The Effect of Different Morphologies of WO3/GO Nanocomposite on Photocatalytic Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Graphene Oxide (GO) Synthesis
2.2. In Situ and Ex Situ Synthesis of WO3/GO Composites
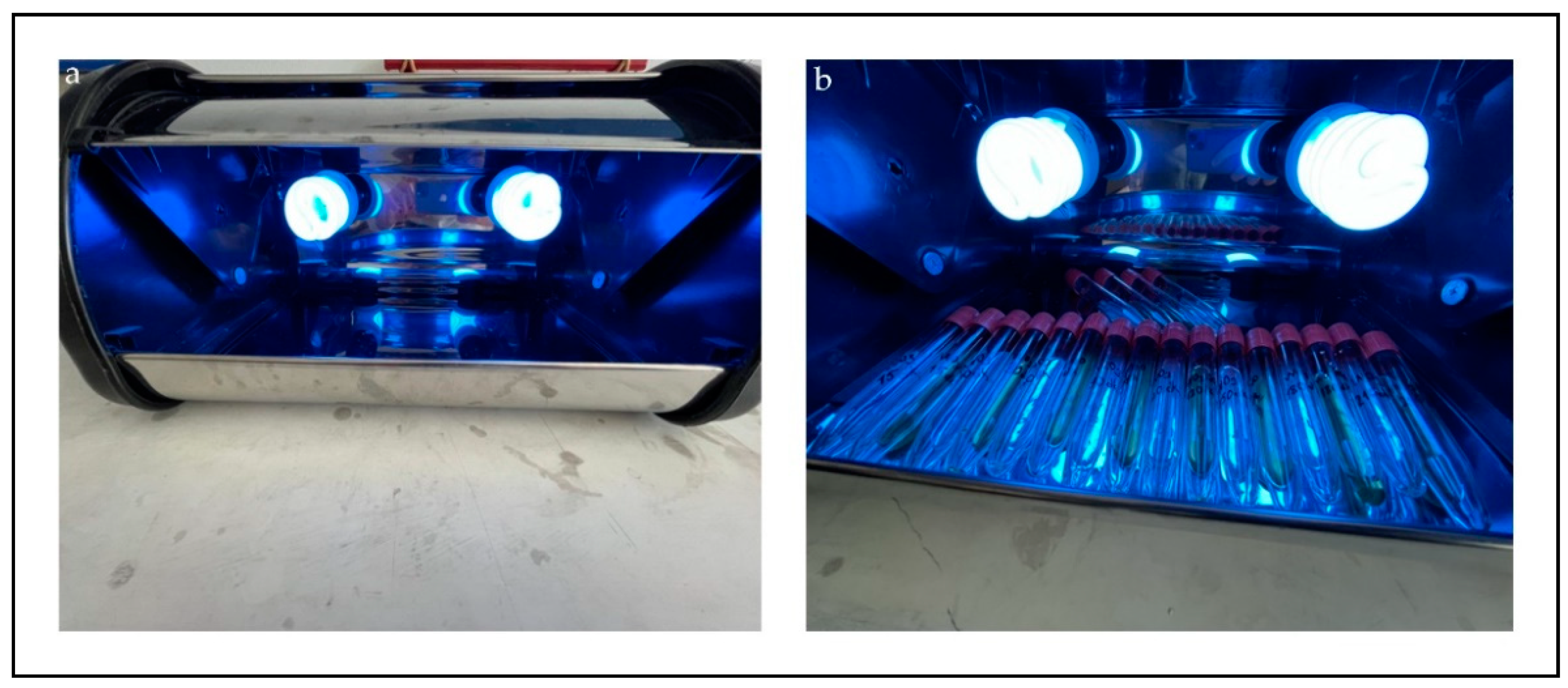
2.3. Photocatalytic Activities of WO3/GO Composites
3. Results
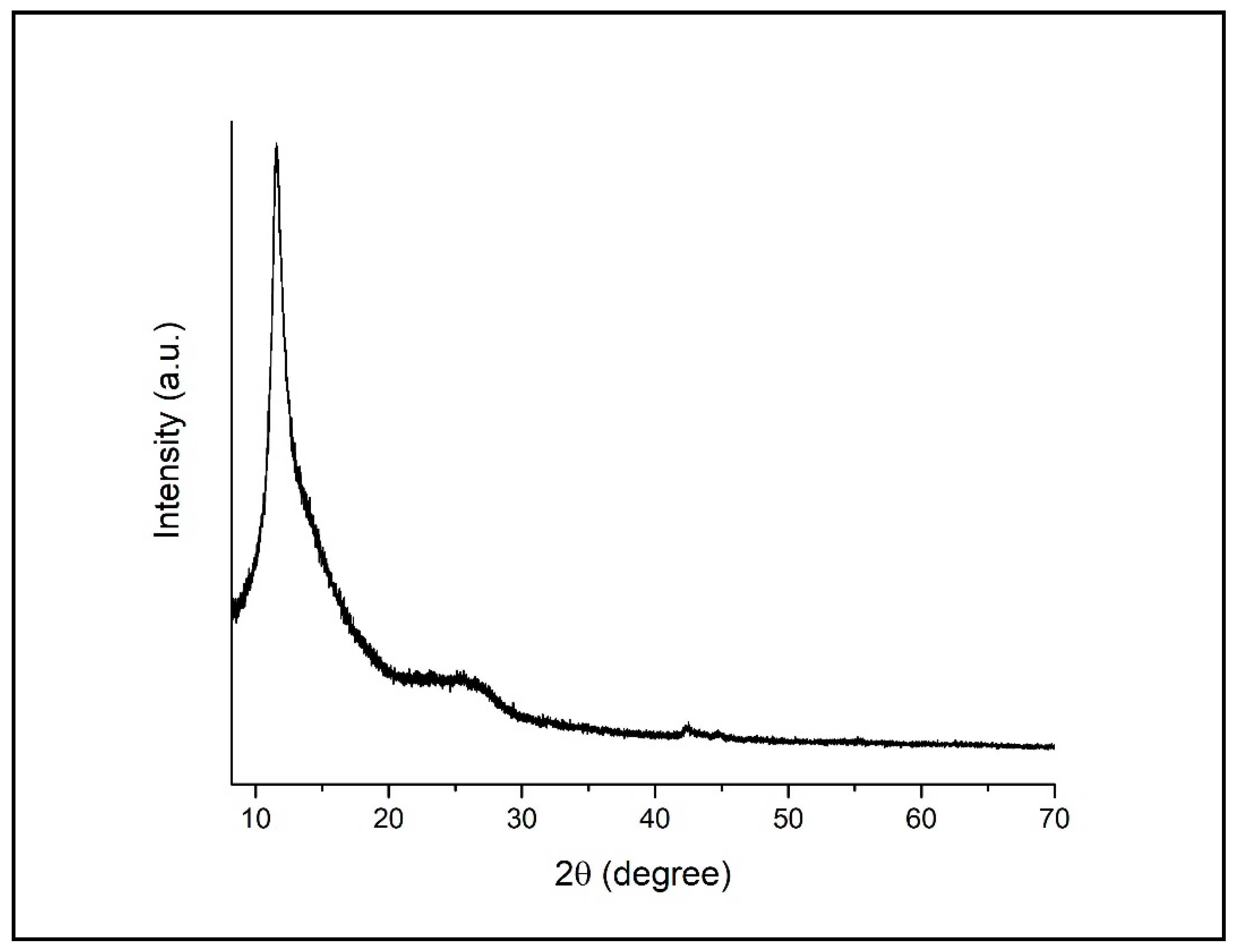
3.1. Characterization of GO
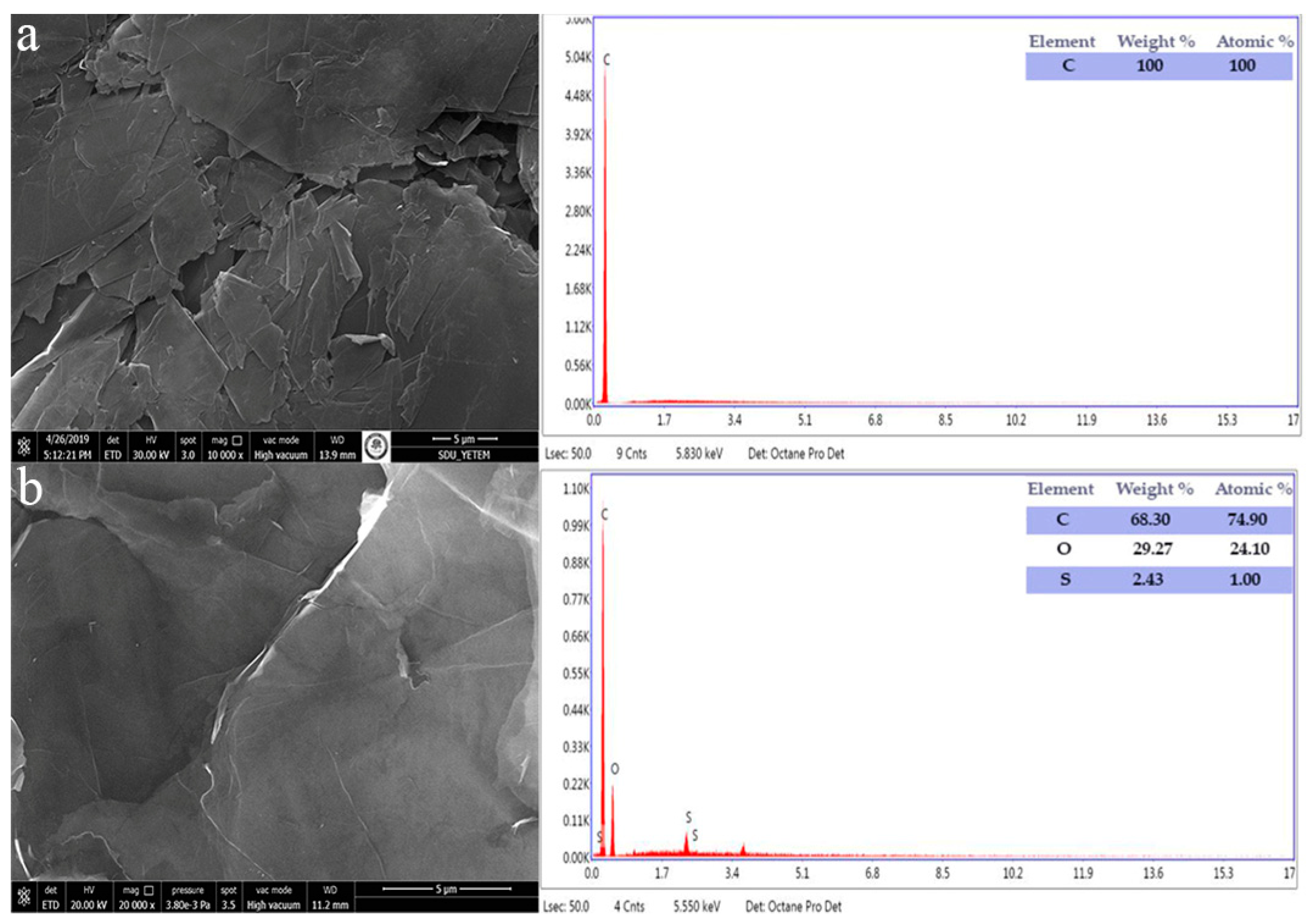
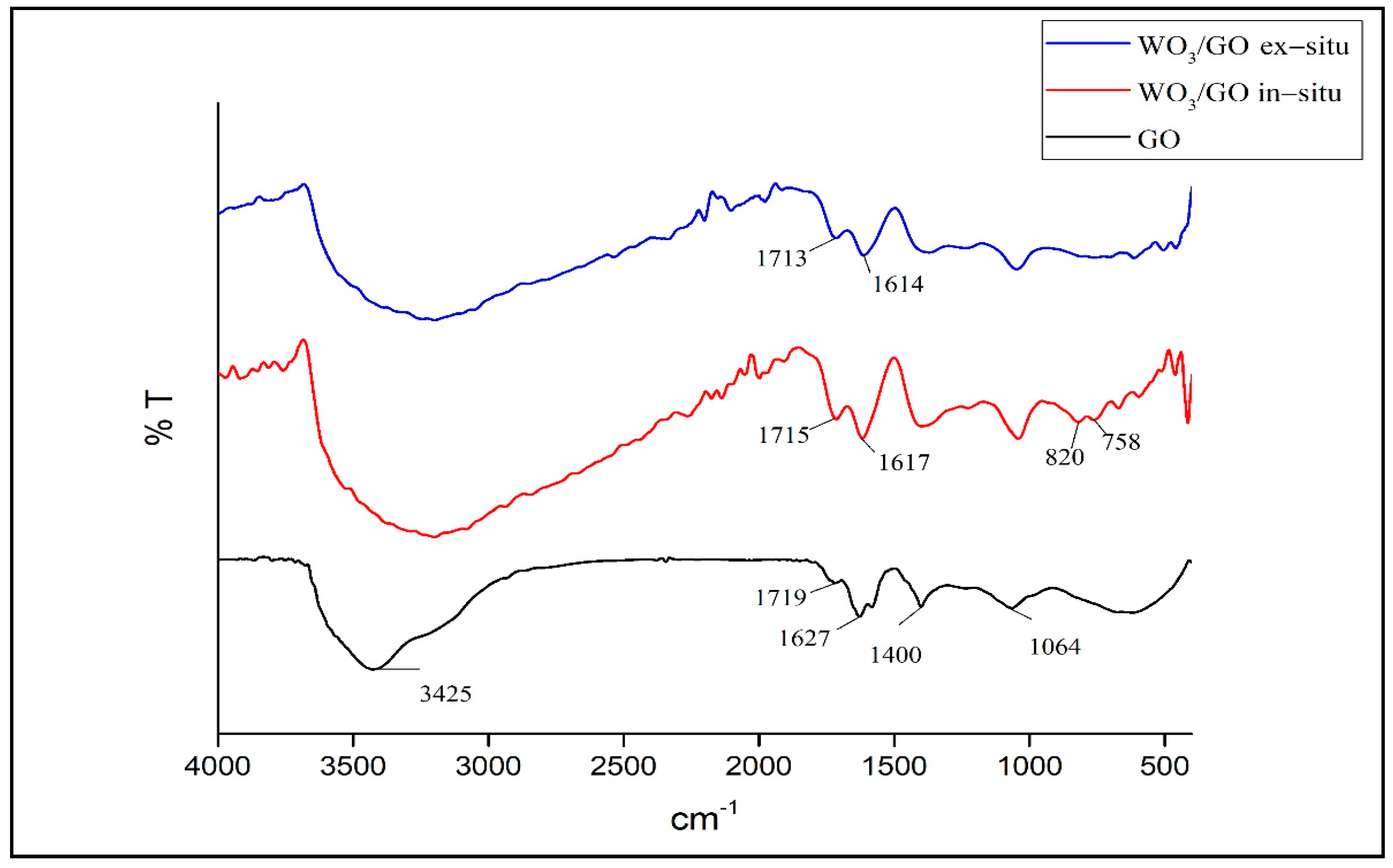
3.2. Characterization of WO3/GO Composites
3.3. Photocatalytic Degradation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alam, M.W.; Aamir, M.; Farhan, M.; Albuhulayqah, M.; Ahmad, M.M.; Ravikumar, C.; Dileep Kumar, V.; Ananda Murthy, H. Green Synthesis of Ni-Cu-Zn Based Nanosized Metal Oxides for Photocatalytic and Sensor Applications. Crystals 2021, 11, 1467. [Google Scholar] [CrossRef]
- Hossain, S.; Chu, W.S.; Lee, C.S.; Ahn, S.H.; Chun, D.M. Photocatalytic performance of few-layer Graphene/WO3 thin films prepared by a nano-particle deposition system. Mater. Chem. Phys. 2019, 226, 141–150. [Google Scholar] [CrossRef]
- Alam, M.W.; Al Qahtani, H.S.; Souayeh, B.; Ahmed, W.; Albalawi, H.; Farhan, M.; Abuzir, A.; Naeem, S. Novel Copper-Zinc-Manganese Ternary Metal Oxide Nanocomposite as Heterogeneous Catalyst for Glucose Sensor and Antibacterial Activity. Antioxidants 2022, 11, 1064. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor. Sens. Actuators B Chem. 