Abstract
The aneurysmal bone cyst (ABC) is a benign cystic and expanding osteolytic lesion consisting of bone-filled spaces of variable size, separated by connective tissue containing trabeculae of bone or osteoid tissue and osteoclast giant cells. Radiographic findings may vary from unicystic or moth-eaten radiolucencies to extensive multilocular lesions with bilateral expansion and destruction of mandibular cortices. Treatment modalities include curettage (with reported recurrences) and resection with immediate reconstruction. The main arterial and feeder vessels may be embolized to prevent profuse intraoperative blood loss and achieve a bloodless surgical field. Failed embolization may necessitate ligation of the external carotid artery of the affected side.
The aneurysmal bone cyst (ABC) was first described by Jaffe and Lichenstein in 1942 [1,2]. Bernier and Bhaskar [3] reported the first case of an ABC involving the mandible. The World Health Organization defined ABCs as an expansive osteolytic lesion consisting of blood-filled spaces and channels divided by connective tissue septa that can contain osteoid tissue and osteoclast-like giant cells [4]. They are often admixed with trabeculae of reactive woven bone [5].
They are rare, benign, expanding, locally destructive, and often misdiagnosed lesions, which can cause rapid bony expansion. ABCs are not true neoplasms [6]. More than 50% of ABCs occur in the long bones and vertebral column. The lesions are frequently seen in the clavicle, rib, innominate bone, skull, and bones of the hand, feet, and other sites. They are usually tender or painful, especially on motion, and this soreness may limit movement of the affected bones. Swelling over the area of the involved bone is common [7]. ABCs can present as asymptomatic radiological findings or rapidly expanding progressive lesions that are destructive and can lead to pathological fractures of the involved bones. They are essentially low-flow macrocystic lesions usually filled with blood [8]. They can occur as either a primary or a secondary process.
The characteristic radiological features of mandibular ABCs can vary from mainly unilocular radiolucencies to multilocular radiolucencies with honeycomb- or soap bubble–like appearances [9]. Treatment modalities include simple curettage, radiotherapy, sclerosing injections, various forms of embolization, as well as resection and immediate reconstruction with grafts [10]. During operation, an intact periosteum and very thin bony shell usually cover the cyst. When these are removed, dark venous blood wells up, which should usually stop. However, bleeding may be difficult to control. Surgery has been complicated by massive hemorrhage in many cases necessitating ligation of the external carotid artery (ECA) [11]. Recent genetic studies proposed that primary ABC is a tumor rather than a reactive lesion. A predisposing genetic defect could be part of a multifactorial pathogenesis in the development of some ABCs. Some primary ABCs exhibited the chromosomal translocation t (16;19) (q22;p13) [12].
Case Report
A 12-year-old girl presented to a maxillofacial and oral surgery practice with swelling of her left mandible (Figure 1a). The swelling was present for several months and progressively increased in size. The large mass made mastication increasingly difficult. Clinical examination revealed a large extraoral swelling extending from the posterior border of the ramus to about the lower left canine region anteriorly. Intraorally, there was an expansile lesion of the left side of the mandible with mobile displaced teeth (Figure 1b). The mass moved on movement of the mandible. There was no bruit on auscultation. Routine hematologic studies included full blood count, urea and electrolyte studies, activated partial thromboplastin time, random glucose and liver function tests. These were found to be within normal limits. HIV serology was nonreactive. There was no further contributory medical history.
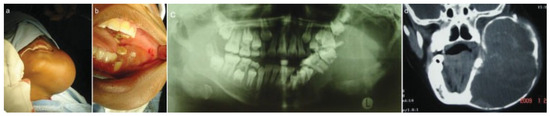
Figure 1.
(a) Preoperative extraoral view. (b) Preoperative intraoral view showing expansion of alveolus. (c) Preoperative orthopantomograph. (d) Computed tomography scan.
