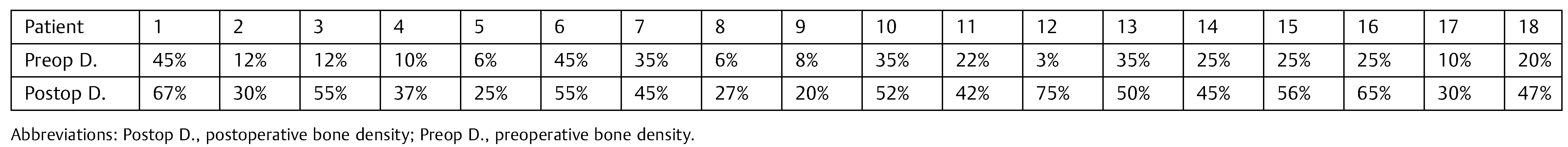Cysts of the jaws are pseudoneoplastic bone lesions characterized by the existence of a cavity lined by an epithelial membrane of odontogenic origin and less often respiratory, and have a liquid or semiliquid contents. This membrane has sometimes added various degrees of inflammation.[
1] Cysts of the jaws are one of the most common pathological processes sit in them, and are clinically important because they are often destructive and produce significant signs and symptoms, especially when are infected. Treatment thereof has been discussed throughout the years in abundance in the literature. Although there are many trends, we can summarize them in the decompression technique,[
2,
3,
4] by which communication of the cyst with the mouth results in decreased volume as a result of the decrease in intracystic pressure. As an alternative to this, there is the total removal of the cyst or enucleation, with or without treatment of residual cavity,[
5,
6] depending on the histological type thereof. Upon resolution of the case, also arise a couple of options. The first one, is the use a bone grafting material for the purpose of achieving higher bone regeneration, such us autologous bone,[
7,
8,
9] bone substitutes [
10,
11,
12,
13,
14] or mixtures of them.[
15,
16,
