Need for Redo Surgery of Maxillofacial Fractures
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Descriptive Statistics
2.3. Study Variables for Data Analyses
2.4. Data Analyses
2.5. Ethical Considerations
3. Results
3.1. Descriptive Statistics
3.2. Data Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Gutta, R.; Tracy, K.; Johnson, C.; James, L.E.; Krishnan, D.G.; Marciani, R.D. Outcomes of Mandible Fracture Treatment at an Academic Tertiary Hospital: A 5-Year Analysis. J. Oral Maxillofac. Surg. 2014, 72, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Raikundalia, M.; Svider, P.F.; Hanba, C.; Folbe, A.J.; Shkoukani, M.A.; Baredes, S.; Anderson Eloy, J. Facial Fracture Repair and Diabetes Mellitus: An Examination of Postoperative Complications. Laryngoscope 2017, 127, 809–814. [Google Scholar] [CrossRef]
- Chen, C.L.; Zenga, J.; Patel, R.; Branham, G. Complications and Reoperations in Mandibular Angle Fractures. JAMA Facial Plast. Surg. 2018, 20, 238–243. [Google Scholar] [CrossRef]
- Daar, D.A.; Kantar, R.S.; Cammarata, M.J.; Rifkin, W.J.; Alfonso, A.R.; Wilson, S.C.; Rodriguez, E.D. Predictors of Adverse Outcomes in the Management of Mandibular Fractures. J. Craniofac. Surg. 2019, 30, 571–577. [Google Scholar] [CrossRef]
- Perez, D.; Ellis, E. Complications of Mandibular Fracture Repair and Secondary Reconstruction. Semin. Plast. Surg. 2020, 34, 225–231. [Google Scholar] [CrossRef]
- Thepmankorn, P.; Choi, C.B.; Haimowitz, S.Z.; Parray, A.; Grube, J.G.; Fang, C.H.; Baredes, S.; Anderson Eloy, J. ASA Physical Status Classification and Complications Following Facial Fracture Repair. Ann. Otol. Rhinol. Laryngol. 2022, 131, 1252–1260. [Google Scholar] [CrossRef]
- Seemann, R.; Schicho, K.; Wutzl, A.; Koinig, G.; Poeschl, W.P.; Krennmair, G.; Ewers, R.; Klug, C. Complication Rates in the Operative Treatment of Mandibular Angle Fractures: A 10-Year Retrospective. J. Oral Maxillofac. Surg. 2010, 68, 647–650. [Google Scholar] [CrossRef]
- Ghosh, R.; Gopalkrishnan, K.J. Facial Fractures. Craniofac. Surg. 2018, 29, e334–e340. [Google Scholar] [CrossRef]
- Oksa, M.; Haapanen, A.; Marttila, E.; Snäll, J. Simple dentate area fractures of the mandible –can we prevent postoperative infections? Acta Odontol. Scand. 2022, 80, 494–500. [Google Scholar] [CrossRef]