Imaging studies included chest radiographs, an orthopantomograph, posteroanterior mandibular radio-graph, computed tomography (CT), and CT angiogram (Figure 1c,d and Figure 2a–c). The chest radiographs were normal. The orthopantomograph showed an expansile lesion of the left side of the mandible involving the ramus and body with associated displaced and floating teeth. CT studies included 1.5 × 3-mm overlapping axial cuts, 5 × 5-mm coronal cuts through the same region, and 5 × 5-mm spiral axial scans through the mandible after contrast administration. Imaging studies showed a large expansile multilocular cystic mass involving the left mandible. The mass measured 8 × 9 cm and 152 to 158 Hounsfield units, which was diagnostically suggestive of a vascular lesion radiologically. There were septae and some enhancing soft tissue components within the mass. The lesion extended to the nasopharynx and oral cavity medially, to the base of the skull superiorly, and to the parotid and carotid spaces posteriorly. There was no intracranial involvement. The patient was booked for incisional biopsy. Prior aspiration of the lesion was positive for blood, which appeared to be of a high-flow nature. To avoid the possibility of uncontrollable intra-operative bleeding, the procedure was abandoned. Based on the imaging studies, a provisional diagnosis of an ABC was made. Attempted shrinkage of the lesion was considered with the use of embolization. The patient was therefore referred to an interventional radiologist for possible embolization of the lesion. Embolization of the internal maxillary artery on the left side was done with 100- to 300- as well as 300- to 500-size contour particles. A silicone SL14 catheter (Jet Medical South Africa, Gauteng, Cape Town, KZN, South Africa) with a Silver Speed guide wire (Micra Therapentics, Inc., Irvine, CA) was used to do superselective embolization of the feeding vessels to the tumor. An angiogram done at this stage showed a highly vascular mass in the left mandible. The patient was unable to keep to the regular appointments due to financial constraints and therefore presented herself 3 months after embolization. The lesion was found to have increased in size. New CT scans confirmed that the lesion had increased in size and was still highly vascular. A decision was taken to surgically remove the lesion after ligation of the ECA with immediate reconstruction of the mandible. Postresection reconstruction was preplanned using stereolithographic models to achieve maximum preoperative symmetry and function.
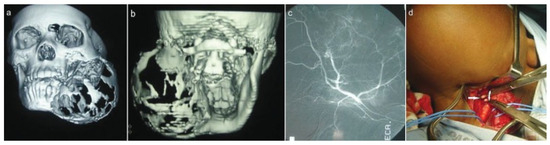
Figure 2.
(a,b) 3-D computed tomography (CT) reconstruction views. (c) CT angiogram during embolization. (d) Exposed external carotid artery (arrows).
The ECA was identified and ligated (Figure 3a). The tumor was then dissected subperiosteally and resected, followed by immediate reconstruction using a titanium heavy reconstruction plate (Figure 3b). The tumor mass was removed via an intraoral approach. Postoperative recovery was un-eventful. The biopsy report confirmed the lesion as being an ABC (Figure 4d). Due to the long span of the defect, it was envisaged that a delayed free fibula reconstruction be done subsequently.
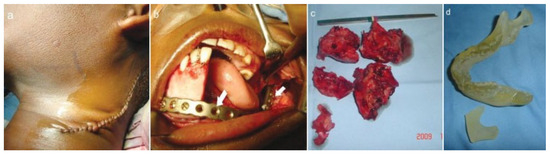
Figure 3.
(a) Approach to external carotid artery. (b) Reconstruction plate after fixation. (c) Resected specimen. (d) Stereolithographic model.
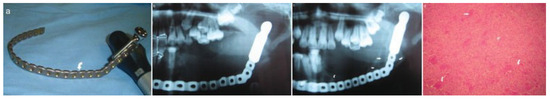
Figure 4.
(a) Prebent reconstruction plate. (b) One-week postoperative orthopantomograph. (c) Eighteenth-month postoperative orthopantomograph showing evidence of osteogenesis (arrows). (d) Micrograph showing multinucleated giant cells (arrows).
