Two Stage Enucleation and Deflation of a Large Unicystic Ameloblastoma with Mural Invasion in Mandible
Abstract
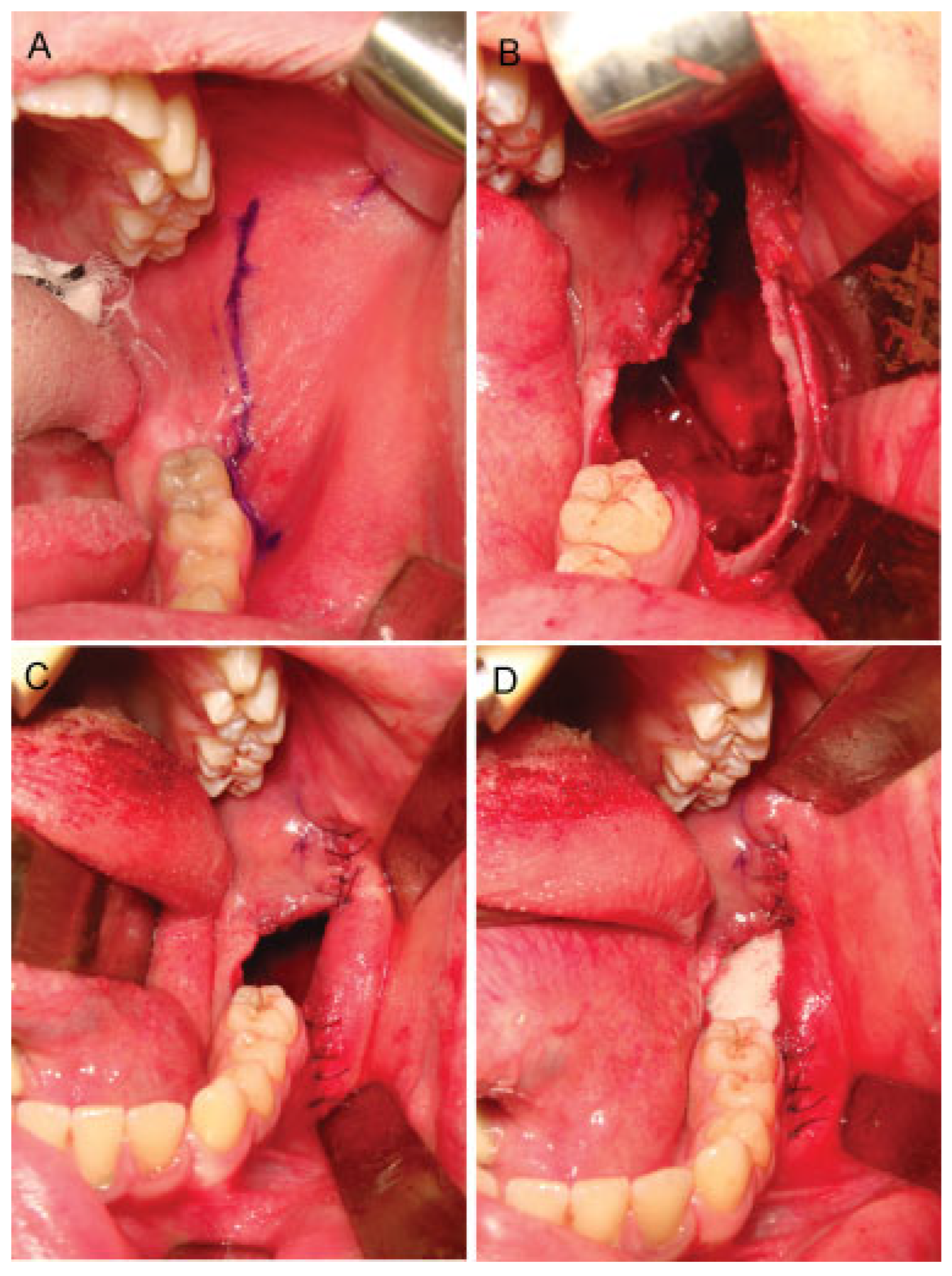
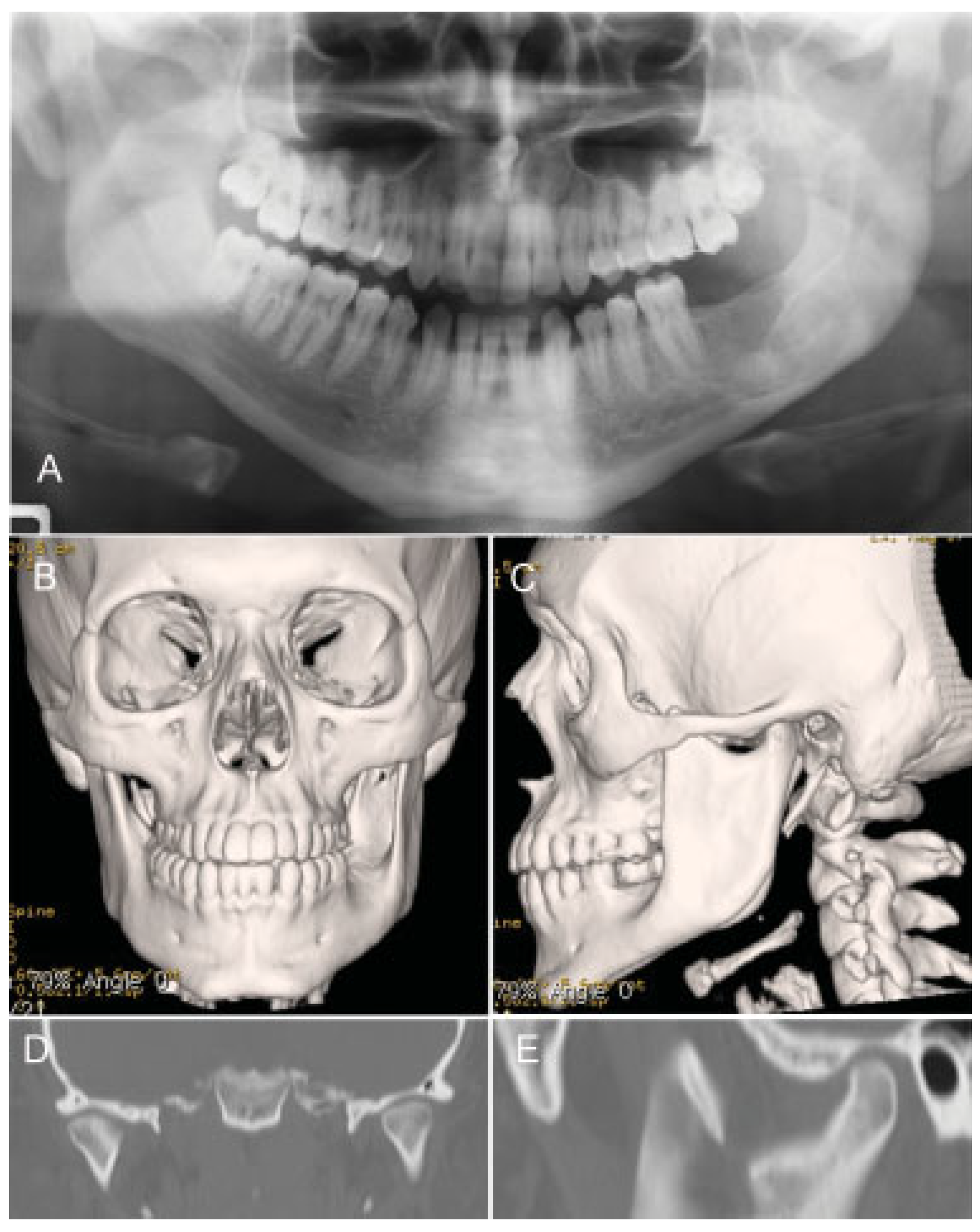
Case Report
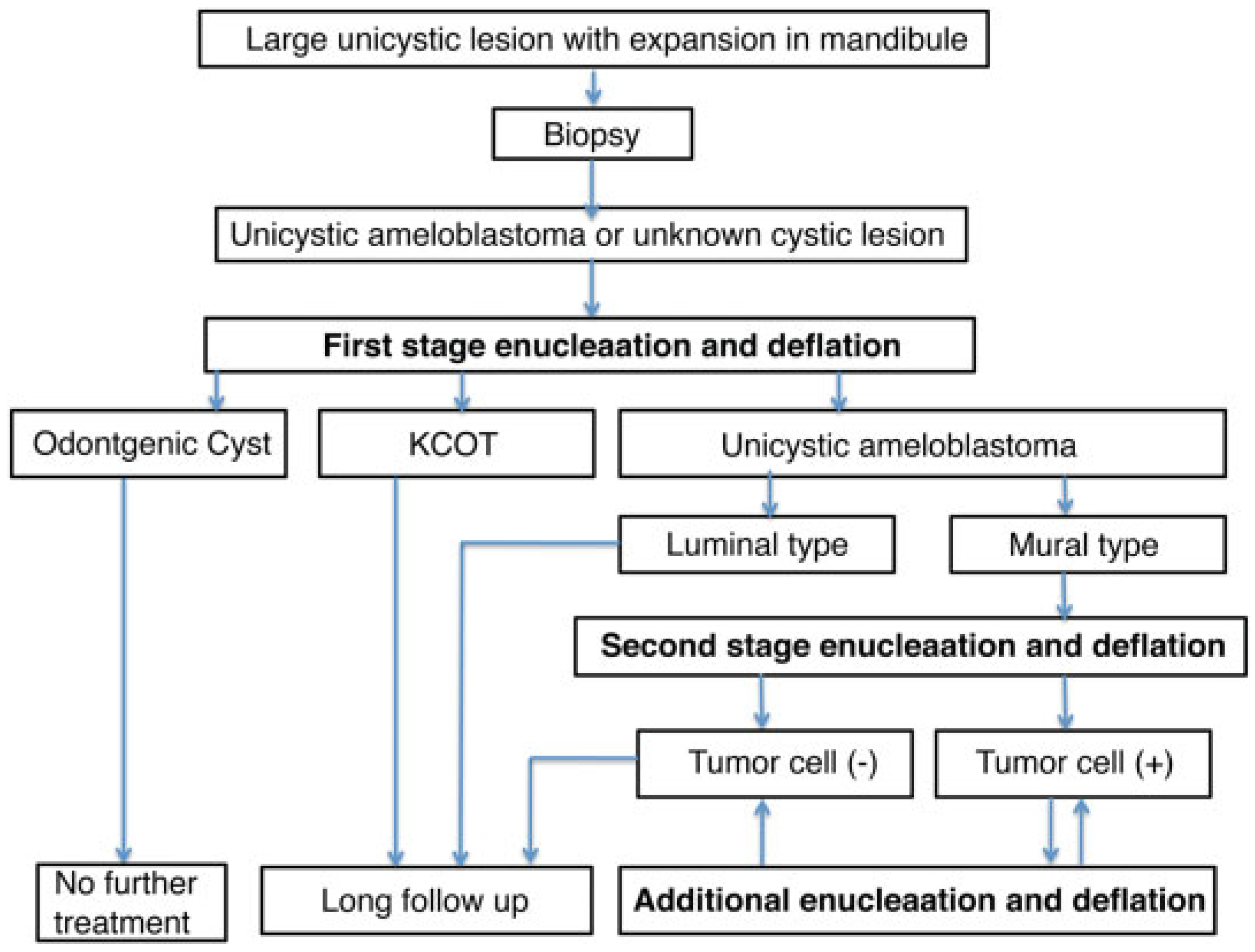
Discussion
Acknowledgments
References
- Vayvada, H.; Mola, F.; Menderes, A.; Yilmaz, M. Surgical management of ameloblastoma in the mandible: segmental mandibulectomy and immediate reconstruction with free fibula or deep circumflex iliac artery flap (evaluation of the long-term esthetic and functional results). J Oral Maxillofac Surg 2006, 64, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Zemann, W.; Feichtinger, M.; Kowatsch, E.; Kärcher, H. Extensive ameloblastoma of the jaws: surgical management and immediate reconstruction using microvascular flaps. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007, 103, 190–196. [Google Scholar] [PubMed]
- Gardner, D.G.; Corio, R.L. Plexiform unicystic ameloblastoma. A variant of ameloblastoma with a low-recurrence rate after enucleation. Cancer 1984, 53, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.A.; Adekeye, E.O. Unicystic ameloblastoma of the mandible: a long-term follow-up. J Oral Maxillofac Surg 1997, 55, 345–348, discussion 349–350. [Google Scholar] [PubMed]
- Philipsen, H.P.; Reichart, P.A. Unicystic ameloblastoma. A review of 193 cases from the literature. Oral Oncol 1998, 34, 317–325. [Google Scholar] [PubMed]
- Ackermann, G.L.; Altini, M.; Shear, M. The unicystic ameloblastoma: a clinicopathological study of 57 cases. J Oral Pathol 1988, 17, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, K.A.; Stoelinga, P.J.; de Wilde, P.C.; Brouns, J.J.; Voorsmit, R.A. Rational approach to diagnosis and treatment of ameloblastomas and odontogenic keratocysts. Br J Oral Maxillofac Surg 2004, 42, 381–390. [Google Scholar] [PubMed]
- Lau, S.L.; Samman, N. Recurrence related to treatment modalities of unicystic ameloblastoma: a systematic review. Int J Oral Maxillofac Surg 2006, 35, 681–690. [Google Scholar] [PubMed]
- Rosenstein, T.; Pogrel, M.A.; Smith, R.A.; Regezi, J.A. Cystic ameloblastoma—behavior and treatment of 21 cases. J Oral Maxillofac Surg 2001, 59, 1311–1316, discussion 1316–1318. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Inoue, N.; Kobayashi, I.; et al. Ameloblastoma treated by “Dredging method”—report of a case. Asian J Oral Maxillofac Surg 1991, 3, 89–93. [Google Scholar]
- Kawamura, M.; Inoue, N.; Kobayashi, I.; et al. “Dredging Method”—a new approach for the treatment of ameloblastoma. Asian J Oral Maxillofac Surg 1991, 3, 81–88. [Google Scholar]
- Sadat, S.M.A.; Ahmed, M. “Dredging Method”—a conservative surgical approach for the treatment of ameloblastoma of jaw. J Bangladesh Coll Phys Surg 2011, 29, 72–77. [Google Scholar]
- Sadat, S.M.A.; Haider, I.A.; Bhuiyan, R.A.; et al. Treatment of ameloblastoma by “Dredging Method”. Int J Oral Maxillofac Surg 2007, 36, 1035. [Google Scholar] [CrossRef]
- Kurokawa, H.; Ishida, C.; Murata, T.; et al. A case report of unicystic ameloblastoma treated with dredging method. J Kyushu Dental Society 2000, 54, 253–257. [Google Scholar] [CrossRef][Green Version]


© 2014 by the author. The Author(s) 2014.
