Abstract
Background Stereolithographic (SLA) models have become a valuable resource in preoperative planning in maxillofacial reconstruction. The objective of this study was to perform a defect specific analysis of the utility of SLA models. The goal was to determine the manner in which the perceived benefit of preoperative modeling translates to measurable clinical advantages. Methods Patients who underwent reconstruction of defects of the mandible or midface using SLA modeling between 2006 and 2011 were identified through billing records. Based on the nature and extent of bony defect, cases requiring nearly identical reconstruction, but without modeling, were matched case by case for comparison. Given the presumed efficiency of SLA modeling, a comparison of total and reconstructive operative times was performed to see if this could offset the cost of the model. Results There were 10 patients each in the “model” and “nonmodel” group. No significant differences were observed for total operative time between groups. Surprisingly, the total reconstructive time was lower in the group not using SLA models (p = 0.05). Conclusions SLA models provide several operative planning advantages, but did not appear to decrease operative time enough to sufficiently offset the cost of the model in this group.
Reconstruction of the head and neck after trauma or surgical tumor extirpation often requires replacement of bone to provide a desirable functional and aesthetic result. Osteocutaneous free flap reconstruction is the primary treatment modality for defects of the mandible and midface. Osteocutaneous radial forearm, fibula, scapula, or iliac crest, have all been described as donor options for such reconstruction. The success of free flap reconstruction, particularly flap survival, is multifactorial. Significant factors in flap survival have been shown to include ischemia time, surgeon experience, and patient comorbidities, among others [1]. Maximizing factors in favor of flap survival is critical for the success of the reconstruction.
Stereolithographic (SLA) models have been available since the 1990s for anatomical modeling. Their application has been widely documented in maxillofacial reconstruction [2,3,4,5]. Advantages of SLA models include decreased surgical time, preoperative planning, prebending implants, surgical re- hearsal, patient education, and ultimately more predictable surgical results. Application in free flap reconstruction has been demonstrated in several case reports and case series [3,6,7].
Efforts to prevent escalating costs in care are a frequent pressure on the surgical team. Free flap reconstruction can be a time-consuming process with high operative costs. The purpose of this study was to attempt to measure the impact of SLA modeling on operative times as a means to offset the cost of the technology.
Methods
A retrospective chart review was performed after protocol approval was obtained from the institutional review board. Patients were identified from 2006 to 2011 who underwent fibula osteocutaneous free flap head and neck reconstruction. Billing records for SLA modeling were used to identify the study cohort. A review of all patients undergoing fibula osteocuta- neous free flap reconstruction was performed, and a control group was created by matching patients who had identical extirpative defects that were reconstructed in the same manner without the use of SLA modeling. Mandibular defects were classified as anterior, anterolateral, lateral, or angle to angle, and matched by length as well as defect location. Patient data recorded included age, site of defect, cause of defect, and previous therapies. The primary outcome measure was operative time, as documented in the operative records. Total operative time was measured as time of incision to conclusion of closure and final application of all dressings. Total flap time was calculated based on the documented incision time for flap harvest to the conclusion of the procedure.
In the absence of a model, plates are either bent intraoperatively in a standard fashion to conform to the native mandible, or if necessary, bent free hand with the use of a temporary operative external fixator. The model group in this cohort primarily represents patients with extensive soft tissue loss and distortion (i.e., trauma) or tumors that distorted the outer cortex of the mandible in such a manner where this was anticipated to be difficult.
Operating room fees are fixed for the first hour, followed by a rate per additional 15 minutes. As all cases exceeded 1 hour, measured costs of operation were calculated based on time after the first hour. The primary time-related fees were general operating room cost and anesthesia cost after the first hour. The time-related cost is then calculated based on each additional 15 minutes. The average cost of operating room time and anesthesia is $195 for each additional 15 minutes. Cost of SLA models at our institution are approximately $1,015 for mandible only and $1,615 for mandible through midface. These prices are based on a contractual rate agreed upon between the SLA model distributor and the institution, and are likely to vary by site. Statistical analysis was then performed using two-sided t tests to examine mean operative time for both the overall case as well as the reconstruction itself. Chi-square testing was used to evaluate differences in categorical values across treatment groups. Stata 9.0 (College Station, Texas, United States) was used for the analysis.