The surgical wounds healed without complications. Review of the patient after 18 months showed no recurrence of the lesion. New bone formation was clinically palpable and evident on radiographs (Figure 4c), which negated the need for grafting. Depending on patient compliance and financial affordability, long-term follow-up visits are planned with a view to obtaining CT scans to assess the degree of new bone formation with histology, temporomandibular joint function and growth.
Discussion
ABCs are classified as pseudocysts as they appear radiographically as a cystlike lesion but microscopically exhibit no epithelial lining. In most cases, mandibular ABCs present as painless expansive masses that rarely result in malocclusion or devitalized teeth. When the expansion is great enough, teeth adjacent to the cyst may be displaced, or pain may develop when an adjacent joint space is encroached or soft tissue impinged. The bicortical erosion resulting from osteolytic effects of pressure expansion suggests cystic evolution into the active growth phase. Stabilization is exemplified by a thin shell of reactive new bone, and hence the “soap bubble” appearance seen on radiography [13]. There is a mandibular predominance, with most arising in the posterior segments [5,14]. On occasion, malocclusion together with mobility, migration, or resorption of involved teeth may be present. Maxillary lesions often bulge into the adjacent sinus, causing nasal obstruction, epistaxis, proptosis, and diplopia [5].
They are generally regarded as representative of a reactive rather than a neoplastic process. Frequently, an unrelated antecedent primary lesion of bone exists that is believed to initiate a vascular, presumably arteriovenous malformation causing significant alteration of hemodynamic forces and resulting in a secondary lesion or ABC. Lichenstein [15] proposed that this phenomenon leads to an increased venous pressure and subsequent development of dilated and engorged vascular bed in the transformed bone area. Resorption of bone then occurs, to which giant cells are related, and this is replaced by connective tissue, osteoid, and new bone [7].
An alternate explanation is that it represents an exuberant attempt at repair of a hematoma of bone, similar to central giant cell granuloma (GCG). But in ABC, it is postulated that the hematoma maintains a circulatory connection with the damaged vessel, which would produce a slow flow of blood through the lesion and account for the clinical welling of blood when the lesion is entered. Therefore, the only difference between the ABC and the GCG is that the GCG fails to retain a circulatory connection with the lesion [7].
Occurrence of the ABC with other lesions has been reported and includes solitary bone cyst, giant cell tumor, osteoclastoma, odontogenic fibroma, hemangioepithelioma, and hemangioma. ABCs were also reported to form secondary to fractures or other bone trauma [5,7]. Raubenheimer et al. reported a case of an ABC associated with an ameloblastoma [16]. Reports document a preexisting fibrous dysplasia in some cases and a central GCG in others. Less commonly, the ABC may arise in association with a nonossifying fibroma, chondroblastoma, or central hemangioma. The association of the ABC with other lesions has been termed ABC “plus” [8].
Radiographically, ABCs show an expansile, often “blowout” type of lesion with thinning of cortical plate and a honeycomb or soap bubble appearance. The inner side of the lesion may demonstrate spurs or ridges. Contrast-enhanced CT scans show thinning of mandibular cortical plates. Magnetic resonance imaging show high signal intensity within the lesion and low signal intensity at the periphery of the lesion. Hypovascular or avascular lesions are noted with arteriography, sometimes together with dilated overlying vessels, which maybe embolized if microvascular free flap reconstruction is not being considered. A manometer test for ABCs have also been described wherein a needle is introduced into the lesion and attached to a manometer. A gradual rise in the column of blood in the manometer occurs, which oscillates with the pulse and confirms the diagnosis of an ABC [9]. In 16 cases with which this technique was utilized, Chari and Reddy [17] showed that a significant 12 cases (75%) had an increase in pressure noted by manometry, and these lesions were all found to be ABCs [9].
The treatment of ABCs consists primarily of curettage. Recurrence rates after curettage have been reported to be as high as 50%. Curettage may be associated with postoperative pain, wound dehiscence, swelling, and sepsis. Cryotherapy is associated with a lower recurrence rate; however, nerve tissue surrounding the lesion is at risk of being damaged.
