Optic Nerve Monitoring
Abstract
Anatomical and Pathophysiologic Considerations
Clinical Appearance of a Traumatic Optic Nerve Injury
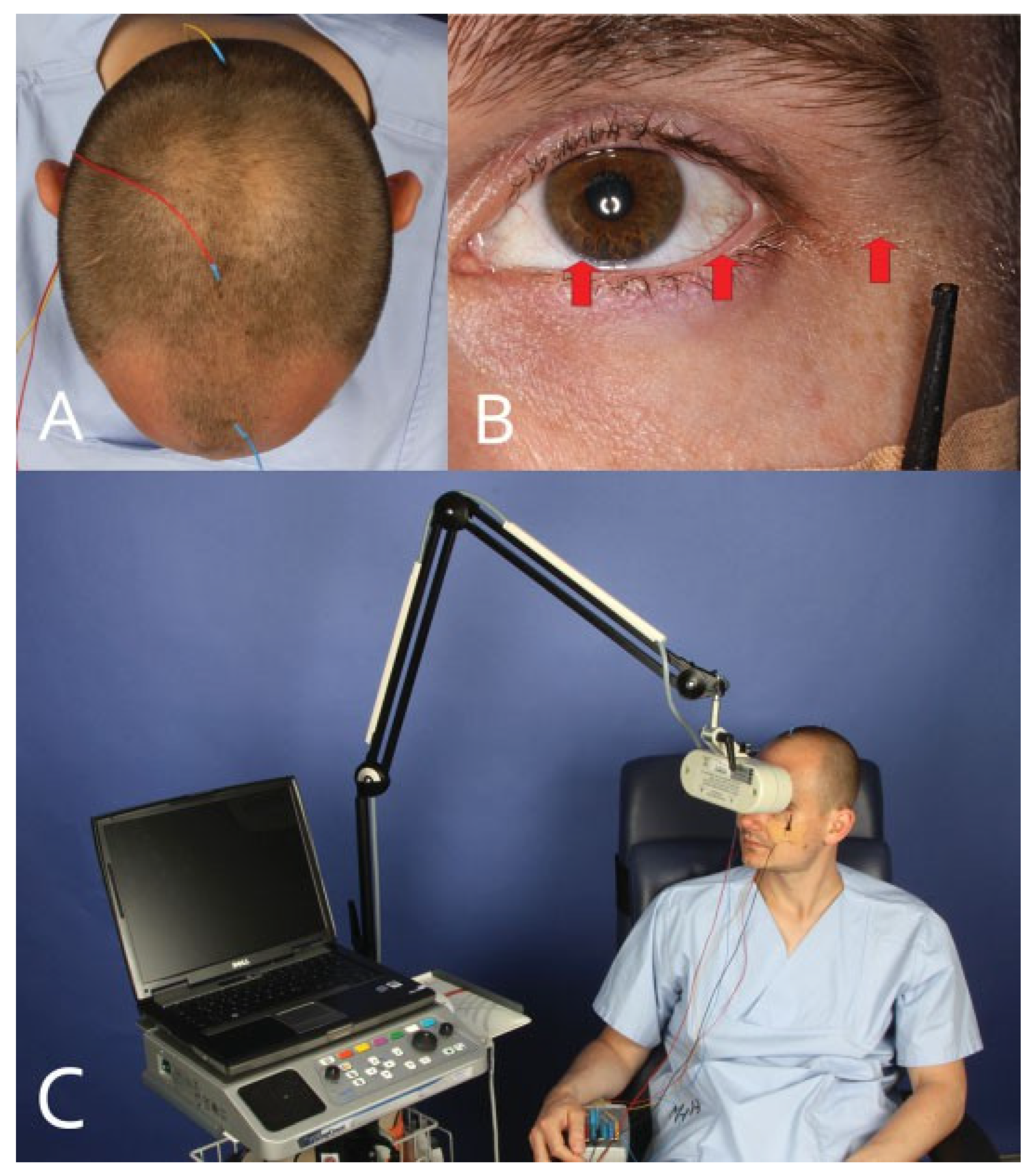
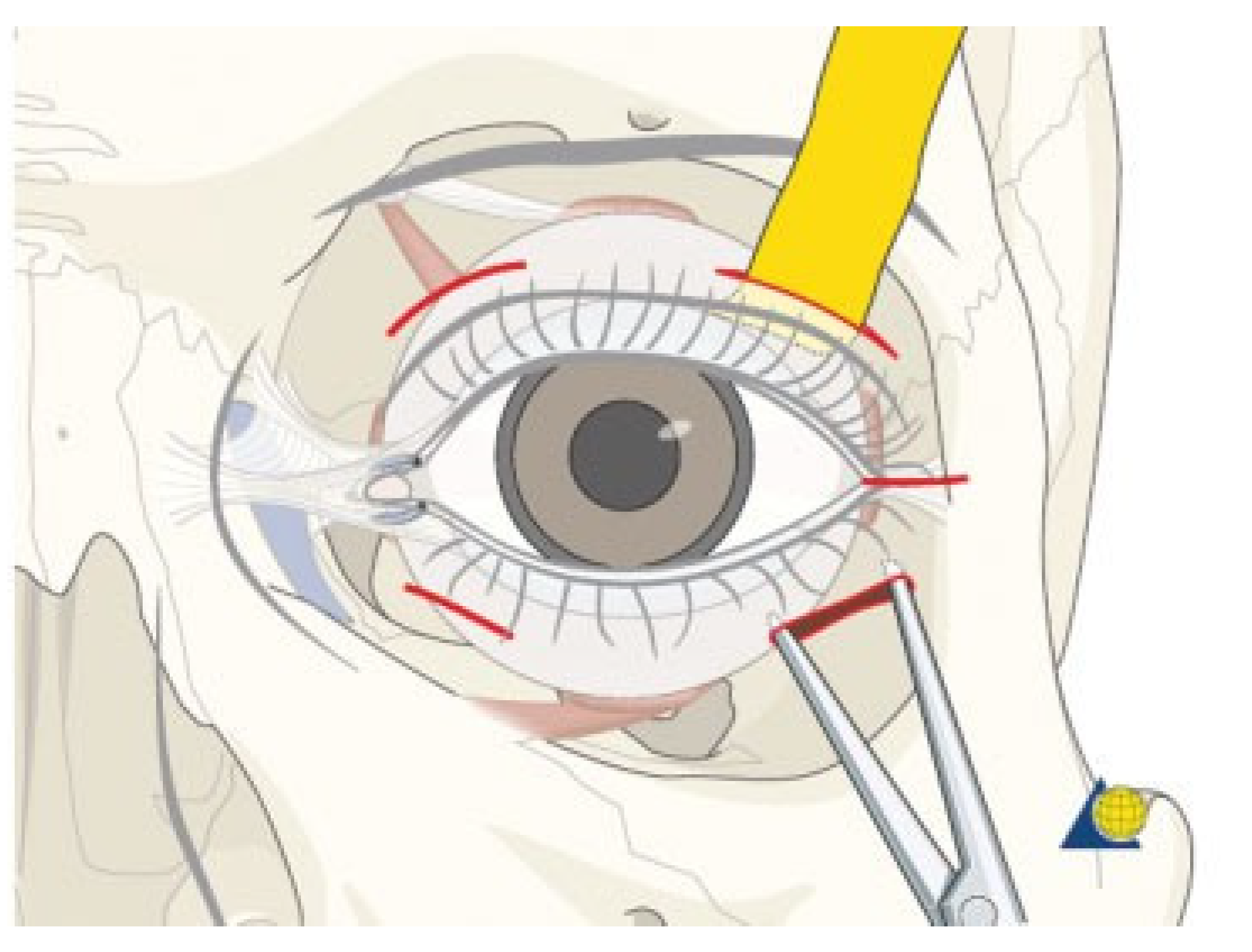
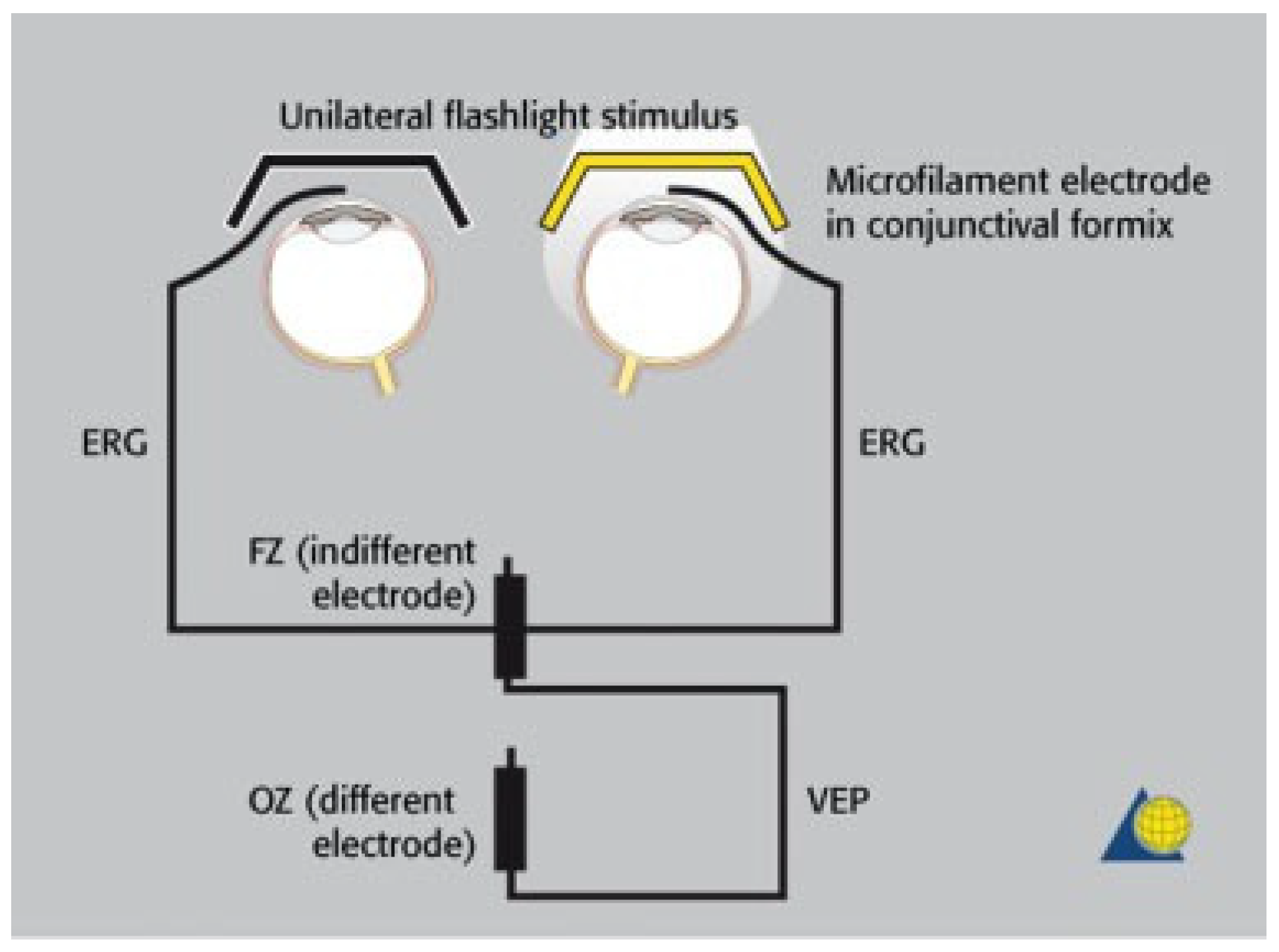
Electrophysiology of the Visual Pathway
Primary Diagnosis with Visual Pathway Testing of Patients with Head Injuries
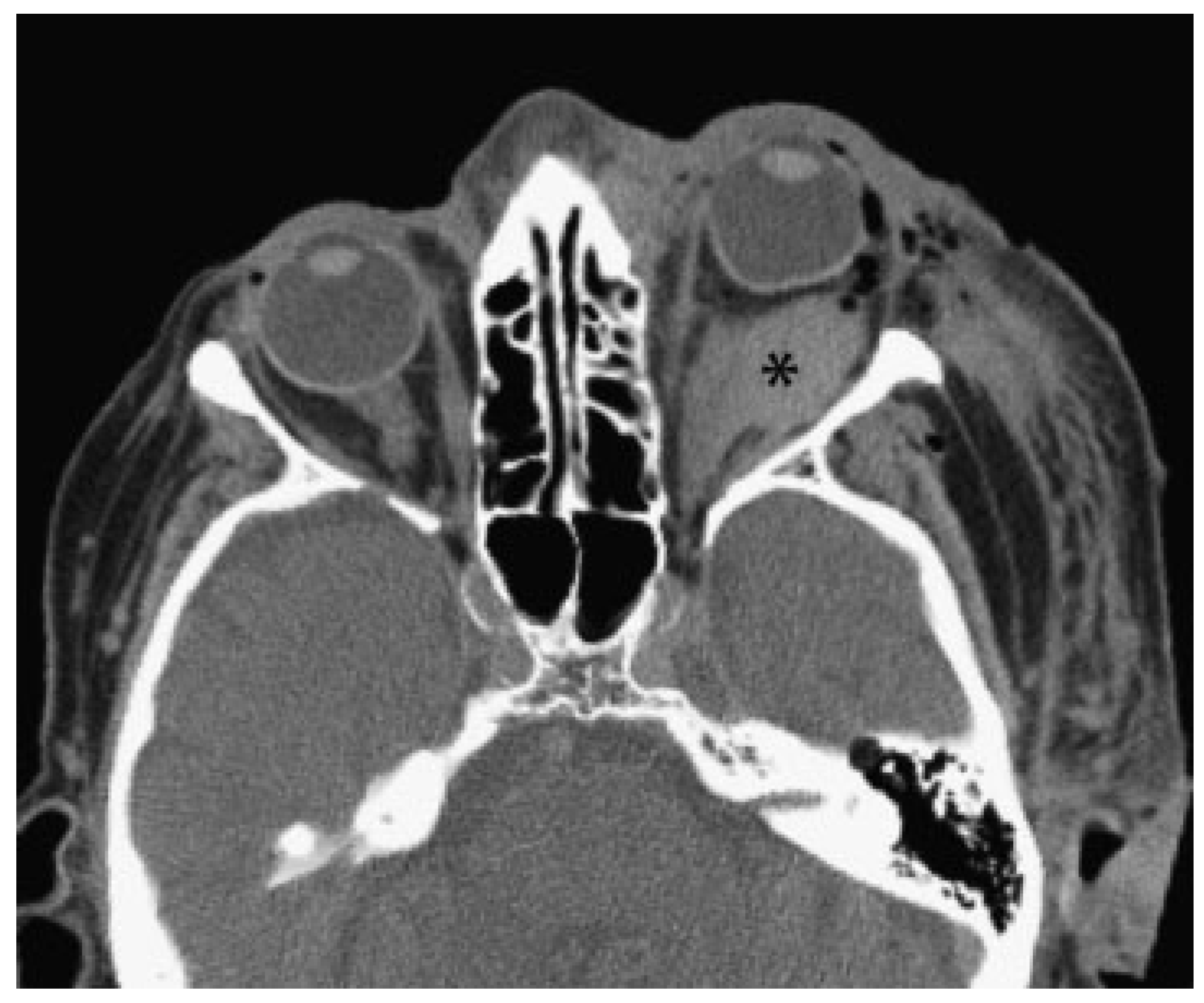
- Primary signs: fracture of the optic nerve canal; fracture in the retrobulbar region; hematoma, swelling, or disconti- nuity of the optic nerve; hematoma in the posterior third of the orbit.
- Secondary signs: shading of the sphenoidal sinus or of the posterior ethmoidal cells; air–fluid level in the maxillary sinus; epidural hematoma of the temporobasal region.
- Concomitant injuries: lamina papyracea; frontal sinus; zygomatic bone; orbital floor; orbital roof; air collection below the frontonasal region, optic chiasm, cavernous sinus, or posterior of the big wing of the sphenoid bone; contusions; subarachnoid or subdural hemorrhage.
Traumatology
Diagnostic Imaging
Optic Nerve Monitoring with Regard to Craniofacial Reconstructions
Therapy for Traumatic Optic Nerve Damage
Recommendations for the Treatment of Traumatic Optic Nerve Lesions
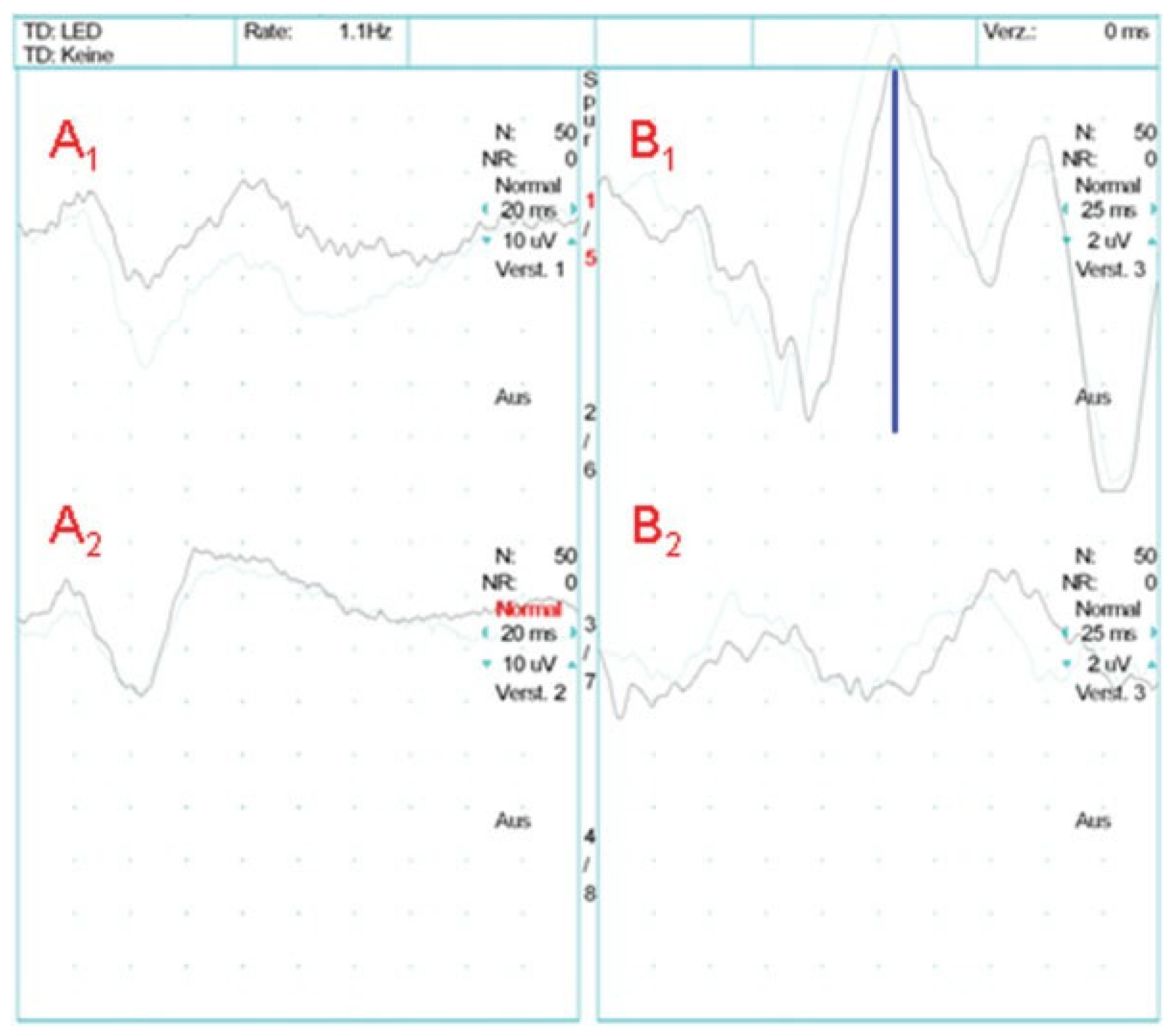
- The integrity of the visual pathway should be tested immediately in every patient with midface or skull base trauma. If clinical examination is not reliable, VEP and ERG recordings should be performed (Figure 7). This applies also in craniofacial surgery with orbital involvement.
- In emergency cases, a normal light reaction is a reliable parameter for an intact visual pathway. However, in primary diagnosis of patients with head injuries, pupillary function is often disturbed (e.g., by opioids, injury to the iris sphincter itself, or bilateral damage of the oculomotor nerve).
- Classification of VEP records as “normal”, “abnormal”, or “not reproducible” is generally sufficient to grade visual pathway function. In cases of deficient neuro-ophthalmo- logic findings, the decision for or against treatment of a visual pathway injury is based on VEP records together with clinical and radiologic findings. With pathologic findings in particular, but also reproducible VEP records, immediate treatment is recommended to avoid additional secondary optic nerve damage.
- If an afferent disorder of the visual pathway is clinically proven or cannot be excluded, or, in cases of a pathologic VEP record, megadose methylprednisolone therapy should be applied. Contraindications have to be considered.
- Prompt surgical optic nerve decompression is indicated in cases of retrobulbar hematoma, provided pulsating exophthalmos and cerebrospinal fluid leak are ruled out.