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Chang, X.; Zhou, Q.; Sun, S.; Shao, C.; Lei, Y.; Liu, T.; Yin, Y. Graphene-tungsten oxide nanocomposites with highly enhanced gas-sensing performance. J. Alloys Compd. 2017, 705, 659–667. [Google Scholar] [CrossRef]
- Tian, W.; Liu, X.; Yu, W. Research Progress of Gas Sensor Based on Graphene and Its Derivatives. Appl. Sci. 2018, 8, 1118. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Le, X.V.; Luu, T.L.A.; Nguyen, H.L.; Nguyen, C.T. Synergistic enhancement of ammonia gassensing properties at low temperature by compositing carbon nanotubes with tungsten oxide nanobricks. Vacuum 2019, 168, 108861. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Ivanov, M.; Cerneavschi, A.; Rodríguez, J.R.; Cirera, A.; Cornet, A.; Morante, J.R. Structural stability of indium oxide films deposited by spray pyrolysis during thermal annealing. Thin Solid Films 2005, 479, 38–51. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemoresistive gas sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Sayama, K.; Hayashi, H.; Arai, T.; Yanagida, M.; Gunji, T.; Sugihara, H. Highly active WO3 semiconductor photocatalyst prepared from amorphous peroxo-tungstic acid for the degradation of various organic compounds. Appl. Catal. B Environ. 2010, 94, 150–157. [Google Scholar] [CrossRef]
- Guo, Y.; Quan, X.; Lu, N.; Zhao, H.; Chen, S. High photocatalytic capability of self-assembled nanoporous WO3 with preferential orientation of (002) planes. Environ. Sci. Technol. 2007, 41, 4422–4427. [Google Scholar] [CrossRef] [PubMed]