Embolization techniques alone have not been universally successful and have often resulted in rapid development of collaterals from surrounding vessels [10]. Furthermore, it may not always be possible to navigate catheters through small vessels for remobilization, especially when the anatomy has been grossly distorted [18]. Radiation therapy has also been used but with the risk of malignant transformation. Sarcomas have been reported to arise from ABCs that were irradiated. The use of autogenous costochondral grafts, cancellous bone chips, as well as microvascular free flap reconstruction to bridge postresection defects has been reported [9]. Although spontaneous regression of an ABC is very rare, several reports have documented healing after open biopsy [1]. A “wait and see” approach can be used in small lesions.
Recurrence prophylaxis with calcitonin nasal spray has been successful in some studies. Calcitonin inhibits osteoclastic activity and promotes trabecular bone formation with increased mineral density [18]. Transcatheter embolization and ligation of the ECA is often undertaken to cut off the blood supply of large hypervascular tumors and for bleeding in severe epistaxis and trauma. This procedure is safe, with no ischemic consequences to tissues in the head and neck region [9].
It is well documented that if the right or left ECA be ligated, blood can reach the ligated side through the opposite ECA or by way of the circle of Willis within days of ligation. Bryant reported 83 cases of ligation of the ECA with no morbidity or mortality associated with the ligation itself [19]. The anastomosis between the middle meningeal artery (a branch from the maxillary artery) and the ophthalmic artery is a well-described anatomic feature but the incidence is not known. In rare cases, the main contribution to the ophthalmic circulation is from the middle meningeal artery. This variation in origin of the ophthalmic artery can result in catastrophic consequences following occlusion of the ECA [4]. Although the loss of vision in one eye is no less important than the removal of a rapidly enlarging vascular tumor, the problems associated with unexpected uncontrollable intraoperative bleeding therefore cannot be taken lightly.
Spontaneous ossification of the ABC is described in the literature, which may explain the new bone formation seen in the case being described [18].
Included among a few of the various options for the treatment of cystic defects believed to stimulate new bone fill may include:
- A combination of Bioplast® (Guidechem, Zhejiang, China) fibrin powder, thrombin, patient’s blood, and antibiotics.
- Platelet-rich fibrin.
- Xenograft bone with aspirate bone marrow.
- Recombinant bone morphogenic protein (BMP)-2 in con-junction with rib graft.
It is worth noting that other substances capable of delivering BMPs to tissues for longer periods, such as fibrin and collagen sponges, hydroxyapatite, calcium sulphates, and synthetic materials like copolymers, have been used as carriers in most studies. They are believed to increase the osteoinductive capacity of BMPs.
Financial constraints did not allow treatment by any such methods and hence the cavity was closed. It was hoped that the patient’s megakaryocyte-derived BMPs, together with patient-replaced osteogenic granulation tissue, would promote bone growth and repair [20].
The defect left behind after resection of the lesion was in excess of 5 cm, which warranted immediate or delayed grafting. Many authors recommend immediate reconstruction of the defect with autogenous grafts. The surgeons decided against immediate grafting based on the patient’s young age and potential for new bone formation and based on the authors’ experience with cases of new bone formation.
The use of stereolithographic models assisted in preoperative contouring of the reconstruction plate so as to match as closely as possible the contralateral contour of the mandible for facial symmetry. These computer-generated hard copies of the mandible at the preoperative stage could assist further, should bone grafting of the defect become necessary.
Countries such as ours in which extensive cross-border migration is rife are faced with the burden of accepting migrants from countries that have political instability, placing added strain on the health care system. This results in failure to provide the latest health care technology to such patients due to their poor socioeconomic conditions. Patients are therefore very often lost to follow-up. This case has significance as poor preoperative planning, especially of highly vascular ABCs, which can be a surgically bloody experience with possible fatal consequences, and the prospect of new bone formation without grafting make it worth considering. Caution is advocated.
Acknowledgments
We thank Professor E. J. Raubenheimer Head of Department Oral Pathology at the School of Oral Health Sciences, Medunsa Campus, University of Limpopo, South Africa, for providing the histopathology slides and report.