- Regarding conscious patients, immediate decompression of the optic canal is indicated in the case of afferent disorders with progressive loss or absence of visual acuity together with direct or indirect radiologic signs of trauma in the retrobulbar region or direct vicinity of the optic nerve canal. In these cases, return of visual acuity was not achieved if VEP records were not reproducible before decompression. Nevertheless, surgical intervention is recommended until studies consisting of large numbers of patients show that recurrence of visual acuity is impossible after finding extinct VEPs.
- Concerning unconscious patients, decompression of the optic canal is also recommended with direct or indirect radiologic signs of trauma in the retrobulbar region or direct vicinity of the optic nerve canal. If the afferent disorder of the visual pathway cannot be clinically proven, surgical intervention is recommended in the case of a pathologic VEP record (reproducible or not).
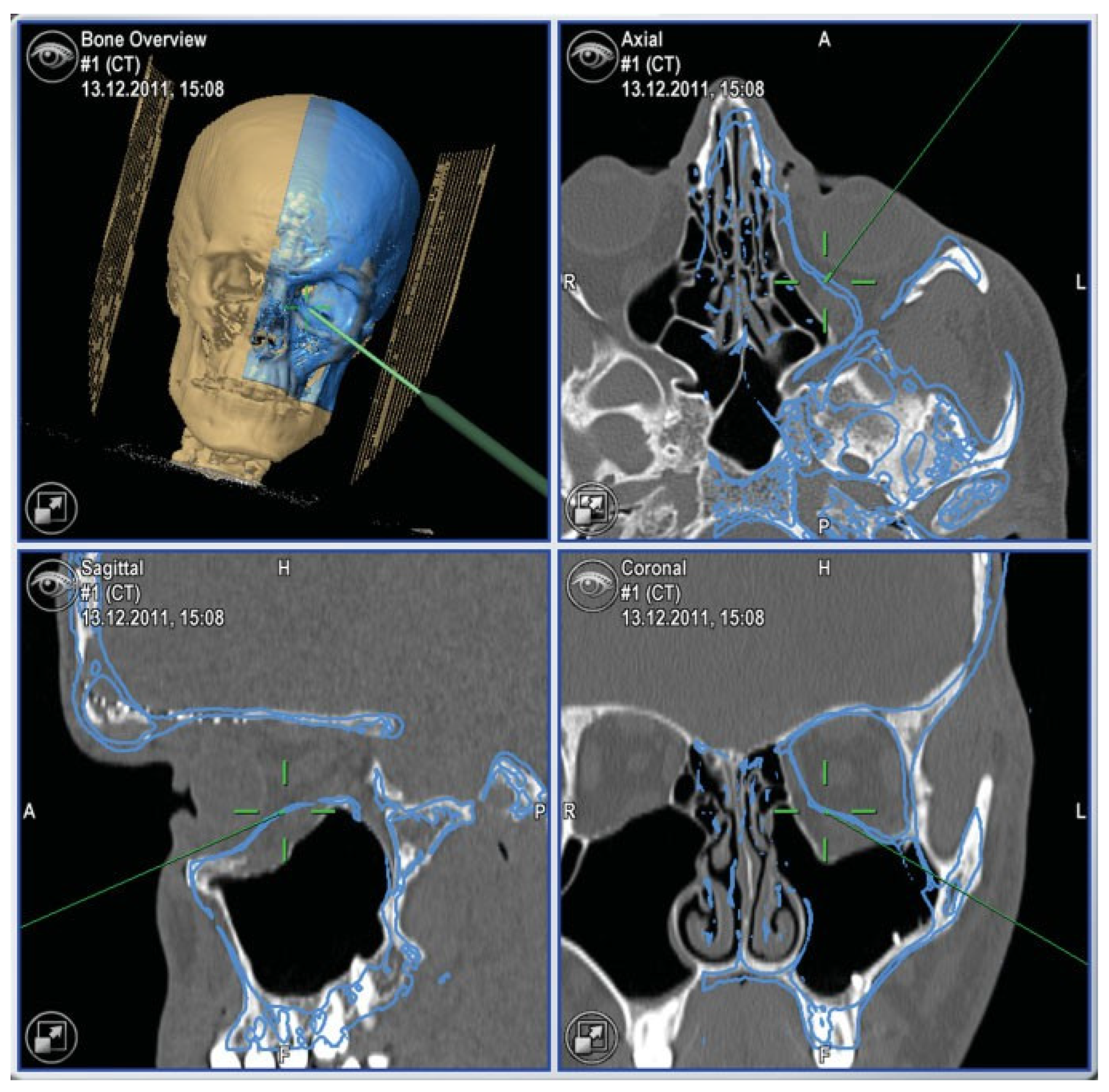
- In the case of optic canal decompression, postoperative evaluation of surgical outcome by using CT with axial and coronal thin-layer reconstructions is mandatory.
References
- Lang, J. Posterior ethmoid cells and their relation to the optic canal. HNO 1988, 36, 49–53. [Google Scholar] [PubMed]
- Osguthorpe, J.D.; Sofferman, R.A. Optic nerve decompression. Otolaryngol Clin North Am 1988, 21, 155–169. [Google Scholar]
- Wolin, M.J.; Lavin, P.J. Spontaneous visual recovery from traumatic optic neuropathy after blunt head injury. Am J Ophthalmol 1990, 109, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Elisevich, K.V.; Ford, R.M.; Anderson, D.P.; Stratford, J.G.; Richardson, P.M. Visual abnormalities with multiple trauma. Surg Neurol 1984, 22, 565–575. [Google Scholar] [PubMed]
- Habal, M.B.; Maniscalco, J.E.; Rhoton, A.L., Jr. Microsurgical anatomy of the optic canal: Correlates to optic nerve exposure. J Surg Res 1977, 22, 527–533. [Google Scholar] [CrossRef]
- Kline, L.B.; Morawetz, R.B.; Swaid, S.N. Indirect injury of the optic nerve. Neurosurgery 1984, 14, 756–764. [Google Scholar] [CrossRef]
- Anderson, R.L.; Panje, W.R.; Gross, C.E. Optic nerve blindness following blunt forehead trauma. Ophthalmology 1982, 89, 445–455. [Google Scholar] [CrossRef]
- Rochels, R. Holographic deformation analysis of the optic canal in blunt cranial trauma. Fortschr Ophthalmol 1990, 87, 182–185. [Google Scholar]
- Stankiewicz, J.A. Blindness and intranasal endoscopic ethmoidec- tomy: Prevention and management. Otolaryngol Head Neck Surg 1989, 101, 320–329. [Google Scholar]
- McCartney, D.L.; Char, D.H. Return of vision following orbital decom- pression after 36 hours of postoperative blindness. Am J Ophthalmol 1985, 100, 602–604. [Google Scholar]