- Sonia, S.; Kumar, P.S.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Influence of growth and photocatalytic properties of copper selenide (CuSe) nanoparticles using reflux condensation method. Appl. Surf. Sci. 2013, 283, 802–807. [Google Scholar] [CrossRef]
- Deng, F.; Pei, X.; Luo, Y.; Luo, X.; Dionysiou, D.D.; Wu, S.; Luo, S. Fabrication of hierarchically porous reduced graphene oxide/SnIn4S8 composites by a low-temperature coprecipitation strategy and their excellent visible-light photocatalytic mineralization performance. Catalysts 2016, 6, 113. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Wang, W.; Zeng, S.; Shang, M.; Zhang, L. Preparation of ordered mesoporous Ag/WO3 and its highly efficient degradation of acetaldehyde under visible-light irradiation. J. Hazard. Mater. 2010, 178, 427–433. [Google Scholar] [CrossRef]
- Zheng, H.; Mathe, M. Hydrogen evolution reaction on single crystal WO3/C nanoparticles supported on carbon in acid and alkaline solution. Int. J. Hydrogen Energy 2011, 36, 1960–1964. [Google Scholar] [CrossRef]
- Cao, L.; Yuan, J.; Chen, M.; Shangguan, W. Photocatalytic energy storage ability of TiO2-WO3 composite prepared by wet-chemical technique. Res. J. Environ. Sci. 2010, 22, 454–459. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Seo, H. Aligned nanotriangles of tantalum doped tungsten oxide for improved photoelectrochemical water splitting. J. Alloys Compd. 