Puerarin Ameliorates 5-Fluorouracil–Induced Intestinal Mucositis in Mice by Inhibiting JAKs. J. Pharmacol. Exp. Ther. 2021, 379, 147–155. [Google Scholar] [CrossRef]
- Gad, M.; Mohammad, Y.; Mohammad, T. Acute and repeated-doses (28 Days) toxicity of Thymol formulation in male albino rats. Aust. J. Basic Appl. Sci. 2013, 7, 594–601. [Google Scholar]
- Meeran, N.; Javed, M.H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for Its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Satti, N.K.; Suri, K.A.; Amina, M.; Bani, S. Stimulatory effects of Cuminum cyminum and flavonoid glycoside on Cyclosporine-A and restraint stress induced immune-suppression in Swiss albino mice. Chem. Biol. Interact. 2010, 185, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Mahran, Y.F.; Badr, A.M.; Aldosari, A.; Bin-Zaid, R.; Alotaibi, H.N. Carvacrol and thymol modulate the cross-talk between TNF-α and IGF-1 signaling in radiotherapy-induced ovarian failure. Oxidative Med. Cell. Longev. 2019, 2019, 3173745. [Google Scholar] [CrossRef] [PubMed]
- Vieceli Dalla Sega, F.; Fortini, F.; Aquila, G.; Campo, G.; Vaccarezza, M.; Rizzo, P. Notch signaling regulates immune responses in atherosclerosis. Front. Immunol. 2019, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Ishihara, S.; Ansary, M.U.; Sonoyama, H.; Tada, Y.; Oka, A.; Kusunoki, R.; Tamagawa, Y.; Fukuba, N.; Mishima, Y. Crosstalk between TLR5 and Notch1 signaling in epithelial cells during intestinal inflammation. Int. J. Mol. Med. 2013, 32, 1051–1062. [Google Scholar] [CrossRef]
- Khan, F.; Singh, V.K.; Saeed, M.; Kausar, M.A.; Ansari, I.A. Carvacrol induced program cell death and cell cycle arrest in androgen-independent human prostate cancer cells via inhibition of notch signaling. Anti-Cancer Agents Med. Chem. 2019, 19, 1588–1608. [Google Scholar] [CrossRef]
- Gholijani, N.; Gharagozloo, M.; Farjadian, S.; Amirghofran, Z. Modulatory effects of thymol and carvacrol on inflammatory transcription factors in lipopolysaccharide-treated macrophages. J. Immunotoxicol. 2016, 13, 157–164. [Google Scholar] [CrossRef]
- Liu, D.; Luo, H.; Qiao, C. SHP-1/STAT3 Interaction Is Related to Luteolin-Induced Myocardial Ischemia Protection. Inflammation 2022, 45, 88–99. [Google Scholar] [CrossRef]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Uyanikgil, Y.; Ozturk, F. Apoptotic effects of thymol, a novel monoterpene phenol, on different types of cancer. Bratisl. Lek. Listy 2020, 121, 122–128. [Google Scholar] [CrossRef]
- Zeng, Q.; Che, Y.; Zhang, Y.; Chen, M.; Guo, Q.; Zhang, W. Thymol Isolated from Thymus vulgaris L. inhibits colorectal cancer cell growth and metastasis by suppressing the Wnt/β-catenin pathway. Drug Des. Dev. Ther. 2020, 14, 2535. [Google Scholar] [CrossRef]
- Sherif, I.O.; Al-Mutabagani, L.A.; Sabry, D.; Elsherbiny, N.M. Antineoplastic activity of chrysin against human hepatocellular carcinoma: New insight on GPC3/SULF2 axis and lncRNA-AF085935 expression. Int. J. Mol. Sci. 2020, 21, 7642. [Google Scholar] [CrossRef]
| Groups | Description |
|---|---|
| Control | Rats received 0.5% dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA) daily by oral gavage. |
| 5-FU | Rats received 0.5% DMSO daily by oral gavage plus 150 mg/kg 5-fluorouracil (5-FU, 50 mg/mL ampoules, Ebewe Pharma, Austria) injected intraperitoneally (i.p.) on the 6th and 7th days to induce intestinal toxicity. |
| 5-FU + Thymol 60 | Rats received 60 mg/kg/day thymol (Purity > 98%, Abcam, Cambridge, UK) in 0.5% DMSO orally, plus 150 mg/kg 5-FU injected i.p. on the 6th and 7th days. |
| 5-FU + Thymol 120 | Rats received 120 mg/kg/day thymol in 0.5% DMSO orally, plus 150 mg/kg 5-FU injected i.p. on the 6th and 7th days. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badr, A.M.; Alkharashi, L.A.; Sherif, I.O.; Alanteet, A.A.; Alotaibi, H.N.; Mahran, Y.F. IL-17/Notch1/STAT3 Pathway Contributes to 5-Fluorouracil-Induced Intestinal Mucositis in Rats: Amelioration by Thymol Treatment. Pharmaceuticals 2022, 15, 1412. https://doi.org/10.3390/ph15111412
Badr AM, Alkharashi LA, Sherif IO, Alanteet AA, Alotaibi HN, Mahran YF. IL-17/Notch1/STAT3 Pathway Contributes to 5-Fluorouracil-Induced Intestinal Mucositis in Rats: Amelioration by Thymol Treatment. Pharmaceuticals. 2022; 15(11):1412. https://doi.org/10.3390/ph15111412
Chicago/Turabian StyleBadr, Amira M., Layla A. Alkharashi, Iman O. Sherif, Alaa A. Alanteet, Hind N. Alotaibi, and Yasmen F. Mahran. 2022. "IL-17/Notch1/STAT3 Pathway Contributes to 5-Fluorouracil-Induced Intestinal Mucositis in Rats: Amelioration by Thymol Treatment" Pharmaceuticals 15, no. 11: 1412. https://doi.org/10.3390/ph15111412
APA StyleBadr, A. M., Alkharashi, L. A., Sherif, I. O., Alanteet, A. A., Alotaibi, H. N., & Mahran, Y. F. (2022). IL-17/Notch1/STAT3 Pathway Contributes to 5-Fluorouracil-Induced Intestinal Mucositis in Rats: Amelioration by Thymol Treatment. Pharmaceuticals, 15(11), 1412. https://doi.org/10.3390/ph15111412