17] The second option is based on the regeneration and recovery from the clot formed in the first instance [
18,
19] without any addition of filling materials, a method that was chosen to evaluate the effects of bone regeneration achieved after surgical cysts enucleation. There are few publications relating to spontaneous bone regeneration without the use of bone grafting materials after cyst enucleation. These include Santamaría et al.,[
18] Chiapasco et al.,[
19] Yih and Morita,[
20] Di Dio et al.,[
21] and Zhao et al.[
22] The specific objective of this retrospective randomized clinical study is to evaluate the spontaneous osteogenesis after cysts enucleation of the jaws, through radiographs using a new computerized method which in turn facilitates the monitoring of this type of pathology after surgery.
Materials and Methods
In this retrospective randomized clinical study, we selected a group of 18 patients with cysts of the jaws, chosen by random number table among a total of 30 patients treated in similar conditions from year 2009 to 2012. All patients were selected based on the following inclusion and exclusion criteria:
Inclusion criteria: (1) Cyst in maxilla or mandible, (2) treatment by enucleation without using bone grafting materials, and (3) radiograph control at least 6 months after surgery.
Exclusion criteria: (1) Pregnancy, (2) aggregate systemic pathologies such as diabetes, thyroid disorders, bone metabolism diseases, among others, and (3) patients taking calcium, bisphosphonates, glucocorticoids, or other drugs that can interfere with bone metabolism.
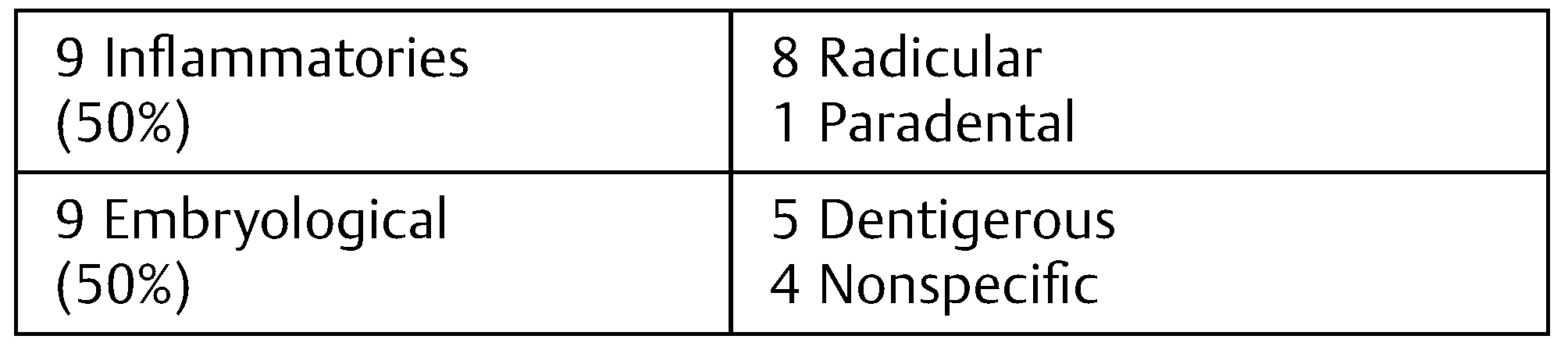
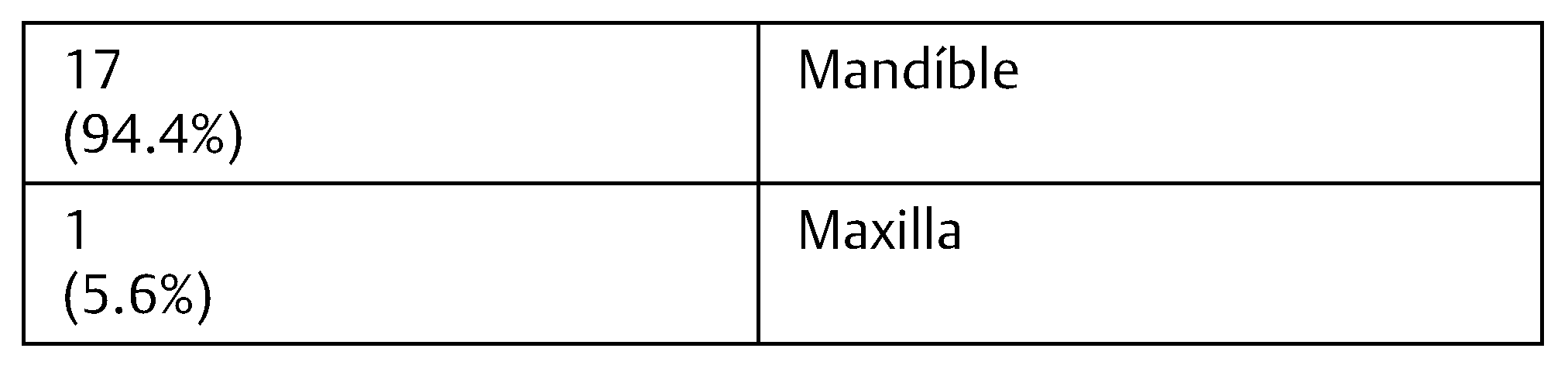
The sample consisted of a group of 18 patients with 8 to 65 years with an average of 31.8 years (standard deviation: 18.88). Of the 18 patients, 11 were men (61.1%) and 7 women (38.8%). Nine (50%) had inflammatory cysts and nine (50%) embryological cysts, all of them of odontogenic origin. Seventeen (94.4%) of the patient had lesions that affected the mandible (
Table 1 and
Table 2). All cases were compared with respect to spontaneous bone regeneration, patient age, and cyst histological type using an analysis of variance with InfoStat Program (InfoStat Software, 2012 version, Córdoba, Argentina).
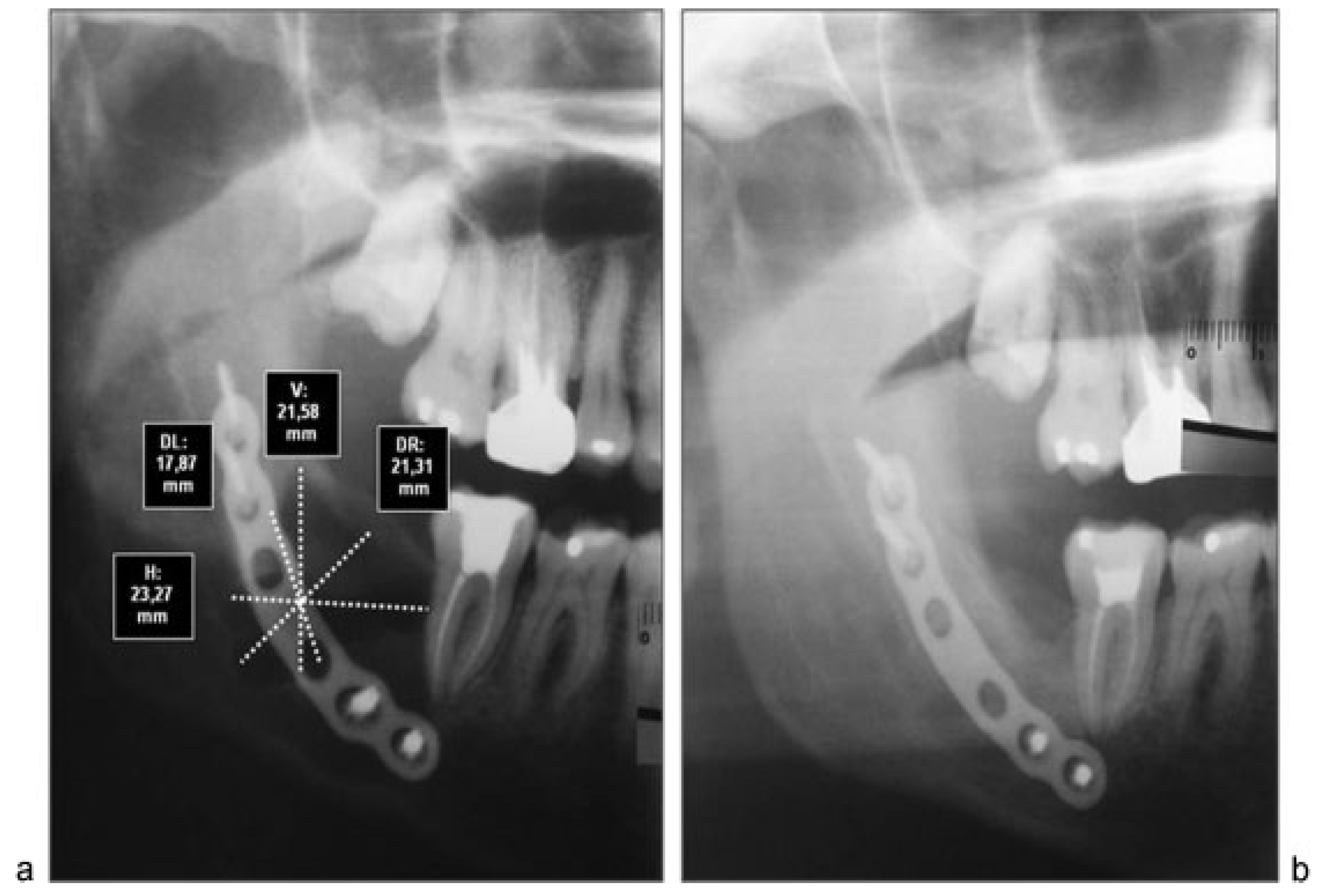
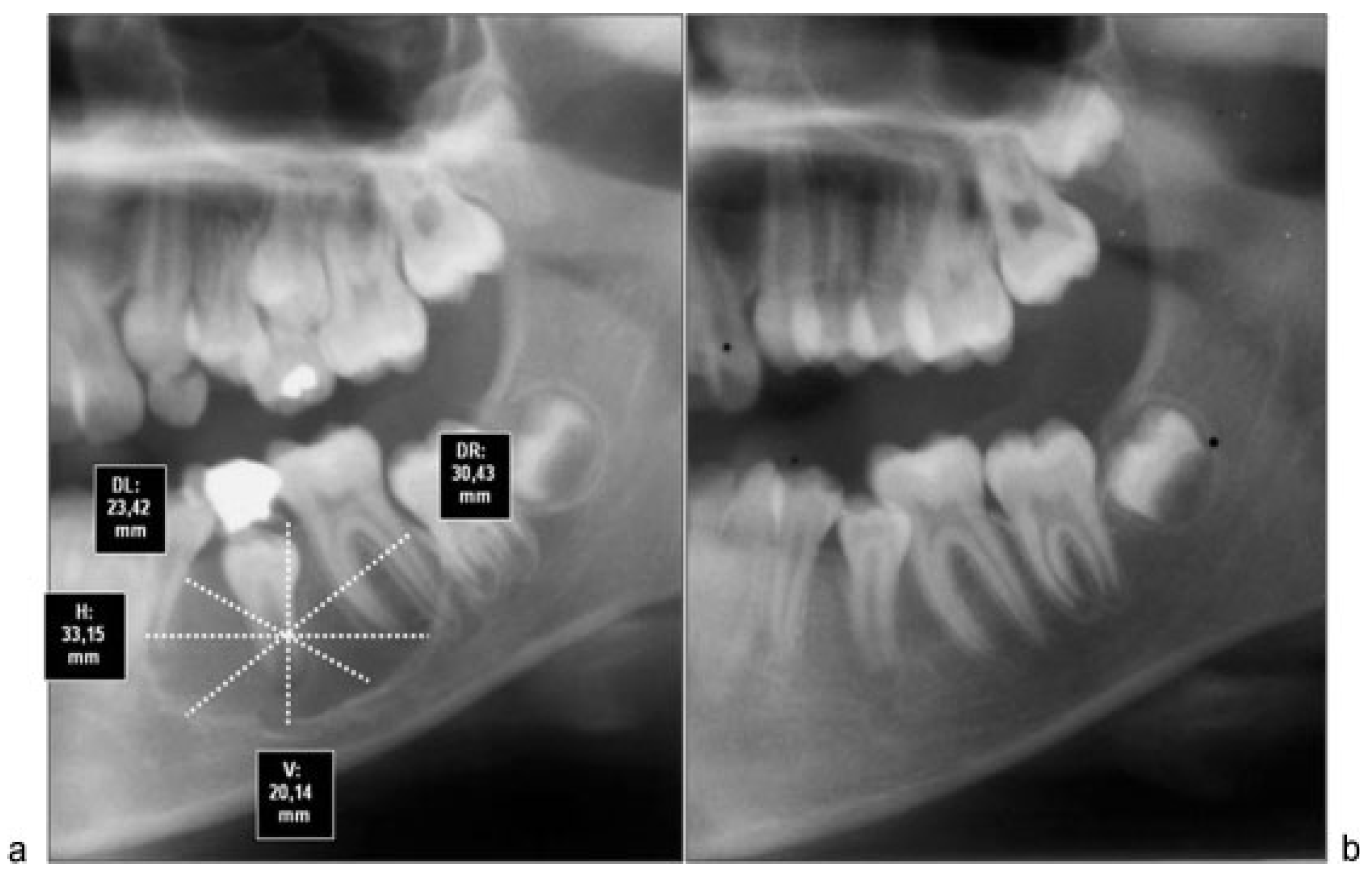
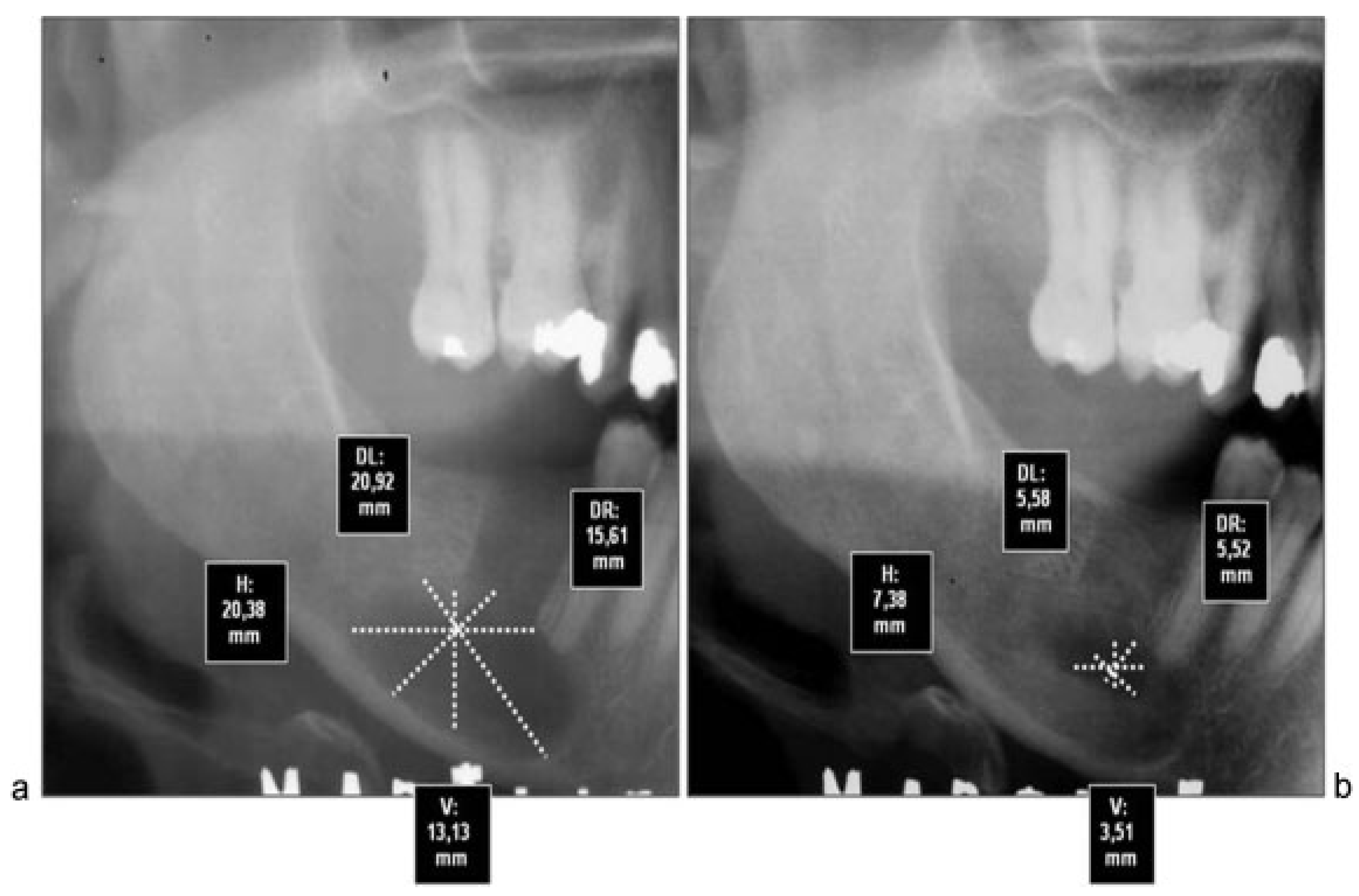
All cases were treated by enucleation and primary closure of the wound, with antibiotic treatment for 7 to 10 days by the same senior surgical team. Radiographic control was performed after 6 months in all patients, with an average time of control of 8.8 months. To objectively evaluate the magnitude of the bone regenerative process in the postoperative time, we proceeded to scan preoperative and postoperative radiographs with the program Nemoceph—NemoStudio NX Pro— Nemotec. The way to standardize the measurements of the radiographs of each patient was to assign parameters to the program’s tools, based on a linear measure of it, magnify it, or reduce it possible to place life size.
Both preoperative and postoperative radiographs from each patient were taken at the same radiographic institute. Once parameterized proceeded to take the following linear measurements in mm (
Figure 1):
Horizontal true that passes through the Ecuador thereof.
Vertical true that passes through the axis thereof.
Diagonals passing through the intersection of the two preceding ones.
We proceeded to measure each of these lines to its intersection with the outer limits of the cyst. These measurements were repeated in both preoperative and postoperative radiographs. After that we proceeded to assess how much had changed each of the measures, purchased with the preoperative. Finally, we calculated the percentage of variation in all patients. In addition, preoperative and postoperative radiographic densities of each patient were assessed with the Nemoceph Densitometric Tool—NemoStudio NX Pro—Nemotec at the point of intersection (PI) of the lines mentioned earlier.
