Radiation injury to tissues occurs as a result of damage to vascular endothelium causing obliterating endarteritis and leading to a hypoxic, hypocellular, and hypovascular state that is characterized by fibrosis and bone necrosis [
1,
2]. Osteoradionecrosis (ORN) can be defined as a condition in which there is an area of exposed, nonviable bone in an irradiated field that has failed to heal with conservative management after a period of 3 months or more [
1,
3]. There are different schools of thought about the etiology of this condition and equally advocates of different techniques to manage it [
3,
4,
5,
6]. What is clear is that ORN is a very difficult condition to treat, and it has been the issue of much debate due to its unpredictable clinical course.
Defect bridging with a reconstruction plate alone is bound to fail sooner or later because of metal fatigue leading to plate fracture [
7]. In ours and most other centers, the free fibula osteocutaneous flap has become the working horse for segmental reconstruction of the mandible [
8,
9,
10,
11]. However, circulatory conditions at the donor site, previous trauma or surgery may contraindicate the application of a fibula flap. Also, extensive and complex, intra-/extraoral soft tissue defects may render a fibula osteocutaneous flap less suitable. The free osteocutaneous iliac crest flap or the free osteocutaneous scapular flap may be used as alternatives, however, these flaps can have limitations in supplying the necessary combination of bone and soft tissue, and both have a significant donor site morbidity.
In the irradiated patient, healing conditions for a free nonvascularized bone graft are less than optimal due to poor vascularity. These issues prompted us to seek new ways to create a recipient bed with adequate vascularity to receive a particulate iliac bone graft with the success of primary healing and subsequent functional bone remodeling.
The technique described in this article introduces the idea of bringing well-vascularised new tissue into a previously irradiated, hypovascular mandibular region by means of a latissimus dorsi (LD) musculocutaneous flap wrapped around the reconstruction plate thus forming a recipient site of nonirradiated, well-vascularised soft tissue that can cover both the intra oral defect with secondary epithelization of the LD muscle, and the external defect with the skin pad, and subsequently promote healing of a free particulate bone graft. The technique may be indicated in patients with short bony defects or in other situations where a fibula flap or an alternative osteocutaneous flap has been deselected. Extensive and complex intra/extraoral soft tissue defects represent another indication for the LD myo-cutaneous flap. Restoration of mandibular body height is an additional benefit from this technique, which favors optimal jaw relations for implant supported dental rehabilitation.
Patients and Methods
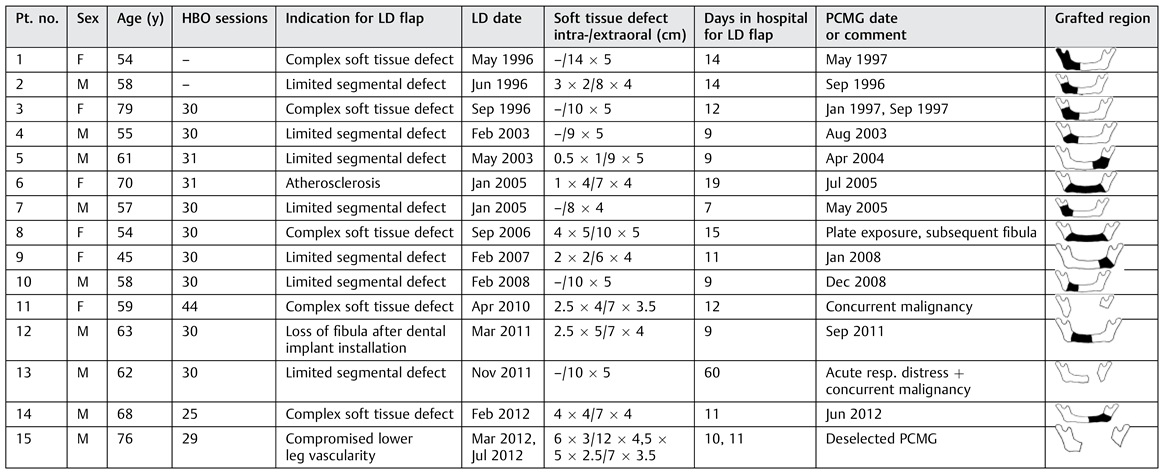
A total of 15 patients, 6 females and 9 males, median age 61 years (range, 46–79 years), were operated on with the LD musculocutaneous flap. Twelve patients underwent a subsequent iliac particulate bone reconstruction during the 16-year period from 1996 to 2012 (
Table 1). The method was selected for the following reasons: bone defect to be bridged less than 5 cm and/or reconstruction of mandibular height essential (
n = 5), compromised vascular supply to lower extremities (
n = 4), size of soft tissue defect (
n = 5), and previous fibula flap lost due to infection after implant treatment (
n = 1).