Results
Ten patients were identified who had had SLA models used in preoperative planning and intraoperative management. Ten “nonmodel” patients were matched to the “model” patients based on nature and size of the bony defect. Demographic data and diagnoses are summarized in Table 1. Ages ranged from 15 to 81 years with 6 females and 14 males. Mean age for the entire cohort was 53.3 19.1 years. Mean age for the nonmodel group was 66.1 3.0 years, and was 40.5 5.6 for the model group (p = 0.008), which was likely a function of the differences in diagnoses across groups. Although match- ing was performed defect to defect, almost the entire non-model group had surgery for squamous cell carcinoma. The lone exception was one patient who presented with an advanced basal cell carcinoma of the chin with invasion of the mandibular symphysis. In contrast, the “modeled” group had a more varied etiology of disease. Four patients underwent surgery for osteoradionecrosis. There were two gunshot wounds with a resulting mandibular defect. The remaining three patients had distinct neoplasms involving the mandible including osteosarcoma, ameloblastoma, and cavernous hemangioma.

Table 1.
Patient demographics and distribution by defect.
The mean operative time for model patients was 556.6 84.3 minutes, with a range of 440 to 669 minutes. The mean operative time for nonmodel patients was 568.1 85.6, with a range of 444 to 677. The p value was 0.77, which was not statistically significant. The mean flap time for model patients was 331.8 52.0, with a range of 265 to 420. The mean flap time for nonmodel patients was 275.4 67.8, with a range of 204 to 411. The p value was less than 0.05, which demonstrates statistical significance. These data are summarized in Table 2.

Table 2.
Comparison of total operative time and reconstruction time using stereolithographic models.
Although none of the patients in the nonmodel group had been treated with prior radiation, five of the patients in the model group had received prior radiation therapy (p = 0.01). Four patients underwent reconstruction for complications stemming from osteoradionecrosis. The other patient (Figure 1) received radiation as part of preoperative therapy for osteosarcoma of the mandible. For model patients, no statistically significant differences were observed for either mean total operative time (p = 0.83) or mean flap time (p = 0.85) when stratified by prior radiation therapy. Out of concern that the disproportionate number of patients in the model group treated with prior radiation may have impacted surgical times, comparisons were made to the nonmodel group using only cases in the model group that did not receive prior radiation. Again, no statistically signifi- cant differences in mean total operative time (p = 0.9) or mean flap time (p = 0.12) were observed.
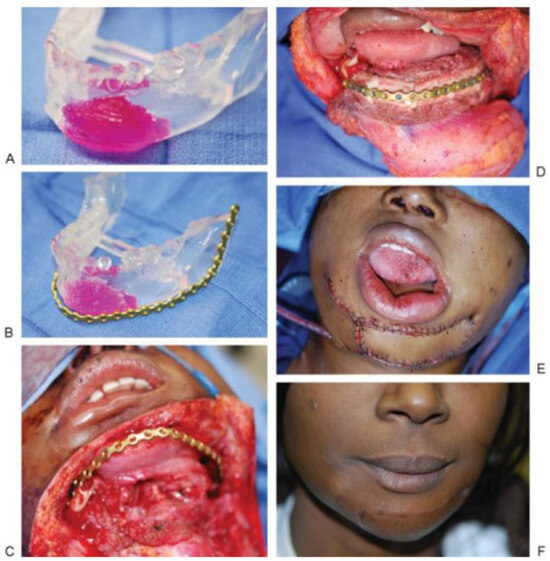
Figure 1.