Results
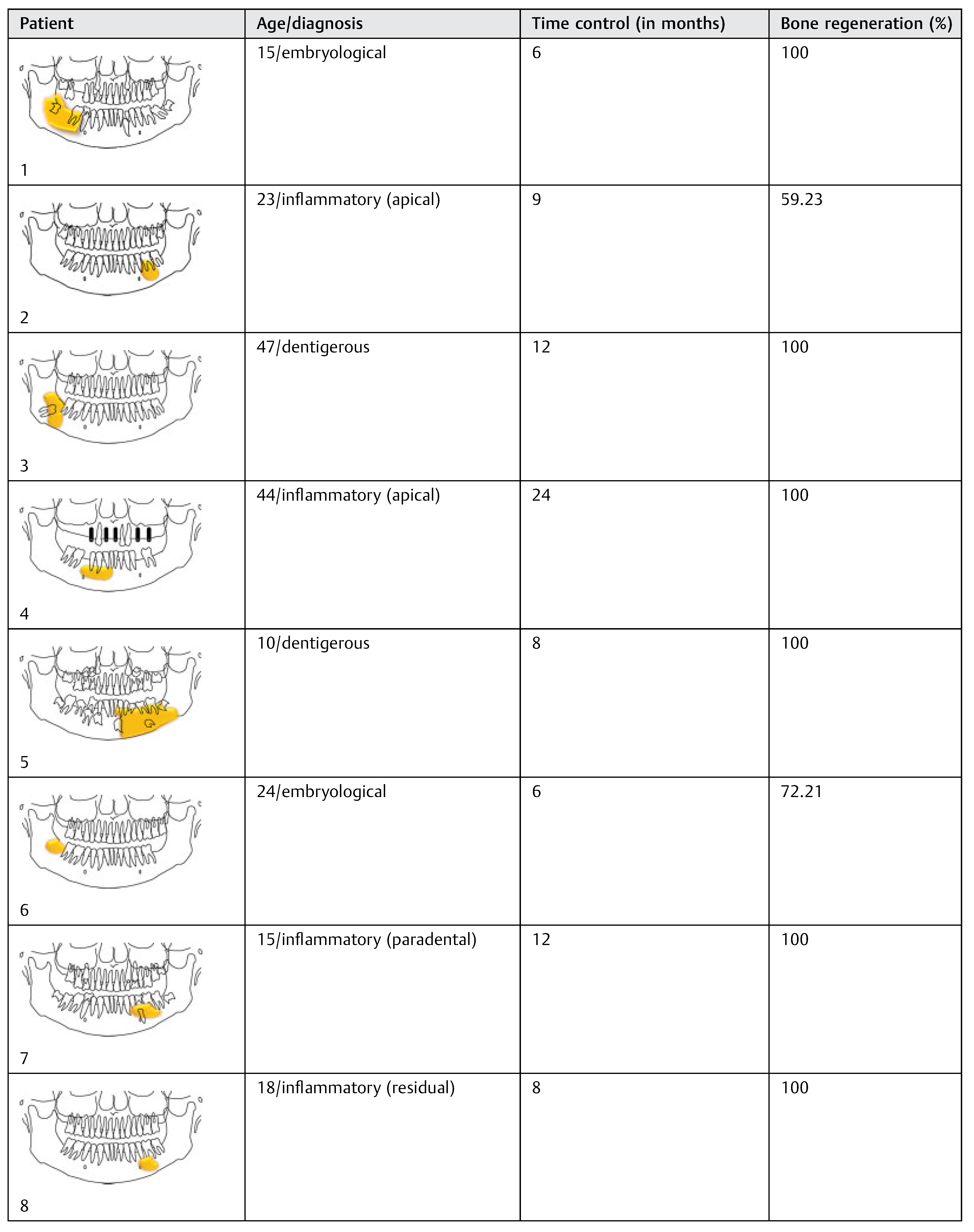
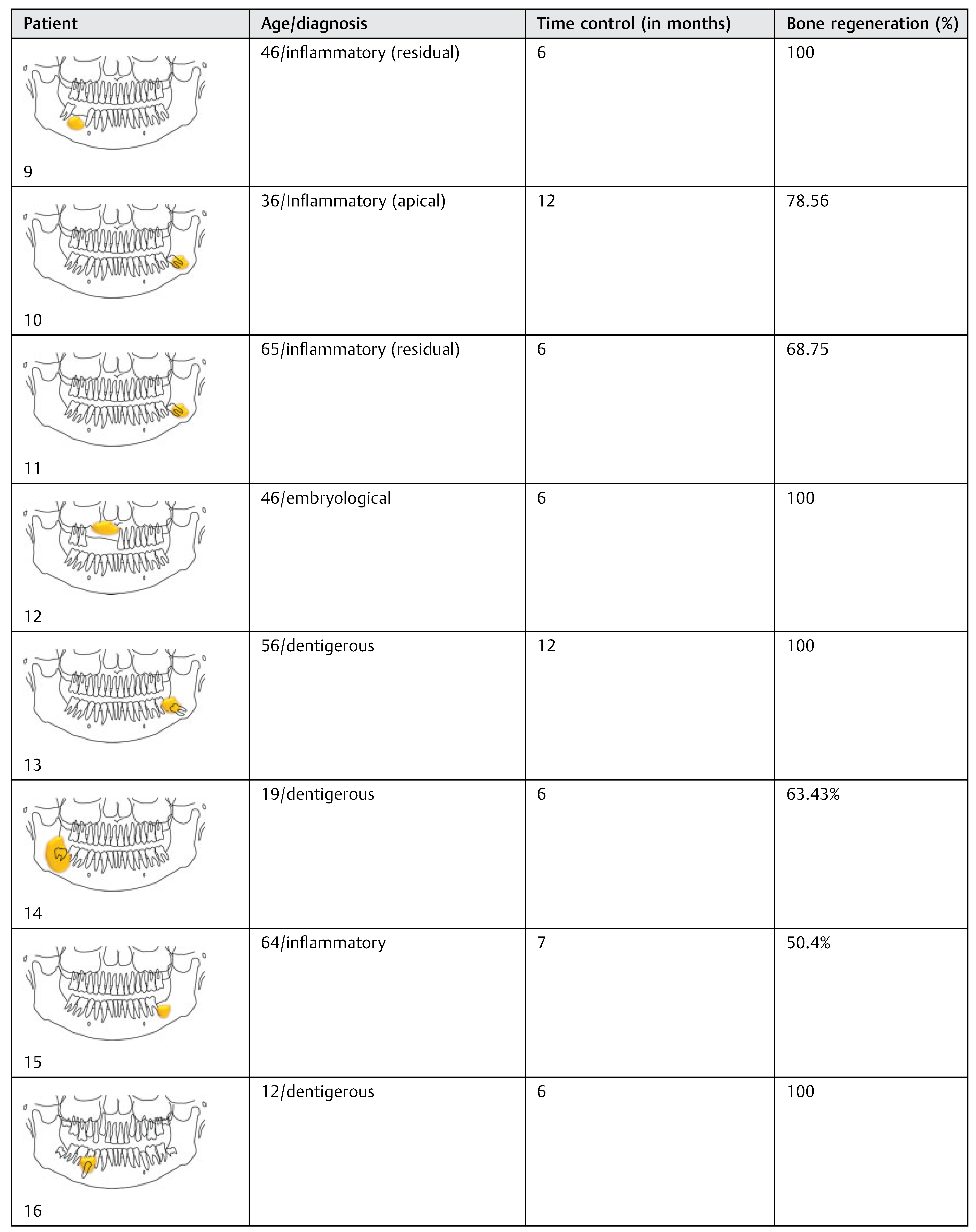
The 18 patients were divided according to age, histopathologic diagnosis, postoperative control time, and percentage of bone regeneration (
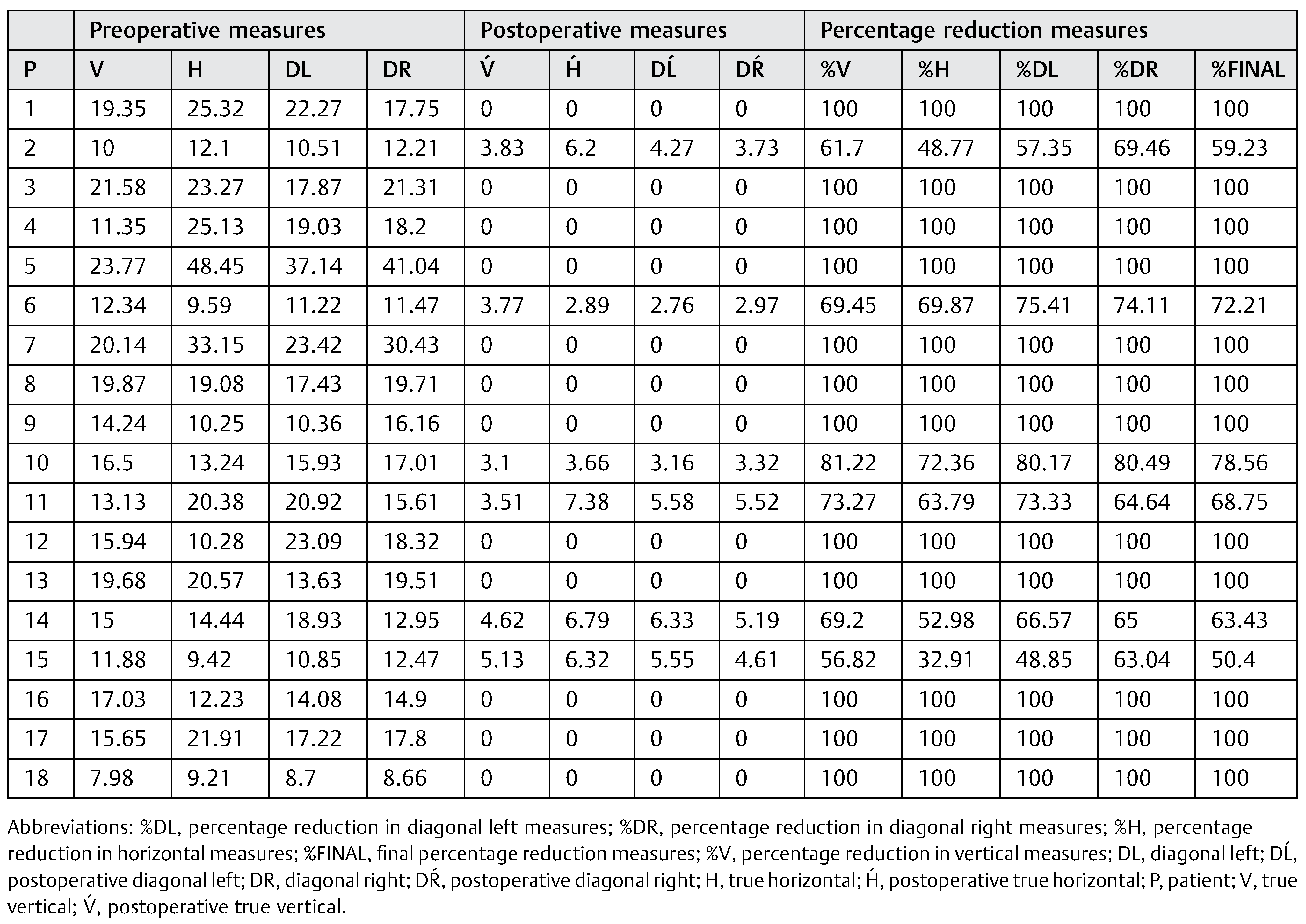
Table 3). The sample analysis showed an average decrease of 85.59% in the horizontal measures, 89.53% in the vertical, and 88.98 and 89.81% in the left and right diagonal, respectively. The percentage of reduction was greater in diagonal and vertical measures compared with the horizontal measures, with an overall average reduction of 88.47%. Twelve patients (66.6%) had 100% bone regeneration after 6 months of cystic enucleation. In the remaining six patients, bone regeneration was approximately 50.4 to 78.56% with an average of 65.43%. In four cases (patients 2, 11, 14, and 15) one of both bone plates (buccal or lingual) were destroyed by the lesion; therefore, bone regeneration was clearly lower (
Table 3 and
Table 4).
Regarding the percentage of spontaneous bone regeneration, we did not find statistically significant differences between embryological and inflammatory cysts after cystic enucleation (t-test; p = 0.66). Similarly, there was no difference between young and adult patients (t-test, p = 0.44).