The protocol of care we describe here essentially involves five stages of treatment consisting of (1) hyperbaric oxygen (HBO) therapy, (2) resection of the affected area, and a two-stage surgical reconstruction consisting of (3) soft tissue reconstruction, and (4) reconstruction with particulate nonvascularized bone, and finally (5) prosthodontic rehabilitation.
In three patients, bone reconstruction was deselected, in two patients due to concurrent malignancy, and one patient refused further surgery.
Hyperbaric Oxygen
At our center comprising the concerted efforts of Plastic Surgery, Oral and Maxillofacial Surgery, and Anaesthesiology, a course of HBO therapy was prescribed to ORN patients facing major surgery regardless of the choice of reconstruction method until 2007 (where after a randomized study HBO/non-HBO was initiated). Of the 15 patients, 13 patients in this sample completed a regimen of 25 to 44 consecutive dives on weekdays, mean 30.7. Each dive was of 90 minutes duration at 2.4 atmospheric pressure (equivalent to a pressure of 16–18 m below sea level). Patients were breathing 100% oxygen through masks or helmets [
12].
Resection
In patients with pathological fracture of the mandible or extensive ORN involving the base of the mandible, a segmental resection causing loss of mandibular continuity was inevitable. We favored a regimen of maintaining midline symmetry and dental occlusion by means of external pin fixation or dental arch bars with elastic traction to prevent collapse toward the resected side thus leaving the resected site clear of foreign bodies such as a reconstruction plate to reduce the risk of further infection.
Reconstruction, Stage 1—Soft Tissue Cover
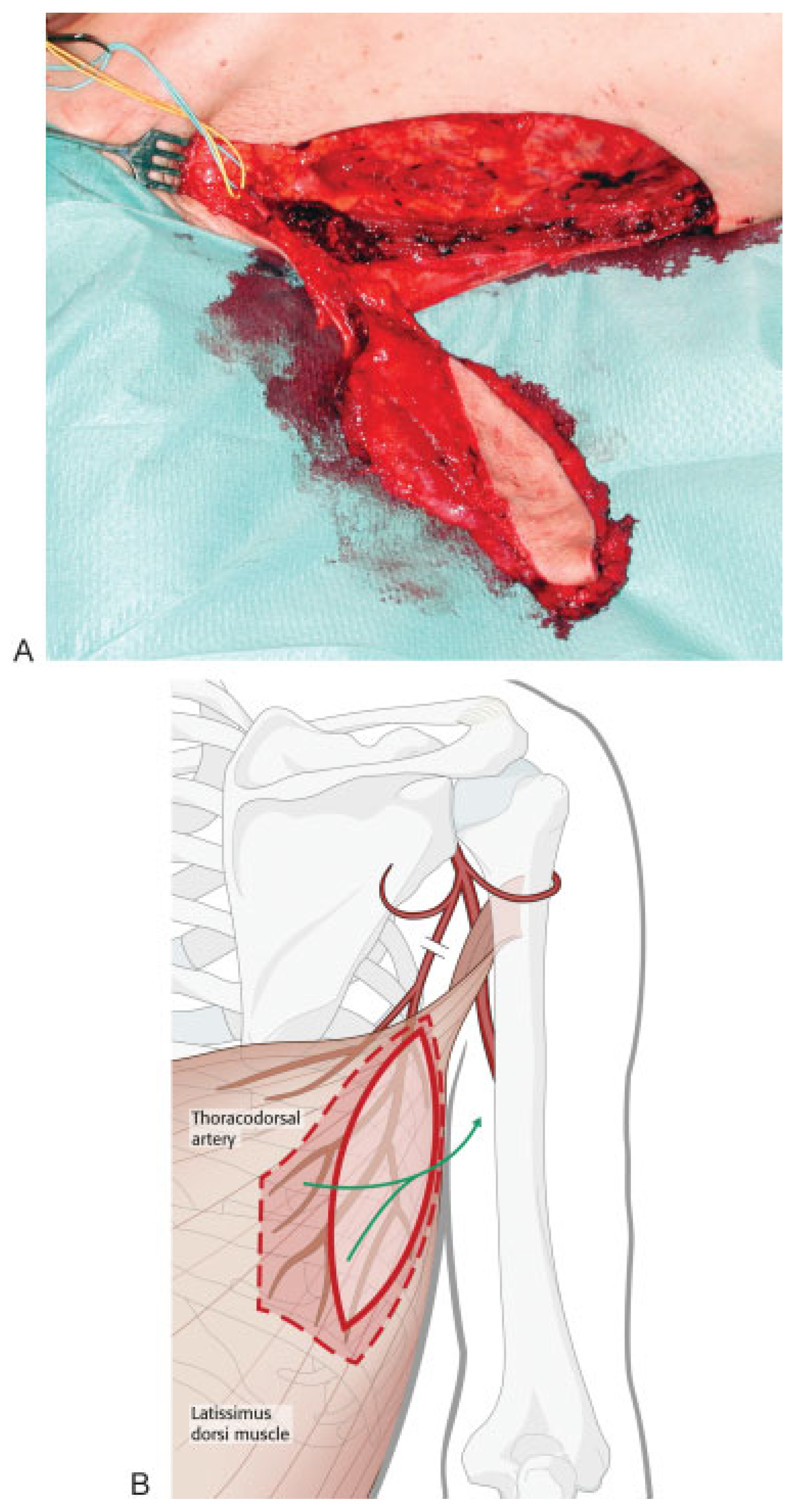
A musculocutaneous LD flap is harvested with the patient in a supine position, the shoulder and hip slightly elevated and the donor arm raised on the side where the flap will be harvested from. We prefer nasoendotracheal intubation for this procedure. Two teams can work simultaneously in this manner—one preparing the recipient site and donor vessels, and the other harvesting the flap.
The superior thyroid artery and a suitable vein are identified to be used as recipient vessels. If an external defect is present, the size is measured and an equally sized skin island is drawn overlying the anterior border of the LD muscle. The flap is harvested together with a muscle fan 1 to 2 cm wider and longer than the defect to be covered in the mandible. This is illustrated in