(A) SLA model of an 18-year-old woman with osteosarcoma of the anterior mandible. She had residual disease after prior chemoradiation. (B) Prebent plate for reconstruction using SLA model. The tumor eroded the mandible anteriorly, making plate approximation to the native mandible difficult. The model was burred down for natural contour and the plate was contoured to the model. (C) View from apron incision from neck with anterior mandible defect with hardware in place. Chin skin was resected as part of soft tissue margin. (D) Inset of fibula free flap, with intraoral and chin skin defect. (E) Cutaneous portion of fibula free flap inset. The flap skin was de-epithelialized to create two skin paddles to reconstruct the chin and oral cavity segments. (F) Three-month postoperative result. Abbreviation: SLA, stereolithographic.
The time needed to be saved to offset the cost of the SLA model was calculated based on 15-minute intervals and cost units at $195. Saving greater than 75 minutes would be required to offset the cost of the mandible SLA model. A savings greater than 120 minutes would be required to offset the cost of the mandible to midface SLA model. The model group actually showed no statistical difference in total oper- ative time and could not offset cost. The distinct free flap time demonstrated a significant statistical difference in favor of the nonmodel group.
Discussion
It is without question that SLA models provide a surgical planning advantage. The ability to plan surgical osteotomies, prebend plating implants, and plan reconstruction with a model is very useful for free flap surgeons. The additional cost of SLA models is only $1,000 to $1,600 depending on the model and institutional contracts. As a percentage of the absolute cost of care of the difficult to reconstruct patient, the impact of the addition of SLA modeling is minimal.
Having been pleased with the use of SLA modeling in our practice, we were surprised to find the relative inability to find a measurable improvement in operative times. What is more difficult to quantify is the portions of the case where time is saved by the use of SLA models. Where the model probably has the greatest utility is with regard to inset time, or the amount of time required putting the flap into anatomic position. Bending of plates requires a quantifiable amount of time, and the ability to do so outside of the operating theater cannot be underestimated. Although total flap time was observed to be higher in the nonmodeled group, what we found to be difficult to measure was the percentage of time dedicated to the inset of the flap relative to the amount of time spent dealing with the microvascular anatomy. Though there was an attempt to compare matched cohorts, the two groups differed in their reasons for treatment (i.e., carcinoma versus benign disease or trauma). In the modeled cohort, 40% of patients were treated for osteoradionecrosis. There is an unquestionable difference in the vasculature of the previous- ly radiated neck, especially when the combination of chemo- therapy and radiation is involved. The differences in anastomotic time in this cohort may well have offset the technical advantages of the SLA model if looking specifically at inset time. Although the difficulties encountered with reconstruction in the previously radiated patient would seem to be the most intuitive explanation for the failure to observe a time-saving effect from using the model, no differ- ences in operative times were observed when using the model in radiated versus nonradiated patients, nor were differences observed with or without the use of the model when comparing only nonradiated patients.
Although not a dollar-for-dollar analysis, the objective of this study was to demonstrate a reduction in operating room time as a justification for the use of SLA models. We have failed to do this. The majority of defects reconstructed at our institution are not done with preoperative modeling, which is mostly a function of individual surgeon experience and the awareness of the increased cost. Surgical experience with specific defect restoration may obviate the need for SLA modeling under many circumstances. It is without question that the group of patients where SLA models were utilized, with a preponderance of patients with prior radiation and extensive trauma, represents a disproportionally greater reconstructive challenge compared with patients reconstructed after primary tumor ablation. Thus, the results of this study may reflect the anticipated difficulty of the cases where SLA modeling was performed. In other words, the observed time to perform the cases in the study group may have been even longer in the absence of SLA modeling and may have differed in some other fashion from the defect- matched controls in a manner other than the matched defect. All other operative variables being equal, the primary means for justifying the utility of SLA models remains a reduction in operating room time. Based on the combined cost of operating room time and anesthesia of $195 per 15 minutes, 75 to 120 minutes of time must be saved with the SLA model to offset this cost. This amount of time can be difficult to offset with the use of the model. What cannot be directly quantified is the difference in operating room time that would have resulted from not using SLA models in cases where they were chosen to be utilized.