- Linberg, J.V. Orbital compartment syndromes following trauma. Adv Ophthalmic Plast Reconstr Surg 1987, 6, 51–62. [Google Scholar] [PubMed]
- Linberg, J.V. Orbital emphysema complicated by acute central retinal artery occlusion: Case report and treatment. Ann Ophthalmol 1982, 14, 747–749. [Google Scholar] [PubMed]
- Nayak, S.R.; Kirtane, M.V.; Ingle, M.V. Transethmoid decompression of the optic nerve in head injuries: An update. J Laryngol Otol 1991, 105, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.R.; Kirtane, M.V.; Ingle, M.V. Fracture line in post head injury optic nerve damage. J Laryngol Otol 1991, 105, 203–204. [Google Scholar] [CrossRef]
- Raveh, J.; Vuillemin, T.; Sutter, F. Subcranial management of 395 combined frontobasal-midface fractures. Arch Otolaryngol Head Neck Surg 1988, 114, 1114–1122. [Google Scholar] [CrossRef]
- Cornelius, C.P.; Altenmüller, E.; Ehrenfeld, M. Flash-evoked visual potentials in patients with craniofacial fractures. Fortschr Kiefer Gesichtschir 1991, 36, 158–162. [Google Scholar]
- Dutton, G.N.; Al-Qurainy, I.; Stassen, L.F.; Titterington, D.M.; Moos, K.F.; El-Attar, A. Ophthalmic consequences of mid-facial trauma. Eye 1992, 6 Pt 1, 86–89. [Google Scholar] [CrossRef]
- Gellrich, N.C.; Zerfowski, M.; Eufinger, H.; Reinert, S.; Eysel, U.T. Interdisciplinary diagnosis and therapy of traumatic optic nerve damage. Mund Kiefer Gesichtschir 1998, 2 (Suppl. 1), S107–S112. [Google Scholar] [CrossRef]
- Frenkel, R.E.; Spoor, T.C. Diagnosis and management of traumatic optic neuropathies. Adv Ophthalmic Plast Reconstr Surg 1987, 6, 71–90. [Google Scholar]
- Joseph, E.; Zak, R.; Smith, S.; Best, W.R.; Gamelli, R.L.; Dries, D.J. Predictors of blinding or serious eye injury in blunt trauma. J Trauma 1992, 33, 19–24. [Google Scholar] [CrossRef]
- Hughes, B. Indirect injury of the optic nerves and chiasma. Bull Johns Hopkins Hosp 1962, 111, 98–126. [Google Scholar] [PubMed]
- Hager, G.; Maruniak, M.; Gerhardt, H.-J. Indications and results of operative exposure of traumatically damaged optic nerves (au- thor’s transl). Klin Monatsbl Augenheilkd 1975, 167, 515–526. [Google Scholar]
- Blanco, R.; Salvador, F.; Galan, A.; Gil-Gibernau, J.J. Aplasia of the optic nerve: Report of three cases. J Pediatr Ophthalmol Strabismus 1992, 29, 228–231. [Google Scholar]
- Sloan, T.; Sloan, H.; Rogers, J. Nitrous oxide and isoflurane are synergistic with respect to amplitude and latency effects on sensory evoked potentials. J Clin Monit Comput 2010, 24, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, I.; Hidaka, S.; Okada, H.; Kubo, T.; Okamura, K.; Kato, T. Effects of sevoflurane and propofol on evoked potentials during neuro- surgical anesthesia. Masui 2006, 55, 692–698. [Google Scholar]