2019, 785, 1097–1105. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Yang, H.; Wen, L.; Yi, Z.; Zhou, Z.; Dai, B.; Zhang, J.; Wu, X.; Wu, P. Multi-mode surface plasmon resonance absorber based on dart-type single-layer graphene. RSC Adv. 2022, 12, 7821–7829. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, R.; Long, F.; Wang, J. Development and application of tetrabromobisphenol A imprinted electrochemical sensor based on graphene/carbon nanotubes three-dimensional nanocomposites modified carbon electrode. Talanta 2015, 134, 435–442. [Google Scholar] [CrossRef]
- Shangguan, Q.; Chen, Z.; Yang, H.; Cheng, S.; Yang, W.; Yi, Z.; Wu, X.; Wang, S.; Yi, Y.; Wu, P. Design of Ultra-Narrow Band Graphene Refractive Index Sensor. Sensors 2022, 22, 6483. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.U.; Kausar, A.; Ullah, H.; Badshah, A.; Khan, W.U. A review of graphene oxide, graphene buckypaper, and polymer/graphene composites: Properties and fabrication techniques. J. Plast. Film Sheeting 2016, 32, 336–379. [Google Scholar] [CrossRef]
- Cheng, Z.; Liao, J.; He, B.; Zhang, F.; Zhang, F.; Huang, X.; Zhou, L. One-Step Fabrication of Graphene Oxide Enhanced Magnetic Composite Gel for Highly Efficient Dye Adsorption and Catalysis. ACS Sustain. Chem. Eng. 2015, 3, 1677–1685. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient synthesis of graphene oxide based on improved hummers method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazıcı, M.; Tiyek, İ.; Ersoy, M.S.; Alma, M.H.; Dönmez, U.; Yıldırım, B.; Salan, T.; Karataş, Ş.; Uruş, S.; Karteri, İ.; et al. Modifiye Hummers Yöntemiyle Grafen Oksit (GO) Sentezi ve Karakterizasyonu. GU J. Sci. Part C 2016, 4, 43–50. [Google Scholar]
- Jeevitha, G.; Abhinayaa, R.; Mangalaraj, D.; Ponpandian, N. Tungsten oxide-graphene oxide (WO3-GO) nanocomposite as an efficient photocatalyst, antibacterial and anticancer agent. J. Phys. Chem. Solids 2018, 116, 137–147. [Google Scholar] [CrossRef]
- Fu, L.; Xia, T.; Zheng, Y.; Yang, J.; Wang, A.; Wang, Z. Preparation of WO3-reduced graphene oxide nanocomposites with enhanced photocatalytic property. Ceram. Int. 2015, 41, 5903–5908. [Google Scholar] [CrossRef]
- Kofuji, Y.; Isobe, Y.; Shiraishi, Y.; Sakamoto, H.; Ichikawa, S.; Tanaka, S.; Hirai, T. Hydrogen peroxide production on a carbon nitride–boron nitride-reduced graphene oxide hybrid photocatalyst under visible light. ChemCatChem 2018, 10, 2070–2077. [Google Scholar] [CrossRef]
- Ismail, A.A.; Faisal, M.; Al-Haddad, A. Mesoporous WO3-graphene photocatalyst for photocatalytic degradation of Methylene Blue dye under visible light illumination. Res. J. Environ. Sci. 2018, 66, 328–337. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, J.; Cui, P. Synthesis and enhanced photocatalytic performance of WO3 nanorods@ graphene nanocomposites. Mater. Lett. 2012, 89, 258–261. [Google Scholar] [CrossRef]