According to this study, it seems to be that cyst histology, cyst size, and age have no statistically significant influence in bone regeneration percentage, as long as bone plates be conserved after cysts enucleation. However, it is important to note that the sample is very small with large data dispersion.
Changes in bone density showed an increase in postoperative radiographs in relation to preoperative radiographs at PI, according to the values obtained with the Nemoceph Densitometry Tool—NemoStudio NX Pro—Nemotec in all patients of the sample (
Table 5).
Discussion
Healing by primary closure of the wound after cysts enucleation has been used very successfully for a long time. Bone regeneration seems to be possible while periosteum is maintained to allow this. Also, endosteum seems to have a leading role in providing sufficient osteoprogenitor cells for spontaneous bone regeneration from residual defect’s walls.
In a controlled clinical prospective randomized trial by Santamaría et al.,[
18] no statistically significant differences have been found between bone regeneration with and without the use of membranes (guided tissue regeneration) after enucleation of inflammatory radicular cysts in 30 patients. Chiapasco et al.[
19] have reported 81.30% reduction of the residual cavity and 91% increase in bone density, at 24 months of control in a study of 27 patients with cysts larger than 40 mm without the use of bone grafting materials. Nevertheless, it has been well established that if both bone plates (buccal and lingual or palatal) were destroyed by the lesion, the area would not completely fill with bone, but leave a residual fibrous “scar” as manifested by a radiolucency.[
18,
19]
In recent years, from the evolution of implantology, and with the appearance of better quality biomaterials begins to be a tendency for the resolution of these processes through the use of them. Who mostly are inclined to this type of treatment, usually made considering the subsequent dental reconstruction using dental implants. Same surgeons prefer the regenerated native bone instead grafted bone at the time of implant placement. However, the nonuse of bone grafts does not preclude the subsequent resolution with implants.