- Hamaguchi, K.; Nakagawa, I.; Hidaka, S.; Uesugi, F.; Kubo, T.; Kato, T. Effect of propofol on visual evoked potentials during neurosurgery. Masui 2005, 54, 998–1002. [Google Scholar]
- Bonkowsky, V.M.; Mang, W.L.; Wendl, F.; Frank, C. Neurologic compli- cations in mid-face fractures. Laryngorhinootologie 1989, 68, 539–542. [Google Scholar] [CrossRef]
- Gossman, M.D.; Roberts, D.M.; Barr, C.C. Ophthalmic aspects of orbital injury. A comprehensive diagnostic and management approach. Clin Plast Surg 1992, 19, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Klopfer, J.; Tielsch, J.M.; Vitale, S.; See, L.C.; Canner, J.K. Ocular trauma in the United States. Eye injuries resulting in hospitalization, 1984 through 1987. Arch Ophthalmol 1992, 110, 838–842. [Google Scholar]
- Ioannides, C.; Treffers, W.; Rutten, M.; Noverraz, P. Ocular injuries associated with fractures involving the orbit. J Craniomaxillofac Surg 1988, 16, 157–159. [Google Scholar]
- Mahapatra, A.K.; Bhatia, R. Predictive value of visual evoked poten- tials in unilateral optic nerve injury. Surg Neurol 1989, 31, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Shokunbi, T.; Agbeja, A. Ocular complications of head injury in children. Childs Nerv Syst 1991, 7, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Brent, B.D.; May, D.R. Orbital apex syndrome after penetrating orbital trauma. Ann Ophthalmol 1990, 22, 267–268. [Google Scholar]
- Hutton, W.L.; Fuller, D.G. Factors influencing final visual results in severely injured eyes. Am J Ophthalmol 1984, 97, 715–722. [Google Scholar] [CrossRef]
- Jorissen, M.; Feenstra, L. Optic nerve decompression for indirect posterior optic nerve trauma. Acta Otorhinolaryngol Belg 1992, 46, 311–324. [Google Scholar]
- Shaked, A.; Hadani, M.; Feinsod, M.C.T. CT and VER follow-up of reversible visual loss with fracture of the optic canal. Acta Neurochir 1982, 62, 91–94. [Google Scholar] [CrossRef]
- Dorfman, L.J.; Gaynon, M.; Ceranski, J.; Louis, A.A.; Howard, J.E. Visual electrical evoked potentials: Evaluation of ocular injuries. Neurology 1987, 37, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, A.K. Visual evoked potentials in optic nerve injury—Does it merit to be mentioned? Indian J Ophthalmol 1991, 39, 20–21. [Google Scholar]
- Halliday, A.M.; Halliday, E.; Kriss, A.; McDonald, W.I.; Mushin, J. The pattern-evoked potential in compression of the anterior visual pathways. Brain 1976, 99, 357–374. [Google Scholar] [CrossRef]
- Uren, S.M.; Stewart, P.; Crosby, P.A. Subject cooperation and the visual evoked response. Invest Ophthalmol Vis Sci 1979, 18, 648–652. [Google Scholar]