References
- Jaffe, H.L. Aneurysmal bone cyst. Bull Hosp Joint Dis 1950, 11, 3–13. [Google Scholar] [PubMed]
- Lichtenstein, L. Aneurysmal bone cyst. Observations on fifty cases. J Bone Joint Surg Am 1957, 39, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Bernier, J.L.; Bhaskar, S.N. Aneursymal bone cysts of the mandible. Oral Surg Oral Med Oral Path 1958, 11, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.M.; Adams, D.; Vanniasingham, P. What Happens to the External Carotid Artery Following Carotid Endarterectomy. BMC Surgery. 2008. Available online: http://www.medscape.com/viewarticle/585886 (accessed on 15 November 2010).
- Neville, B.W.; Damm, D.D.; Allen, C.M.; Bouquot, J.E. Oral and Maxillofacial Pathology; W.B. Saunders: Philadelphia, PA, USA, 2002; pp. 551–552. [Google Scholar]
- Perrotti, V.; Rubini, C.; Fioroni, M.; Piattelli, A. Solid aneurysmal bone cyst of the mandible. Int J Pediatr Otorhinolaryngol 2004, 68, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Shafer, G.W.; Maynard, K.H.; Barnet, M.; Charles, E.T. Benign and malignant tumours of the oral cavity. In A Textbook of Oral Pathology, 4th ed.; W.B. Saunders: Philadelphia, PA, USA, 1983; pp. 149–152. [Google Scholar]
- Padwa, B.L.; Denhart, B.C.; Kaban, L.B. Aneurysmal bone cyst-“plus”: A report of three cases. J Oral Maxillofac Surg 1997, 55, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Wiatrak, B.J.; Myer, C.M.I.I.I.; Andrews, T.M. Alternatives in the management of aneurysmal bone cysts of the mandible. Int J Pediatr Otorhinolaryngol 1995, 31, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, M.H.; Behnia, H.; Motamedi, M.R. Surgical technique for the treatment of high-flow arteriovenous malformations of the mandible. J Craniomaxillofac Surg 2000, 28, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Struthers, P.J.; Shear, M. Aneurysmal bone cyst of the jaws. (I). Clinicopathological features. Int J Oral Surg 1984, 13, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.J.; Sun, H.L.; Yang, R.L.; Zwahlen, R.A.; Zhao, Y.F. Aneurysmal bone cysts of the jaws. Int J Surg Pathol 2009, 17, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Arden, R.L.; Bahu, S.J.; Lucas, D.R. Mandibular aneurysmal bone cyst associated with fibrous dysplasia. Otolaryngol Head Neck Surg 1997, 117, S153–S156. [Google Scholar] [CrossRef] [PubMed]
- Regezi, J.A.; Scuibba, J. Cysts of the oral region. In Oral Pathology-Clinical Pathologic Correlations, 2nd ed.; W.B. Saunders: Philadelphia, PA, USA, 1993; pp. 303–361. [Google Scholar]
- Lichtenstein, L. Aneurysmal bone cyst. Observations on fifty cases. J Bone Joint Surg Am 1957, 39, 873. [Google Scholar] [CrossRef] [PubMed]
- Raubenheimer, E.J.; van Heerden, W.F.; Noffke, C.E. Infrequent clinico-pathological findings in 108 ameloblastomas. J Oral Pathol Med 1995, 24, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Chari, P.R.; Reddy, C.R. A clinical test to differentiate aneurysmal bone cyst from other benign osseous cystic lesions. Aust NZ Surg 1980, 50, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Ettl, T.; Ständer, K.; Schwarz, S.; Reichert, T.E.; Driemel, O. Recurrent aneurysmal bone cyst of the mandibular condyle with soft tissue extension. Int J Oral Maxillofac Surg 2009, 38, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.D. Ligation of the external carotid artery with remarks on the history of the operation. Ann Surg 1887, 6, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Perumal, C.J. An unusually large destructive nasopalatine duct cyst: A case report. J Maxillofac Oral Surg 2011. [CrossRef] [PubMed]
© 2012 by the author. The Author(s) 2012.