- Oksa, M.; Haapanen, A.; Marttila, E.; Furuholm, J.; Snäll, J. Postoperative wound dehiscence in mandibular fractures. Acta Odontol. Scand. 2023, 81, 555–561. [Google Scholar] [CrossRef]
- Nikunen, M.; Rajantie, H.; Marttila, E.; Snäll, J. Implant malposition and revision surgery in primary orbital fracture reconstructions. J. Craniomaxillofac. Surg. 2021, 49, 837–844. [Google Scholar] [CrossRef]
- Persson, A.A.E.; Lifa, H.M.; Falk-Delgado, A.; Nowinski, D. Treatment of orbital fractures—A critical analysis of ophthalmic outcomes and scenarios for re-intervention. J. Plast. Surg. Hand Surg. 2023, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.C.; Dierks, E.J.; Kademani, D.; Holmgren, E.; Bell, R.B.; Hommer, L.; Potter, B.E. Application of a Facial Injury Severity Scale in Craniomaxillofacial Trauma. J. Oral Maxillofac. Surg. 2006, 64, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Hirvikangas, R.; Bertell, J.; Marttila, E.; Löfgren, M.; Snäll, J.; Uittamo, J. Patient injury-related alcohol use—Underestimated in patients with facial fractures? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.; Perez, D. An algorithm for the treatment of isolated zygomatico-orbital fractures. J. Oral Maxillofac. Surg. 2014, 72, 1975–1983. [Google Scholar] [CrossRef]
- Snäll, J.; Kormi, E.; Koivusalo, A.M.; Lindqvist, C.; Suominen, A.L.; Törnwall, J.; Thorén, H. Effects of perioperatively administered dexamethasone on surgical wound healing in patients undergoing surgery for zygomatic fracture: A prospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 685–689. [Google Scholar] [CrossRef]
- Gawande, M.J.; Pravin, N.; Lambade, P.N.; Bande, C.; Gupta, M.K.; Mahajan, M.; Dehankar, T. Two-Point versus Three-Point Fixation in the Management of Zygomaticomaxillary Complex Fractures: A Comparative Study. Ann. Maxillofac. Surg. 2021, 11, 229–235. [Google Scholar] [CrossRef]
- Arjmand, H.; Fialkov, J.A.; Whyne, C.M. Modeling stability post zygomatic fracture reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2024, 91, 241–248. [Google Scholar] [CrossRef]
- Pons, M.; Lutz, J.-C.; Chatelain, B.; Weber, E.; Barrabe, A.; Meyer, C.; Sigaux, N.; Louvrier, A. Impact of intraoperative cone beam computed tomography in the management of zygomatic fractures. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 349–354. [Google Scholar] [CrossRef]