- Jayle, G.E.; Tassy, A.F. Prognostic value of the electroretinogram in severe recent ocular trauma. Br J Ophthalmol 1970, 54, 51–58. [Google Scholar] [PubMed]
- Gellrich, N.C.; Gellrich, M.M.; Zerfowski, M.; Eufinger, H.; Eysel, U.T. Clinical and experimental study of traumatic optic nerve damage. Ophthalmologe 1997, 94, 807–814. [Google Scholar]
- Greenberg, R.P.; Becker, D.P.; Miller, J.D.; Mayer, D.J. Evaluation of brain function in severe human head trauma with multimodality evoked potentials. Part 2, localization of brain dysfunction and correlation with posttraumatic neurological conditions. J Neurosurg 1977, 47, 163–177. [Google Scholar]
- Meyer, B.; François, M.; Lacau Saint-Guily, J.; Lacombe, H.; Laroche, L. Post-traumatic blindness and spontaneous decompression of optical nerve (author’s transl). Ann Otolaryngol Chir Cervicofac 1982, 99, 53–55. [Google Scholar] [PubMed]
- Rochels, R. Ultrasonic diagnosis of fractures of the bony orbit. Fortschr Kiefer Gesichtschir 1987, 32, 144–147. [Google Scholar] [PubMed]
- Chirico, P.A.; Mirvis, S.E.; Kelman, S.E.; Karesh, J.W. Orbital “blow-in” fractures: Clinical and CT features. J Comput Assist Tomogr 1989, 13, 1017–1022. [Google Scholar]
- Luka, B.; Brechtelsbauer, D.; Gellrich, N.C.; König, M. 2D and 3D CT reconstructions of the facial skeleton: An unnecessary option or a diagnostic pearl? Int J Oral Maxillofac Surg 1995, 24 Pt 2, 76–83. [Google Scholar]
- Behrens-Baumann, W.; Chilla, R. Drug and surgical therapy of traumatic optic nerve compression. Fortschr Ophthalmol 1984, 81, 87–89. [Google Scholar]
- Spoor, T.C.; Hartel, W.C.; Lensink, D.B.; Wilkinson, M.J. Treatment of traumatic optic neuropathy with corticosteroids. Am J Ophthalmol 1990, 110, 665–669. [Google Scholar] [CrossRef]
- Purvin, V. Evidence of orbital deformation in indirect optic nerve injury. Weight lifter’s optic neuropathy. J Clin Neuroophthalmol 1988, 8, 9–11. [Google Scholar]
- Bilyk, J.R.; Dallow, R.L.; Ojemann, R.G.; Linggood, R.M.; Shore, J.W. Man- agement of lesions at the cranioorbital junction. Int Ophthalmol Clin 1992, 32, 73–93. [Google Scholar]
- Manfredi, S.J.; Raji, M.R.; Sprinkle, P.M.; Weinstein, G.W.; Minardi, L.M.; Swanson, T.J. Computerized tomographic scan findings in facial fractures associated with blindness. Plast Reconstr Surg 1981, 68, 479–490. [Google Scholar] [PubMed]
- Mahapatra, A.K.; Tandon, D.A. Traumatic optic neuropathy in chil- dren: A prospective study. Pediatr Neurosurg 1993, 19, 34–39. [Google Scholar]
- Seiler, T.; Bende, T.; Schilling, A.; Wollensak, J. Magnetic resonance tomography in ophthalmology. II. Manifestations of edema of the optic nerve. Klin Monatsbl Augenheilkd 1989, 195, 72–78. [Google Scholar] [PubMed]