Although the use of platelet rich plasma (PRP) in conjunction with autologous bone grafts has been well studied and the literature reports encouraging results with it use in bone defects,[
23] the use of recombinant human bone morphogenetic protein-2 (rhBMP-2) alone or in combination with bone grafts is still under study.[
24]
Spontaneous bone regeneration of residual cavities in the jaws seems to have several biological foundations undoubtedly complex. The restitution of the integrity of any tissue depends on the type of involved tissue and the nature of tissue damage. When restitution occurs by means of tissue that is structurally and functionally indistinguishable from native tissue, regeneration has taken place. However, if tissue integrity is re-established primarily through the formation of fibrotic scar tissue, then repair has occurred. Repair by scarring is the body’s version of a spot weld and the replacement tissue is coarse and has a lower cellular content than native tissue. With the exception of bone and liver, tissue disruption invariably results in repair rather than regeneration. At the cellular level, the rate and quality of tissue healing depend on whether the constitutive cells are labile, stable, or permanent. Labile cells, including the keratinocytes of the epidermis and epithelial cells of the oral mucosa, divide throughout their life span. Stable cells such as fibroblasts exhibit a low rate of duplication but can undergo rapid proliferation in response to injury. For example, bone injury causes pluripotential mesenchymal cells to speedily differentiate into osteoblasts and osteoclasts. On the contrary, permanent cells such as specialized nerve and cardiac muscle cells do not divide in postnatal life. The surgeon’s expectation of “normal healing” should be correspondingly realistic and based on the inherent capabilities of the injured tissue. Although a fibrous scar is normal for skin wounds, it is suboptimal in the context of bone healing.[
25]
Tissue healing takes place by the action of more than 40 growth factors with effects on many general and specific cell types. These proteins or peptides released locally, influence cellular functions by binding to cell membrane receptors. This interaction between growth factors and membrane receptors induce intracellular signal transduction specific, like a waterfall, which target gene expression, such as nuclear factor kappa B involved in the healing process wounds. The bone heals through the three phases of the normal wound healing. Once the cyst enucleated, start an inflammatory phase, where the blood clot will serve as biological scaffold for migration and proliferation of inflammatory cells initially, and subsequently bone through various growth factors such as interleukin-1, platelet-derived growth factor, tumor necrosis factor (TNF), and TGF β (transforming growth factor β), among others. The proliferative phase is characterized by angiogenesis from preexisting vessels into the center of the clot, and transformed into the granulation tissue. This angiogenesis is stimulated by early wound hypoxia, which through growth factor induces a balance between mitosis and apoptosis of endothelial cells, giving rise to outbreaks of new blood vessels. The growth factors that induce apoptosis include TNF, TGF β and those that induce mitosis include vascular endothelial growth factor, basic fibroblast growth factor, and nitric oxide.[
26,
27]
Remodeling phase is where the bone differs from other tissues, as it should mineralize their matrix, at the expense of providing osteogenic cells in the periosteum and endosteum in intimate contact with the defect. This is where it carries out the regeneration process due to its stable cell populations stimulated by injury, cystic enucleation in this case. Initially, there is osteoid tissue, which then is mineralized to form plexiform or immature bone, which will be replaced over time by laminillar or mature bone.[
28,
29]
Notably, the immature bone differs from mature histologically, not by having a lower degree of mineralization, but by an irregular distribution of collagen fibers of bone extracellular matrix. This gives a more disorganized mature bone, and therefore their tissue resistance is lower. The replacement of the immature by mature bone results from a complex cellular phenomenon known as bone remodeling balance, which is possible thanks to the bone metabolic unit, a mechanism of interplay between cells with blastic and clastic activity regulating the process through chemical mediators. The main mechanism mediating this process is via RANK/ RANKL. The RANKL (RANK ligand), a protein with an active site on the plasmatic membrane induces osteoclast bone resorption, which exposes the BMP and insulin-like growing factors 1 and 2, three proteins with a receptor on the osteoblast plasmatic membrane, inducing osteogenesis. In turn, there would be a downregulation of this process by osteoprotegerin, another protein released by osteoblasts that inhibits the clastic action directly competing with RANKL, thus reducing the range of bone resorption. This complex biological mechanism on balance seems to be in charge of allowing bone tissue remodeling in physiological conditions.[
29,
30]
Regarding that to the date there are not clinical or images signs that indicate when a cavity must be grafted, the blood clot seems to be an excellent natural biological filling of the residual cavities, when is able to conserve residual bone walls provided by periosteum and endosteum to protect it. In our series of 18 patients, we did not find any blood clot infection after primary closure of the wound, even in large residual cavities.
Although computed tomography provides better information about bone regeneration after surgery, to prevent high radiation dose panoramic radiographs can be considered enough for the purpose of postoperative control, so that we choose this last option (
Figure 2a,b,
Figure 3a,b and
Figure 4a,b).
It seems that in younger patients a greater percentage of bone regeneration is achieved, so that in such cases bone grafting materials could be dispensable. In our series, six of seven patients younger than 20 years old showed 100% of bone regeneration. However, we found no statistically significant difference in this regard.
To avoid subjectivity in deciding whether bone tissue regenerated or not, we proceeded to perform the battery of measures outlined earlier. We did not chose to calculate the surface thereof, as the cysts forms adopted not always fall within a recognizable geometric shape, so we decided on linear measurements. While not attempted any scientific proof about them because of a very small sample size, their use could give us a simple tool that allows mathematically evaluate the regeneration of bone tissue after cyst enucleation. The biological mechanisms described in the literature seem to explain the reasons for which the bone has a great regenerative and remodeling capacity from the blood clot. Spontaneous osteogenesis after cystic enucleation seems to be feasible, and the measurements described in this article provide a simple method that objectifies complementing the clinical results.
In summary, residual bone defects after cystic enucleation seems to regenerate spontaneously despite size of the cavity, histological type, and age, when it is able to conserve residual bone plates provided by periosteum and endosteum.
Nevertheless and whereas the small sample size, the retrospective character and the absence of three-dimensional measures in this study, further randomized double blind trials are needed to allow a decision based on evidence, when choosing a therapeutic approach regarding the use or nonuse of bone grafting materials in residual bone defects.