- Bragaglia, M.; Paleari, L.; Lamastra, F.R.; Puglia, D.; Fabbrocino, F.; Nanni, F. Graphene nanoplatelet, multiwall carbon nanotube, and hybrid multiwall carbon nanotube–graphene nanoplatelet epoxy nanocomposites as strain sensing coatings. J. Reinf. Plast. Compos. 2021, 40, 632–643. [Google Scholar] [CrossRef]
- Pittella, E.; D’Alvia, L.; Palermo, E.; Piuzzi, E. Microwave Characterization of 3D Printed PLA and PLA/CNT Composites. In Proceedings of the 2021 IEEE 6th International Forum on Research and Technology for Society and Industry (RTSI), Rome, Italy, 7–9 June 2021. [Google Scholar] [CrossRef]
- Paleari, L.; Bragaglia, M.; Fabbrocino, F.; Nanni, F. Structural Monitoring of Glass Fiber/Epoxy Laminates by Means of Carbon Nanotubes and Carbon Black Self-Monitoring Plies. Nanomaterials 2021, 11, 1543. [Google Scholar] [CrossRef]
- Izadi, R.; Tuna, M.; Trovalusci, P.; Ghavanloo, E. Torsional Characteristics of Carbon Nanotubes: Micropolar Elasticity Models and Molecular Dynamics Simulation. Nanomaterials 2021, 11, 453. [Google Scholar] [CrossRef] [PubMed]
- Hanifah, M.F.R.; Jaafar, J.; Aziz, M.; Ismail, A.F.; Rahman, M.A.; Othman, M.H.D. Synthesis of kaphene oxide nanosheets via modified hummers method and ıts physicochemical properties. J. Technol. 2015, 74, 189–192. [Google Scholar] [CrossRef] [Green Version]