- Crowe, N.W.; Nickles, T.P.; Troost, B.T.; Elster, A.D. Intrachiasmal hemor- rhage: A cause of delayed post-traumatic blindness. Neurology 1989, 39, 863–865. [Google Scholar]
- Kakisu, Y.; Adachi-Usami, E.; Fujimoto, N. Pattern visually evoked cortical potential and magnetic resonance imaging in optic neuritis. J Clin Neuroophthalmol 1991, 11, 205–212. [Google Scholar] [PubMed]
- Lipkin, A.F.; Woodson, G.E.; Miller, R.H. Visual loss due to orbital fracture. The role of early reduction. Arch Otolaryngol Head Neck Surg 1987, 113, 81–83. [Google Scholar]
- Lam, B.L.; Weingeist, T.A. Corticosteroid-responsive traumatic optic neuropathy. Am J Ophthalmol 1990, 109, 99–101. [Google Scholar]
- Seiff, S.R. High dose corticosteroids for treatment of vision loss due to indirect injury to the optic nerve. Ophthalmic Surg 1990, 21, 389–395. [Google Scholar]
- Hall, E.D.; Braughler, J.M. Glucocorticoid mechanisms in acute spinal cord injury: A review and therapeutic rationale. Surg Neurol 1982, 18, 320–327. [Google Scholar]
- Panje, W.R.; Gross, C.E.; Anderson, R.L. Sudden blindness following facial trauma. Otolaryngol Head Neck Surg 1981, 89, 941–948. [Google Scholar] [CrossRef]
- Bracken, M.B.; Holford, T.R. Effects of timing of methylprednisolone or naloxone administration on recovery of segmental and long- tract neurological function in NASCIS 2. J Neurosurg 1993, 79, 500–507. [Google Scholar]
- Bracken, M.B.; Shepard, M.J.; Collins, W.F.; et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med 1990, 322, 1405–1411. [Google Scholar] [PubMed]
- Osguthorpe, J.D. Transethmoid decompression of the optic nerve. Otolaryngol Clin North Am 1985, 18, 125–137. [Google Scholar]
- Ord, R.A.; El Attar, A. Acute retrobulbar hemorrhage complicating a malar fracture. J Oral Maxillofac Surg 1982, 40, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Dolman, P.J.; Glazer, L.C.; Harris, G.J.; Beatty, R.L.; Massaro, B.M. Mechanisms of visual loss in severe proptosis. Ophthal Plast Reconstr Surg 1991, 7, 256–260. [Google Scholar]
- Katz, B.; Herschler, J.; Brick, D.C. Orbital haemorrhage and prolonged blindness: A treatable posterior optic neuropathy. Br J Ophthalmol 1983, 67, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Takenoshita, Y.; Hasuo, K.; Matsushima, T.; Oka, M. Carotid-cavernous sinus fistula accompanying facial trauma. Report of a case with a review of the literature. J Craniomaxillofac Surg 1990, 18, 41–45. [Google Scholar]
- Zachariades, N.; Papavassiliou, D. Traumatic carotid-cavernous sinus fistula. J Craniomaxillofac Surg 1988, 16, 385–388. [Google Scholar]
- Ardouin, M.; Urvoy, M.; Bourdinière, J.; Guillou, B.; Eon, J.Y. What should be one’s attitude toward trans-sinus latero-orbital decompressive surgery in optic nerve injuries? Bull Soc Ophtalmol Fr 1976, 76, 895–905. [Google Scholar]