Figure 1.
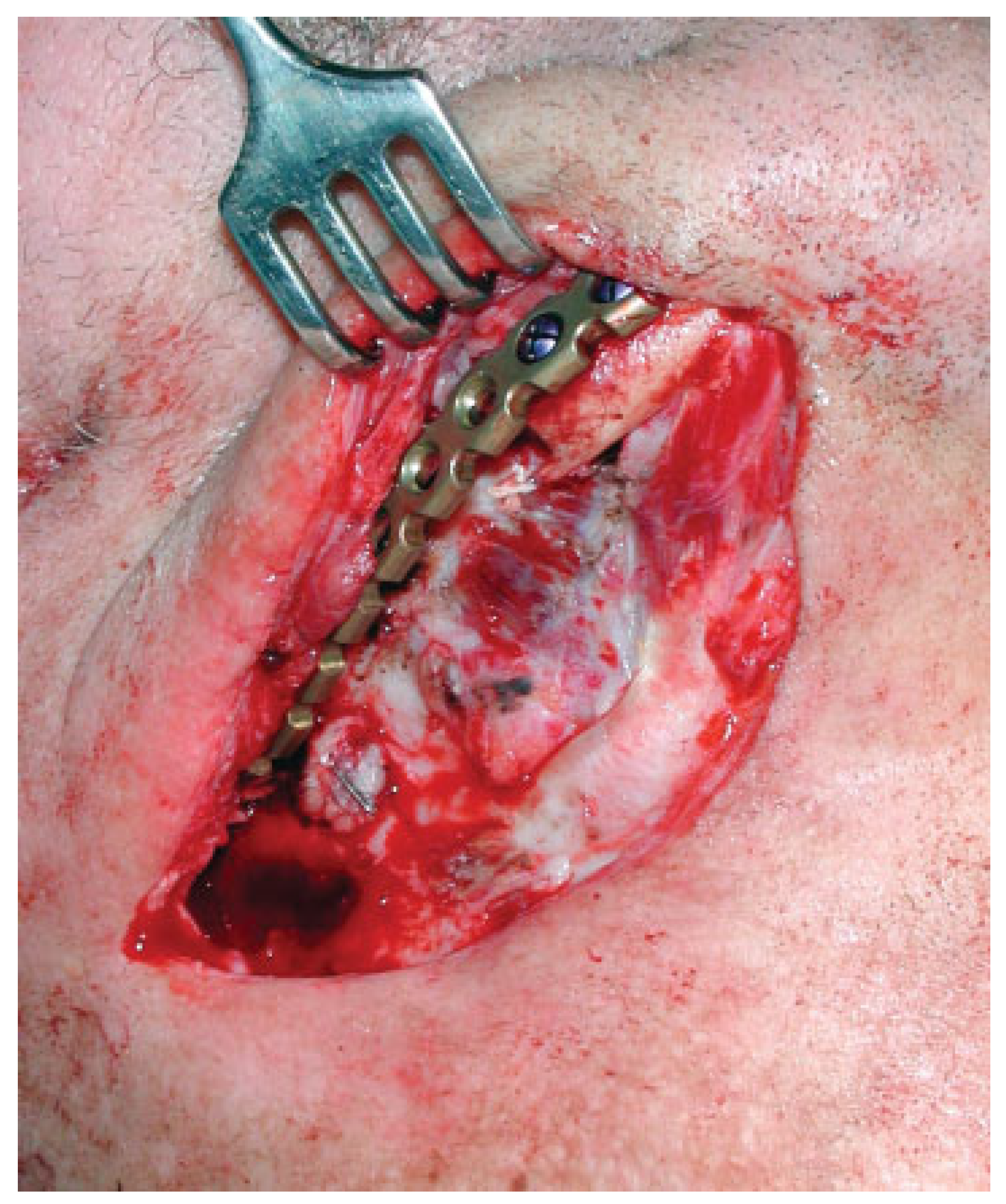
A submandibular incision is made well beneath the body and angle of the mandible to expose the site to be reconstructed, avoiding the mandibular branch of the facial nerve. Supplementary resection is performed if needed. In dentate patients, the teeth are brought in occlusion and the mandible is immobilized through maxillomandibular fixation. A reconstruction plate is then fixed onto the mandible, preferably with three screws placed on either side of the resection stumps according to recognized AO principles [
13] (
Figure 2). The flap is inserted as a muscle fan with the skin island covering the external defect and the internal aspect sutured under the mucosal defect if present. The muscle fan is wrapped around the plate and the mandibular stumps, and sutured in place (
Figure 3). The oral soft tissue wound will shrink and epithelize quickly obviating the need for a skin graft.
Reconstruction, Stage 2—Bone Grafting
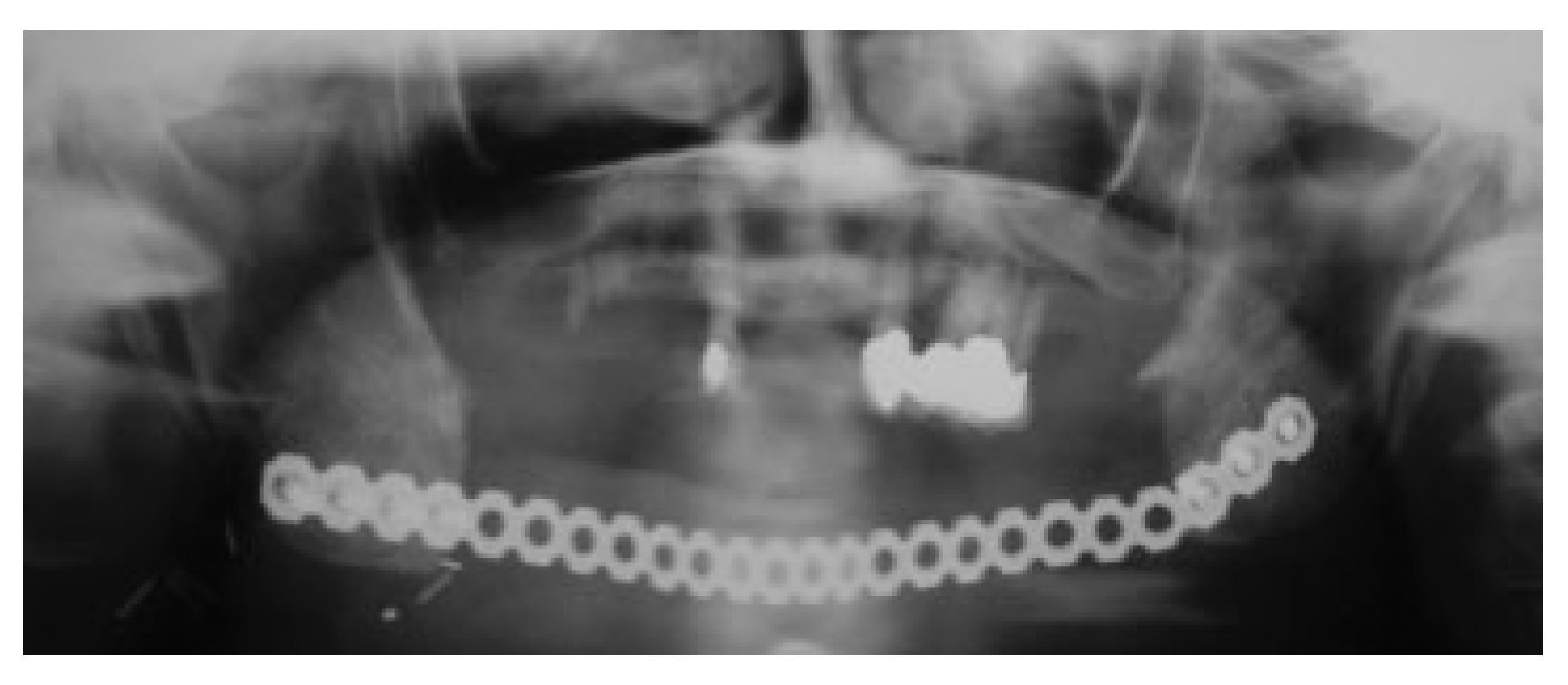