- Murugan Vadivel, A.; Muraliganth, T.; Manthiram, A. Rapid, Facile Microwave-Solvothermal Synthesis of Graphene Nanosheets and Their Polyaniline Nanocomposites for Energy Strorage. Chem. Mater. 2009, 21, 5004–5006. [Google Scholar] [CrossRef]
- Hu, X.; Xu, P.; Gong, H.; Yin, G. Synthesis and Characterization of WO3/Graphene Nanocomposites for Enhanced Photo-catalytic Activities by One-Step In-Situ Hydrothermal Reaction. Materials 2018, 11, 147. [Google Scholar] [CrossRef] [Green Version]
- Vakhrushev, A.V. Synthesis of WO3 Nanostructures and Their Nanocomposites with Graphene Derivatives via Novel Chemical Approach. In Nanomechanics—Theory and Application; IntechOpen: London, UK, 2021; 146p. [Google Scholar] [CrossRef]
- Tie, L.; Yu, C.; Zhao, Y.; Chen, H.; Yang, S.; Sun, J.; Dong, S.; Sun, J. Fabrication of WO3 na-norods on reduced graphene oxide sheets with augmented visible light photocatalytic ac-tivity for efficient mineralization of dye. J. Alloys Compd. 2018, 769, 83–91. [Google Scholar] [CrossRef]
- Derradji, M.; Mehelli, O.; Liu, W.; Fantuzzi, N. Sustainable and Ecofriendly Chemical Design of High Performance Bio-Based Thermosets for Advanced Applications. Front. Chem. 2021, 9, 691117. [Google Scholar] [CrossRef]

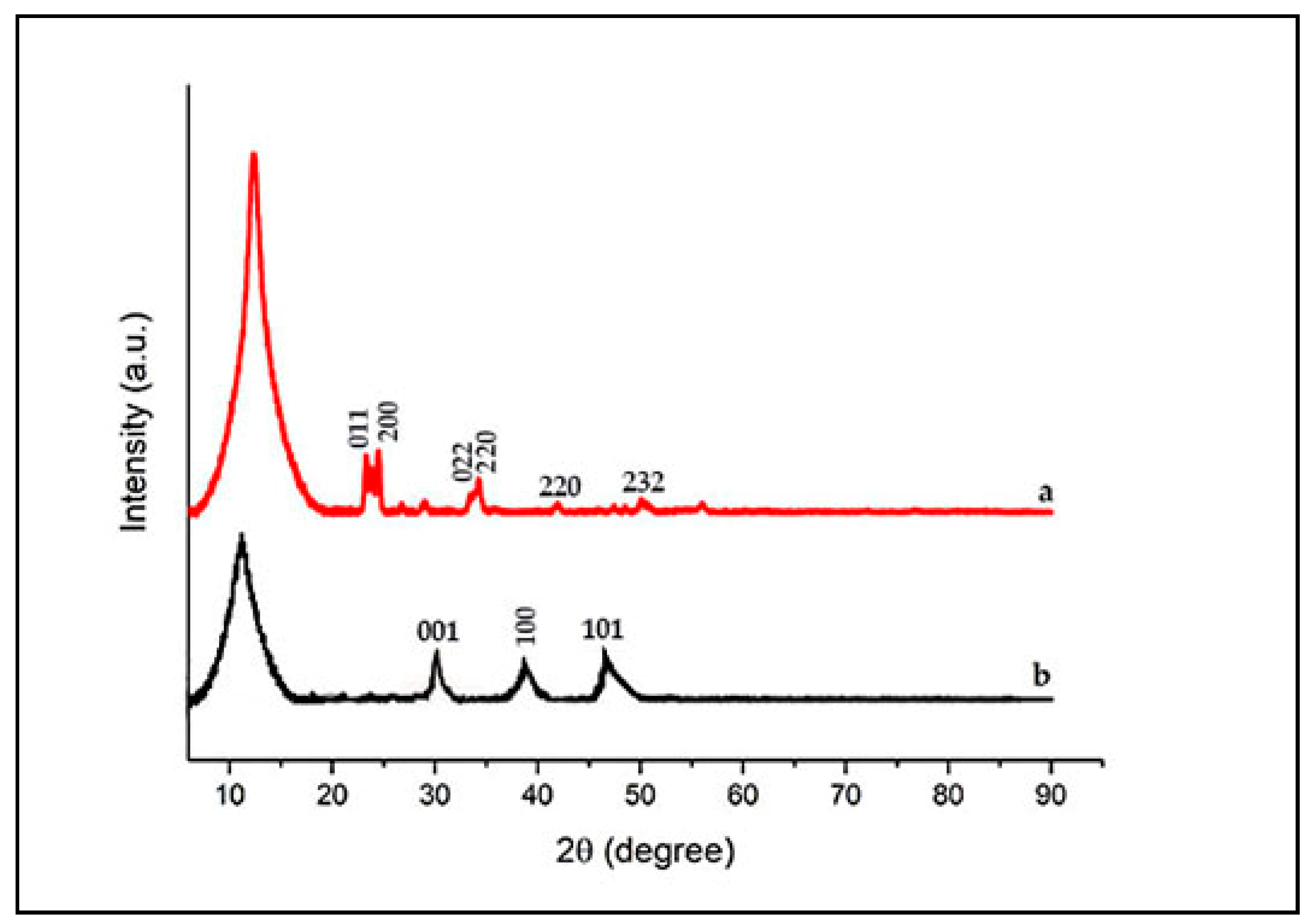
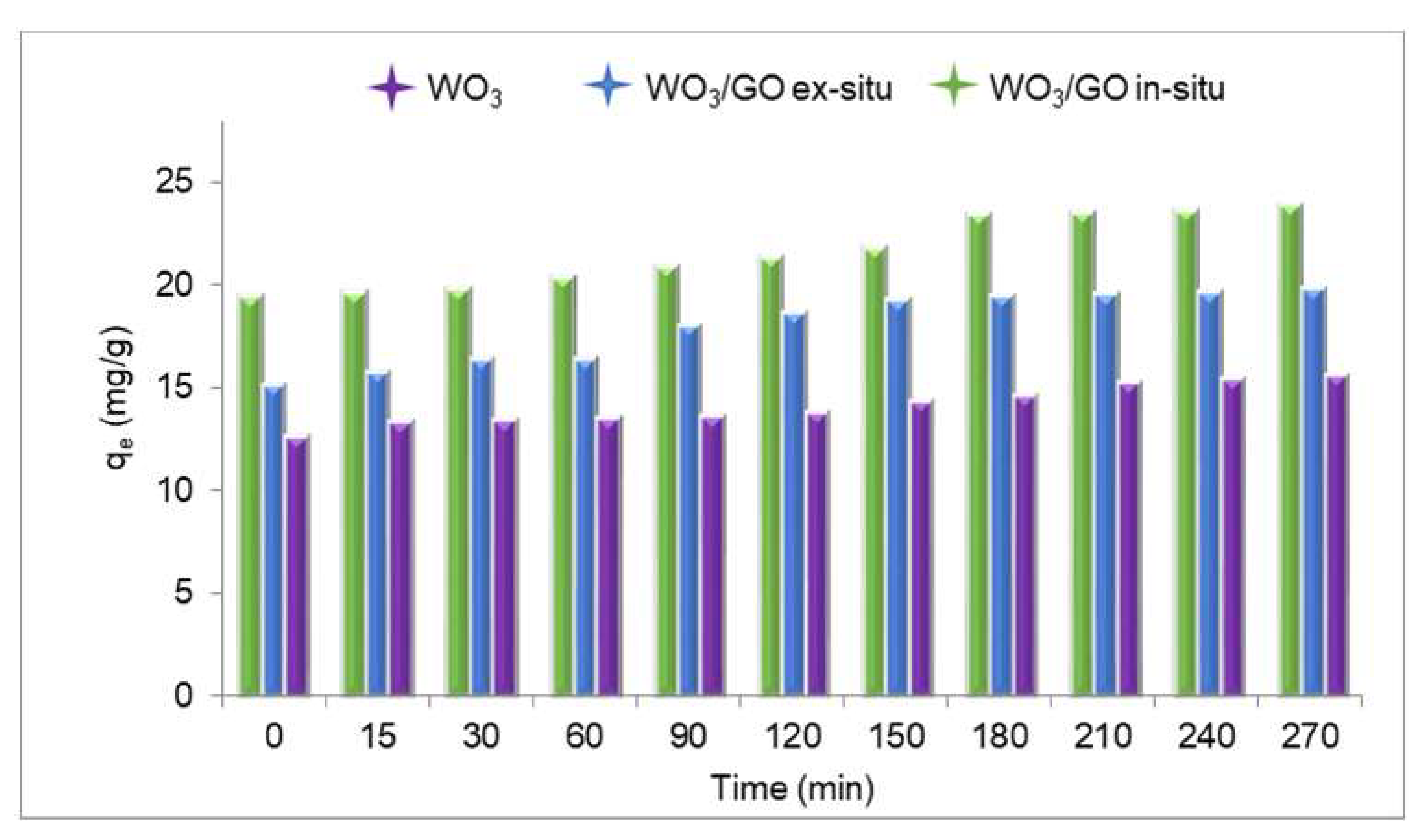

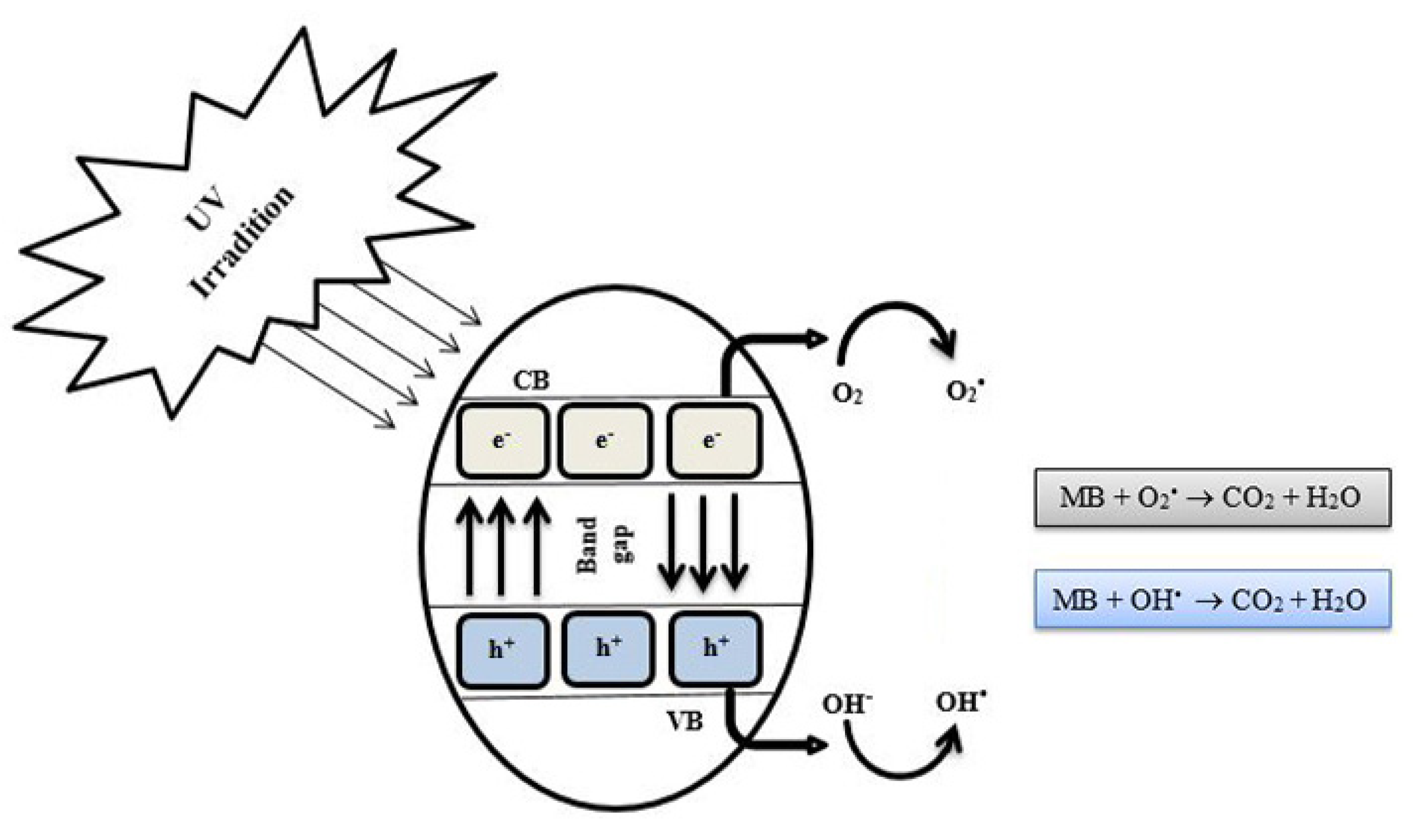
| Samples | % at. | ||
|---|---|---|---|
| Oxygen | Tungsten | Carbon | |
| WO3 | 20.65 | 79.35 | - |
| WC | - | 90.34 | 9.66 |
| WO3/GO ex situ | 41.10 | 8.62 | 50.28 |
| WO3/GO in situ | 26.97 | 16.71 | 56.32 |