- Karnik, P.P.; Maskati, B.T.; Kirtane, M.V.; Tonsekar, K.S. Optic nerve decompression in head injuries. J Laryngol Otol 1981, 95, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Behrens-Baumann, W.; Miehlke, A. Rhinobasal deompression of the optic nerve following trauma (author’s transl). Klin Monatsbl Augenheilkd 1979, 175, 584–591. [Google Scholar] [PubMed]
- Funk, G.F.; Stanley, R.B. Jr.; Becker, T.S. Reversible visual loss due to impacted lateral orbital wall fractures. Head Neck 1989, 11, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Kennerdell, J.S.; Amsbaugh, G.A.; Myers, E.N. Transantral-ethmoidal decompression of optic canal fracture. Arch Ophthalmol 1976, 94, 1040–1043. [Google Scholar] [CrossRef]
- Laroche, L.; Lacombe, H.; Meyer, B.; Saraux, H. Trans-ethmoidal- sphenoidal decompression and optic nerve injury. Bull Soc Ophtalmol Fr 1984, 84, 431–432. [Google Scholar]
- Girard, B.; Bouzas, E.; Lamas, G.; Topouzis, F.; Soudant, J. X-ray comput- ed tomography in surgical indication of physiological section of the optic nerve. Apropos of 15 cases. J Fr Ophtalmol 1992, 15, 93–101. [Google Scholar]
- Waga, S.; Kubo, Y.; Sakakura, M. Transfrontal intradural microsurgical decompression for traumatic optic nerve injury. Acta Neurochir (Wien) 1988, 91, 42–46. [Google Scholar] [CrossRef]
- Sofferman, R.A. An extracranial microsurgical approach to the optic nerve. J Microsurg 1979, 1, 195–202. [Google Scholar] [CrossRef]
- Mann, W.; Rochels, R.; Bleier, R. Microsurgical endonasal decompres- sion of the optic nerve. Fortschr Ophthalmol 1991, 88, 176–177. [Google Scholar]
- Takahashi, M.; Itoh, M.; Kaneko, M.; Ishii, J.; Yoshida, A. Microscopic intranasal decompression of the optic nerve. Arch Otorhinolaryngol 1989, 246, 113–116. [Google Scholar] [CrossRef]
- Slamovits, T.L.; Gardner, T.A. Neuroimaging in neuro-ophthalmology. Ophthalmology 1989, 96, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.; Hollmann, K.; Millesi, W. Results of surgically and conservatively treated cases of traumatic optic nerve lesions. Klin Monatsbl Augenheilkd 1986, 189, 421–422. [Google Scholar] [CrossRef] [PubMed]
- Kolenda, H.; Schröder, M.; Mühlendyck, H.; Rama, B.; Markakis, E. Transethmoidal decompression of the optic nerve in the case of craniocerebral trauma. Neurosurg Rev 1988, 11, 39–43. [Google Scholar] [CrossRef]
- Guyer, D.R.; Miller, N.R.; Long, D.M.; Allen, G.S. Visual function following optic canal decompression via craniotomy. J Neurosurg 1985, 62, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Siegl, H. Decompression of the optic nerve. Laryngol Rhinol Otol (Stuttg) 1985, 64, 118–120. [Google Scholar] [CrossRef]