Usually 3 to 6 months after soft tissue reconstruction, the resection site is re-entered from a submandibular approach and dissected, by identifying the plane between the two layers of the muscle wrap to open a recipient site pouch.
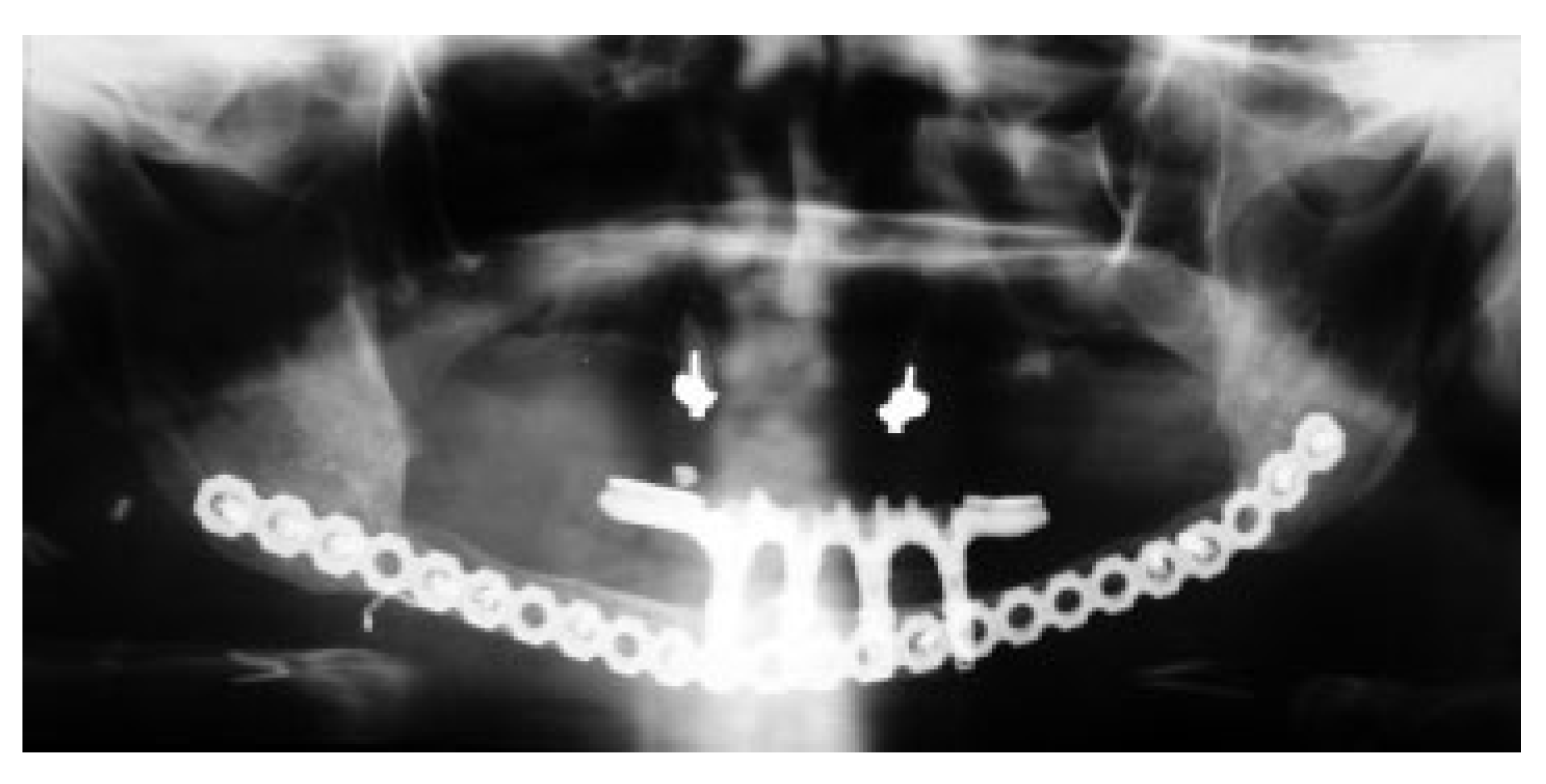
Figure 4 shows en example of an extensive resection with immediate insertion of a reconstruction plate and a LD flap. Six months later, the resection stumps were exposed, and the defect was grafted with a strut of cortical bone that acts as a sheet pile wall for the particulate corticocancellous bone harvested from the ileum. Platelet-rich autologous fibrin glue may be added to help containment and molding of the bone graft mass [
14] (
Figure 5).
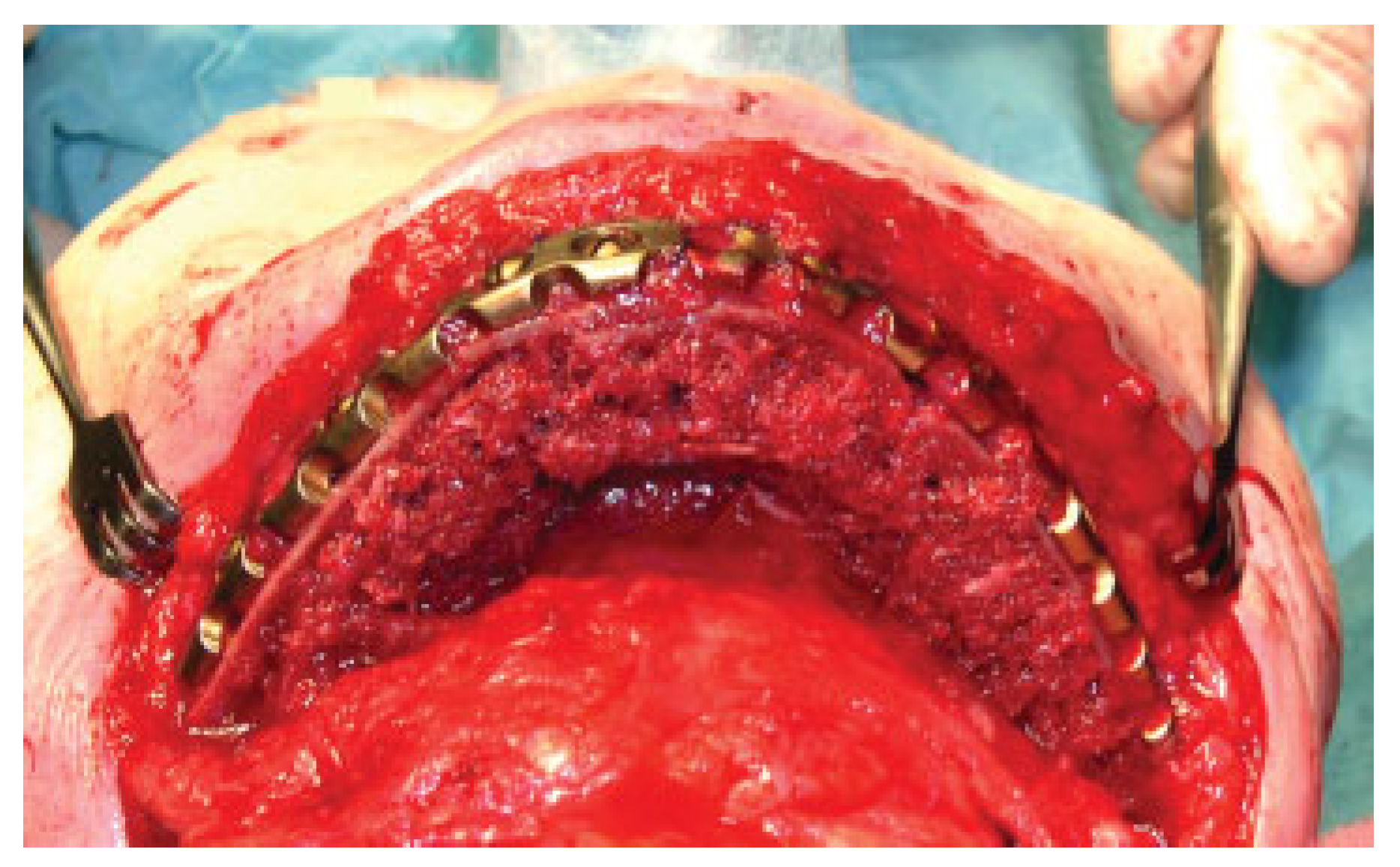
Prosthodontic Rehabilitation
Approximately 4 months later, implants may be inserted into the matured bone graft according to a treatment plan conceived in collaboration with the maxillofacial prosthodontist who will conduct the subsequent rehabilitation with an implant supported dental prosthesis (
Figure 6 and
Figure 7).
Results
Ten patients met the success criteria of uneventful graft healing with restitution of osseous continuity, full or partial restoration of mandibular height, symmetry and function, and avoidance of reconstruction plate fracture. Combined intra- and extraoral soft tissue defects were managed with the soft tissue flap. In one patient, the iliac graft eventually resorbed (