| Photocatalyst | Methods of Synthesis | Photodecomposition | Photocatalytic Effciency | References |
|---|---|---|---|---|
| WO3/GO | In situ ex situ chemical oxidation | MB | 96.30% 90.52% | Current work |
| WO3/GO | Ultrasonication Method | MB | 97.03% | [28] |
| WO3/GO | Sol-gel method | MB | 82% | [31] |
| WO3/GO | Photo-reduction method | MO | 92.7% | [32] |
| WO3/GR | Hydrothermal method | MB | 83% | [39] |
| WO3/rGO | In situ slvothermal method | MB | 94% | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esencan Türkaslan, B.; Çelik, A.K.; Dalbeyler, A.; Fantuzzi, N. The Effect of Different Morphologies of WO3/GO Nanocomposite on Photocatalytic Performance. Materials 2022, 15, 8019. https://doi.org/10.3390/ma15228019
Esencan Türkaslan B, Çelik AK, Dalbeyler A, Fantuzzi N. The Effect of Different Morphologies of WO3/GO Nanocomposite on Photocatalytic Performance. Materials. 2022; 15(22):8019. https://doi.org/10.3390/ma15228019
Chicago/Turabian StyleEsencan Türkaslan, Banu, Aziz Kerim Çelik, Ayça Dalbeyler, and Nicholas Fantuzzi. 2022. "The Effect of Different Morphologies of WO3/GO Nanocomposite on Photocatalytic Performance" Materials 15, no. 22: 8019. https://doi.org/10.3390/ma15228019
APA StyleEsencan Türkaslan, B., Çelik, A. K., Dalbeyler, A., & Fantuzzi, N. (2022). The Effect of Different Morphologies of WO3/GO Nanocomposite on Photocatalytic Performance. Materials, 15(22), 8019. https://doi.org/10.3390/ma15228019