- Sritharan, R.; Arya, R.; Abdelrahman, A.; Parmar, S.; Sharp, I.; Breeze, J. Justifying the implementation of intraoperative computed tomography for midface fracture treatment in improving outcomes. Br. J. Oral Maxillofac. Surg. 2023, 61, 315–319. [Google Scholar] [CrossRef]
- Liu, Y.; Enin, K.; Sciegienka, S.; Hardi, A.; Spataro, E. Intraoperative Computed Tomography Use in Orbital Fracture Repair: A Systematic Review and Meta-Analysis. Facial Plast. Surg. Aesthet. Med. 2023, 25, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, C.F.; Ghali, G.E. Late Correction of Orbital Deformities. Oral Maxillofac. Surg. Clin. N. Am. 2012, 24, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Chepurnyi, Y.; Chernogorskyia, D.; Kopchak, A.; Petrenko, O. Clinical efficacy of peek patient-specific implants in orbital reconstruction. J. Oral Biol. Craniofac. Res. 2020, 10, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Blumer, M.; Pejicic, R.; Gander, T.; Johner, J.P.; Held, U.; Wagner, M.E. Customized Titanium Reconstruction of Orbital Fractures Using a Mirroring Technique for Virtual Reconstruction and 3D Model Printing. J. Oral Maxillofac. Surg. 2021, 79, 200.e1–200.e9. [Google Scholar] [CrossRef]
- Timoshchuk, M.-A.; Murnan, E.J.; Chapple, A.G.; Christensen, B.J. Do Patient-Specific Implants Decrease Complications and Increase OrbitalVolume Reconstruction Accuracy in Primary Orbital Fracture Reconstruction? J. Oral Maxillofac. Surg. 2022, 80, 669–675. [Google Scholar] [CrossRef]
- Salli, M.; Nikunen, M.; Snäll, J. Primary reconstruction of extensive orbital fractures using two-piece patient-specific implants: The Helsinki protocol. Oral Maxillofac. Surg. 2023, 27, 333–340. [Google Scholar] [CrossRef]
- Lander, D.P.; Lee, J.J.; Kallogjeri, D.; Stwalley, D.; Olsen, M.A.; Piccirillo, J.F.; Spataro, E.A. The Impact of Treatment Delay on Malunion and Nonunion After Open Reduction of Mandible Fractures. Facial Plast. Surg. Aesthet. Med. 2021, 23, 460–466. [Google Scholar] [CrossRef]
- Maruthappu, M.; Gilbert, B.J.; El-Harasis, M.A.; Nagendran, M.; McCulloch, P.; Duclos, A.; Carty, M.J. The Influence of Volume and Experience on Individual Surgical Performance. A Systematic Review. Ann. Surg. 2015, 261, 642–647. [Google Scholar] [CrossRef]

| Mandible | Points |
|---|---|
| Dentoalveolar | 1 |
| Each fracture of symphysis/body/angle/ramus | 2 |