Table 1, patient no. 2), and a subsequent fibula reconstruction was performed. In another patient (no. 8) soft tissue shrinkage and plate exposure precluded reconstruction with iliac particulate bone, and a fibula osseocutaneous flap was applied.
Endosseous dental implants were installed in the reconstructed area for successful implant fixed dental restoration in three patients (nos. 6, 12, and 14). The remodeling of bone during healing and function of the reconstructed mandible seemed to enhance a change of morphology of the grafted corticocancellous and particulate bone into a tubular bone with an outer cortex very similar to the pristine mandible. Whereas the grafted bone followed the mandibular horseshoe shape when grafted to the anterior region (
Figure 5,
Figure 6 and
Figure 7), a reconstruction of the angle of the mandible proved more problematic with this technique as functional remodeling tends to lead to a straight bony connection uniting the resection stumps.
Discussion
Jacobson et al. [
15] provided an excellent review on paradigm shifts in the management of ORN of the mandible, and we concur with their view that advanced ORN requires aggressive surgical therapy, and that HBO alone is of minimal if any benefit in the treatment of advanced ORN. Contrary to the Jacobson et al. protocol of immediate reconstruction, we prefer a period of observation to determine whether or not the resection has been sufficient in eradicating progressive ORN whenever possible, that is, in segmental defects not including the symphyseal region. The lesson learned was that resections including the interforaminal mandibular symphyseal region should be managed with immediate bone reconstruction, in all practicality a fibula if at all possible, to avoid plate exposure through soft tissue shrinkage. If a fibula is not an option, we have succeeded by placing the LD skin pad intraoral to cover the reconstruction plate.
Patients with refractory ORN who have suffered extensive tissue loss have been shown to benefit from the use of a vascularised free fibula flap for segmental defect bridging and soft tissue coverage [
9]. The technique described here utilizes vascularised tissue (free LD flap) [
16], to enhance survival of nonvascularised bone (particulate bone graft).