- Stoll, W.; Busse, H.; Kroll, P. Visual recovery following orbital and optic nerve decompressions. Laryngol Rhinol Otol (Stuttg) 1987, 66, 577–582. [Google Scholar]
- Girard, B.; Lamas, G.; Bouzas, E.; Topouzis, F.; Soudant, J. Surgical decompression of the optic nerve in intracanal injuries. Indica- tions and results. J Fr Ophtalmol 1992, 15, 83–92. [Google Scholar]
- Machtens, E.; Heuser, L. Basic and graduated procedures in the roentgen diagnosis of midfacial trauma in relation to degree of severity and localization. Fortschr Kiefer Gesichtschir 1991, 36, 21–25. [Google Scholar]
- Seiff, S.R.; Berger, M.S.; Guyon, J.; Pitts, L.H. Computed tomographic evaluation of the optic canal in sudden traumatic blindness. Am J Ophthalmol 1984, 98, 751–755. [Google Scholar] [CrossRef]
- Kurzeja, A.; Wenzel, M.; Korves, B.; Mösges, R. Decompression of the optic nerve after fractures of the rhino-basal skull with computer- assisted surgery. Laryngorhinootologie 1994, 73, 274–276. [Google Scholar] [CrossRef]
- Cook, M.W.; Levin, L.A.; Joseph, M.P.; Pinczower, E.F. Traumatic optic neuropathy. A meta-analysis. Arch Otolaryngol Head Neck Surg 1996, 122, 389–392. [Google Scholar] [PubMed]
- Habal, M.B.; Maniscalco, J.E. Surgical relations of the orbit and optic nerve: An anatomical study under magnification. Ann Plast Surg 1980, 4, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Janáky, M.; Benedek, G. Visual evoked potentials during the early phase of optic nerve compression in the orbital cavity. Doc Ophthalmol 1992, 81, 197–208. [Google Scholar]
- Obertacke, U.; Joka, T.; Härting, F.; Nau, H.E.; Sauerwein, W. Amaurosis caused by traumatic damage to the optic nerve. Unfallchirurg 1986, 89, 132–137. [Google Scholar] [PubMed]

© 2013 by the author. The Author(s) 2013.
Share and Cite
Schumann, P.; Kokemüller, H.; Tavassol, F.; Lindhorst, D.; Lemound, J.; Essig, H.; Rücker, M.; Gellrich, N.-C. Optic Nerve Monitoring. Craniomaxillofac. Trauma Reconstr. 2013, 6, 75-85. https://doi.org/10.1055/s-0033-1343783
Schumann P, Kokemüller H, Tavassol F, Lindhorst D, Lemound J, Essig H, Rücker M, Gellrich N-C. Optic Nerve Monitoring. Craniomaxillofacial Trauma & Reconstruction. 2013; 6(2):75-85. https://doi.org/10.1055/s-0033-1343783
Chicago/Turabian StyleSchumann, Paul, Horst Kokemüller, Frank Tavassol, Daniel Lindhorst, Juliana Lemound, Harald Essig, Martin Rücker, and Nils-Claudius Gellrich. 2013. "Optic Nerve Monitoring" Craniomaxillofacial Trauma & Reconstruction 6, no. 2: 75-85. https://doi.org/10.1055/s-0033-1343783
APA StyleSchumann, P., Kokemüller, H., Tavassol, F., Lindhorst, D., Lemound, J., Essig, H., Rücker, M., & Gellrich, N.-C. (2013). Optic Nerve Monitoring. Craniomaxillofacial Trauma & Reconstruction, 6(2), 75-85. https://doi.org/10.1055/s-0033-1343783