In our otherwise high-volume reconstructive practice, we have admittedly been slow to adopt the use of SLA modeling. This article does not represent our total reconstructive experience, but rather, an attempt to directly compare operative efficiency using a defect to defect comparison, with SLA modeling as the only specific variable. As much as we still support the use of SLA modeling in specifically selected cases, we cannot demonstrate a clinically relevant justification for their use based on operative times.
It cannot be overemphasized that this study can only report the differences in total flap time. In the end, unfortunately, this is the most accurate measurement of “on table” time. What is difficult to measure from this retrospective review is total ischemia time, as well as the proportion of that time that is dedicated to the inset of the flap. It remains our postulate that the use of SLA modeling improves the ability to inset flaps in a time-saving, efficient, and effective manner. We have chosen to use SLA models in cases where it was anticipated that the reconstruction would be difficult. The amount of time saved with the inset of the flap has no impact on the subsequent difficulty encountered with anastomotic revascularization. In the previously chemoradiated neck vasculature, these difficulties are well described, and time saved on inset allows for greater leeway when needed for reperfu- sion. Under these circumstances, the judicious use of SLA modeling may provide advantages not measurable by the confines of this study. Similarly, complexity of soft tissue reconstruction may add significantly to operative time and is unlikely to be overcome with the assistance of a bony SLA model. A representative example of the utility of SLA modeling is demonstrated in Figure 1A–F.
Our future aspirations are to perform a randomized pro-spective trial, which would account for the variables mentioned previously that limit this current study. Specific measures of time should be accounted for in the operating room such as plate application time and inset time. Future studies could also include the overall cost of hospitalization related to complications of free flap surgery with and without model use, survey of surgeon confidence and adequacy of anatomic reconstruction, and comparative cosmetic evaluation of reconstructive outcomes, including ease of dental reconstruction. Complication rates in all patients reported in this study were minimal, with all flaps surviving and healing uneventfully.
Despite the inability to demonstrate a quantifiable reduction in operative time with the use of SLA models in this study, the authors still advocate the use of SLA models. The ability to preoperatively plan is critical in complex reconstruction using osteocutaneous free flaps. Every minute counts and preoperative planning using the SLA model may save critical minutes of ischemia time during a complex reconstruction, even when they cannot be measured on the operative record.
References
- Singh, B.; Cordeiro, P.G.; Santamaria, E.; Shaha, A.R.; Pfister, D.G.; Shah, J.P. Factors associated with complications in microvascular reconstruction of head and neck defects. Plast Reconstr Surg 1999, 103, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.K.; Cheung, L.K. The usefulness of stereomodels in maxillo- facial surgical management. J Oral Maxillofac Surg 2007, 65, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, L.L., Jr.; Madsen, M.J.; Peterson, G. Stereolithographic modeling technology applied to tumor resection. J Oral Maxillofac Surg 2005, 63, 873–878. [Google Scholar] [PubMed]
- Kernan, B.T.; Wimsatt, J.A., III. Use of a stereolithography model for accurate, preoperative adaptation of a reconstruction plate. J Oral Maxillofac Surg 2000, 58, 349–351. [Google Scholar] [PubMed]
- Mehra, P.; Miner, J.; D’Innocenzo, R.; Nadershah, M. Use of 3-D stereolithographic models in oral and maxillofacial surgery. J Maxillofac Oral Surg 2011, 10, 6–13. [Google Scholar] [PubMed]
- Antony, A.K.; Chen, W.F.; Kolokythas, A.; Weimer, K.A.; Cohen, M.N. Use of virtual surgery and stereolithography-guided osteotomy for mandibular reconstruction with the free fibula. Plast Reconstr Surg 2011, 128, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Chopra, K.; Manson, P.N.; Gastman, B.R. Stereolithographic modeling in reconstructive surgery of the craniofacial skeleton after tumor resection. Plast Reconstr Surg 2012, 129, 743e–745e. [Google Scholar] [PubMed]
© 2013 by the author. The Author(s) 2013.