Mandibular reconstruction using particulate iliac bone graft with a soft tissue cover has been reported in the literature mainly in nonirradiated patients [
17], and to a lesser extent, in irradiated cancer patients [
18,
19,
20]. The healing capacity in patients with manifest ORN is impaired, and hypovascularity in the irradiated region may influence the success of free bone grafting. For that reason, the authors thought that the creation of a recipient bed for the free bone graft reconstruction with adequately vascularised, nonirradiated tissue would prove beneficial for the success of the procedure.
A few studies report the use of the LD muscle (in situ) to accommodate a scaffold with iliac bone, or a bone substitute primed with a bone morphogenetic growth factor, for revascularization and maturation of the bone graft and later transplantation en bloc into the mandibular segmental defect with microvascular technique [
21,
22,
23]. A fascinating but extremely demanding technique.
One of the features of using particulate bone grafting in favor of osteocutaneous flaps is that the bone graft readily heals in a given shape through creeping substitution to stimulate the formation of a mandible like bone structure when placed in a well-vascularized recipient bed [
24]. The presence of an adequate bony foundation allows the possibility to offer the patient osseointegrated dental implants to support fixed or removable prosthetic appliances with excellent function.
Mandibular reconstruction with a free osteocutaneous fibula flap is still a highly predictable “work horse” in many centers worldwide and indeed in ours alike, and the osteocutaneous fibula has become a gold standard for mandibular segmental defect repair [
9,
10,
25]. However, atherosclerosis and other conditions that affect the circulation in the extremities may contraindicate a fibula reconstruction. Moreover, soft tissue defects may override the size that can be covered by the skin island associated with a osteocutaneous fibula flap. Alternative free osteocutaneous flaps from the iliac crest or the scapula can be useful under such circumstances, however, these options have limitations in the quality of bone and soft tissue available. In this situation, a musculocutaneous LD flap may offer a relevant alternative in combination with particulate bone graft.
There are pros and cons with either mode of reconstruction, microvascular, and free bone grafting. The AO
Manual of Internal Fixation in the Cranio-Facial Skeleton advices that very large defects may be reconstructed with microvascular grafts, whereas smaller defects may be repaired with cancellous bone from the iliac crest [
26]. Some authors consider the maximum length of a segmental mandibular gap to be grafted with a nonvascularized bone graft to be 4 to 6 cm. This limit was considerably surpassed in our case no. 6) shown in
Figure 4 and
Figure 5. Conversely, a fibula reconstruction for a defect less than 4 cm may be overdoing fibula reconstruction, and very short segments of fibula may suffer a critical blood supply depending on the location of perforators. Furthermore, mandibular height in dentate regions of the mandible is not easily achieved with a fibula, unless a double barrel reconstruction is performed [
27]. Adequate mandibular height will provide a vertical and transverse jaw relation to favor position and bony support for dental endosseous implants and offer conditions for sufficient oral hygiene. Fixed or removable prostheses will aid speech and mastication, allowing patients to regain their physical appearance and social function, thus leading to a better quality of life.
The authors acknowledge that the use of the free fibula flap to restore continuity of the mandible is commonplace and, in most situations, offers excellent reconstructive results in large or bilateral resections. However, in our opinion, this technique does not necessarily translate to a good cosmetic and functional result due to the low height of the fibula (between 13 and 14mm) and the large soft tissue donor defect that follows after harvesting the fibula either with a transverse or vertical skin island. Even though successful results have been reported with the use of a double-barrel technique to increase the flap height [
27], this is a more complicated technique with a limited potential in large defects.
The technique described here can act as a “life boat” for reconstruction in this group of patients, should there be any contraindications to using the fibula or other microvascular bone flaps. Moreover, the LD flap has proved useful as a rescue after unsuccessful reconstruction with other techniques, and a size of the segmental gap size exceeding 5 cm is no absolute barrier to reconstruction with particulate iliac bone.
Conclusion
We have reported a technique that may be adopted as an alternative reconstruction method for the surgical management of segmental defects in ORN of the mandible, especially in cases where there is a large and complex soft tissue internal and/or external defect to be covered, or when peripheral circulatory conditions may rule out the use of an osteocutaneous free fibular flap.
Concurrent to addressing the clinical problem, any surgical intervention should always aim to address the effects on the patient’s rehabilitation and quality of life thereafter. Although we have reported successful results using our technique, ORN of the mandible remains a challenging interdisciplinary medical and surgical problem. The described technique has proven a safe and useful “life boat” for patients in need of soft tissue coverage beyond that of a fibula flap and/or mandibular height.