Share and Cite
Sasaki, R.; Watanabe, Y.; Ando, T.; Akizuki, T. Two Stage Enucleation and Deflation of a Large Unicystic Ameloblastoma with Mural Invasion in Mandible. Craniomaxillofac. Trauma Reconstr. 2014, 7, 139-142. https://doi.org/10.1055/s-0033-1364197
Sasaki R, Watanabe Y, Ando T, Akizuki T. Two Stage Enucleation and Deflation of a Large Unicystic Ameloblastoma with Mural Invasion in Mandible. Craniomaxillofacial Trauma & Reconstruction. 2014; 7(2):139-142. https://doi.org/10.1055/s-0033-1364197
Chicago/Turabian StyleSasaki, Ryo, Yorikatsu Watanabe, Tomohiro Ando, and Tanetaka Akizuki. 2014. "Two Stage Enucleation and Deflation of a Large Unicystic Ameloblastoma with Mural Invasion in Mandible" Craniomaxillofacial Trauma & Reconstruction 7, no. 2: 139-142. https://doi.org/10.1055/s-0033-1364197
APA StyleSasaki, R., Watanabe, Y., Ando, T., & Akizuki, T. (2014). Two Stage Enucleation and Deflation of a Large Unicystic Ameloblastoma with Mural Invasion in Mandible. Craniomaxillofacial Trauma & Reconstruction, 7(2), 139-142. https://doi.org/10.1055/s-0033-1364197