| Each fracture of the condyle/coronoid | 1 |
| Mid-face | |
| Dentoalveolar | 1 |
| Maxillary sinus (not involved in other complex) | 1 |
| Zygomatico-orbital complex | 1 |
| Orbital floor +/− medial wall (not involved in other complex) | 1 |
| Nasal (not involved in other complex) | 1 |
| Le Fort I | 2 |
| Le Fort II | 4 |
| Le Fort III | 6 |
| (Unilateral Le Fort fractures are assigned half the value) | |
| Naso-orbito-ethmoid | 3 |
| Upper face | |
| Orbital roof/rim | 1 |
| Frontal bone | 2 |
| Posterior wall of frontal sinus | 2 |
| All Patients (n = 1176) | % of 1176 | Redo Patients (n = 25) | % of 25 | |
|---|---|---|---|---|
| Sex | ||||
| Male | 907 | 77.1 | 18 | 72.0 |
| Female | 269 | 22.9 | 7 | 28.0 |
| Age (years) | ||||
| ≤36.75 | 588 | 50.0 | 12 | 48.0 |
| >36.75 | 588 | 50.0 | 13 | 52.0 |
| Mean (range) | 40.1 (3.8–93.8) | 39.2 (14.8–82.0) | ||
| FISS | ||||
| ≤2 | 600 | 51.0 | 12 | 48.0 |
| ≥3 | 576 | 49.0 | 13 | 52.0 |
| Mean (range) | 2.9 (1–26) | 4.0 (1–14) | ||
| Delay from injury to primary surgery (days) | ||||
| <3 | 536 | 45.6 | 11 | 44.0 |
| ≥3 | 640 | 54.4 | 14 | 56.0 |
| Mean (range) | 4.2 (0–62) | 4.5 (0–19) | ||
| Site of primary surgery | ||||
| Only mandible | 648 | 55.1 | 12 | 48.0 |
| Only midface | 498 | 42.3 | 11 | 44.0 |
| Combined mandible + midface | 30 | 2.6 | 2 | 8.0 |
| Patients (n = 25) | Redo Sites (n = 28) | Pure Fractures *** | Reasons for Redo Surgery (28 Sites) | ||
|---|---|---|---|---|---|
| Delay from primary surgery to redo surgery | |||||
| Mean 22 days (range 1–147 days) | |||||
| < 1 week | 12 | ||||
| <1–2 weeks | 7 | ||||
| 2–4 weeks | 3 | ||||
| >4 weeks | 3 | ||||
| Number of fracture sites per patient needing redo surgery | |||||
| 1 fracture site | 22 | ||||
| 2 fracture sites | 3 | ||||
| Site of redo-surgery according to lower and middle facial thirds | |||||
| Only mandible | 12 | ||||
| Only midface | 12 | ||||
| Mandible + midface | 1 | ||||
| Fractures needing redo surgery | |||||
| Mandible | 13 | ||||
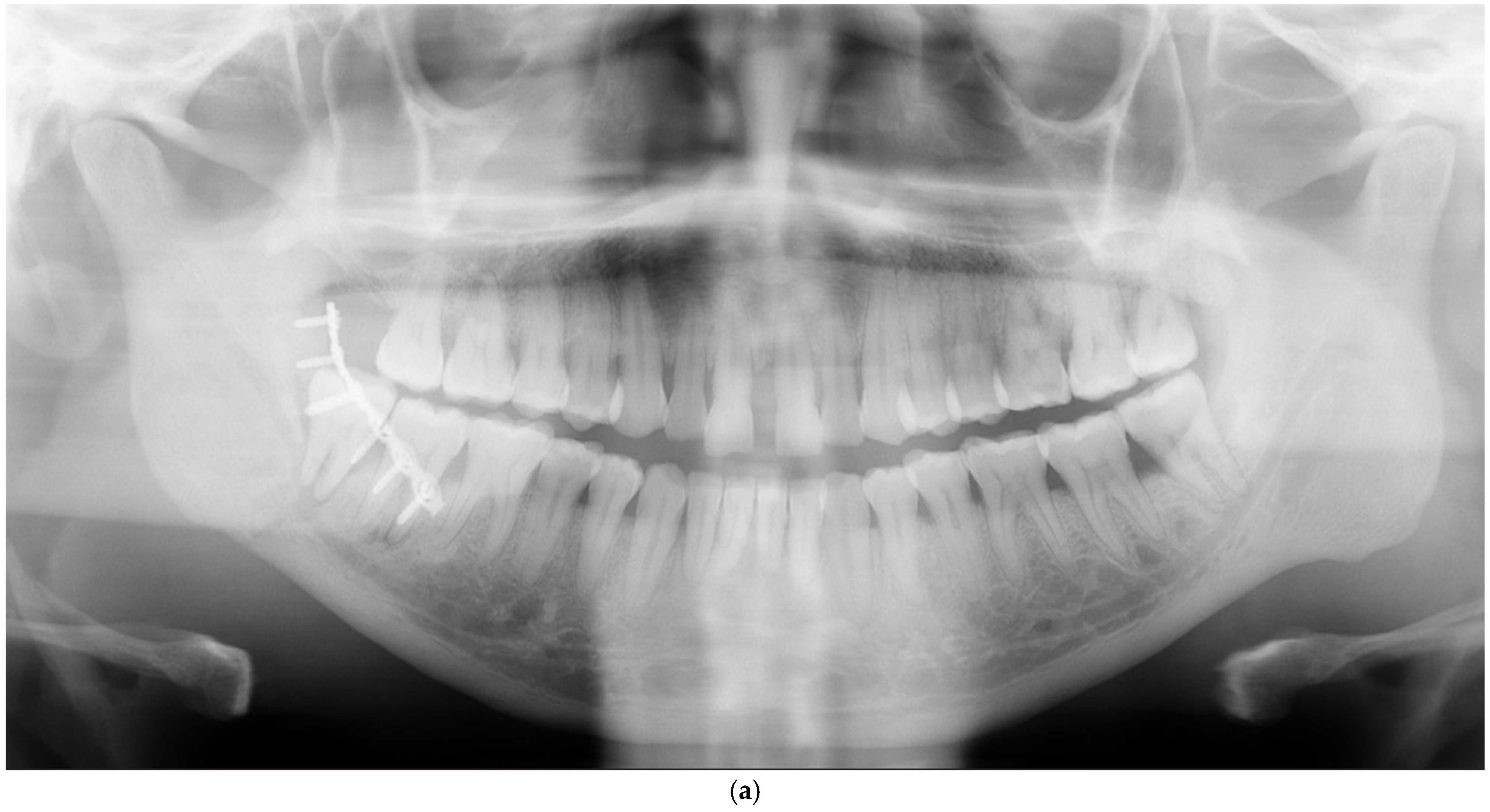
| Anterior part (symphysis/parasymphysis) | 5 | 2 | 3 inadequate reductions, 2 nonunions with infection | ||
| Angle | 3 | 2 | 1 inadequate reduction, 1 infection, 1 nonunion with infection | ||
| Body | 3 | 0 | 3 inadequate reductions | ||
| Condyle | 3 | 1 | 2 inadequate reductions, 1 broken plate | ||
| Midface | 13 | ||||
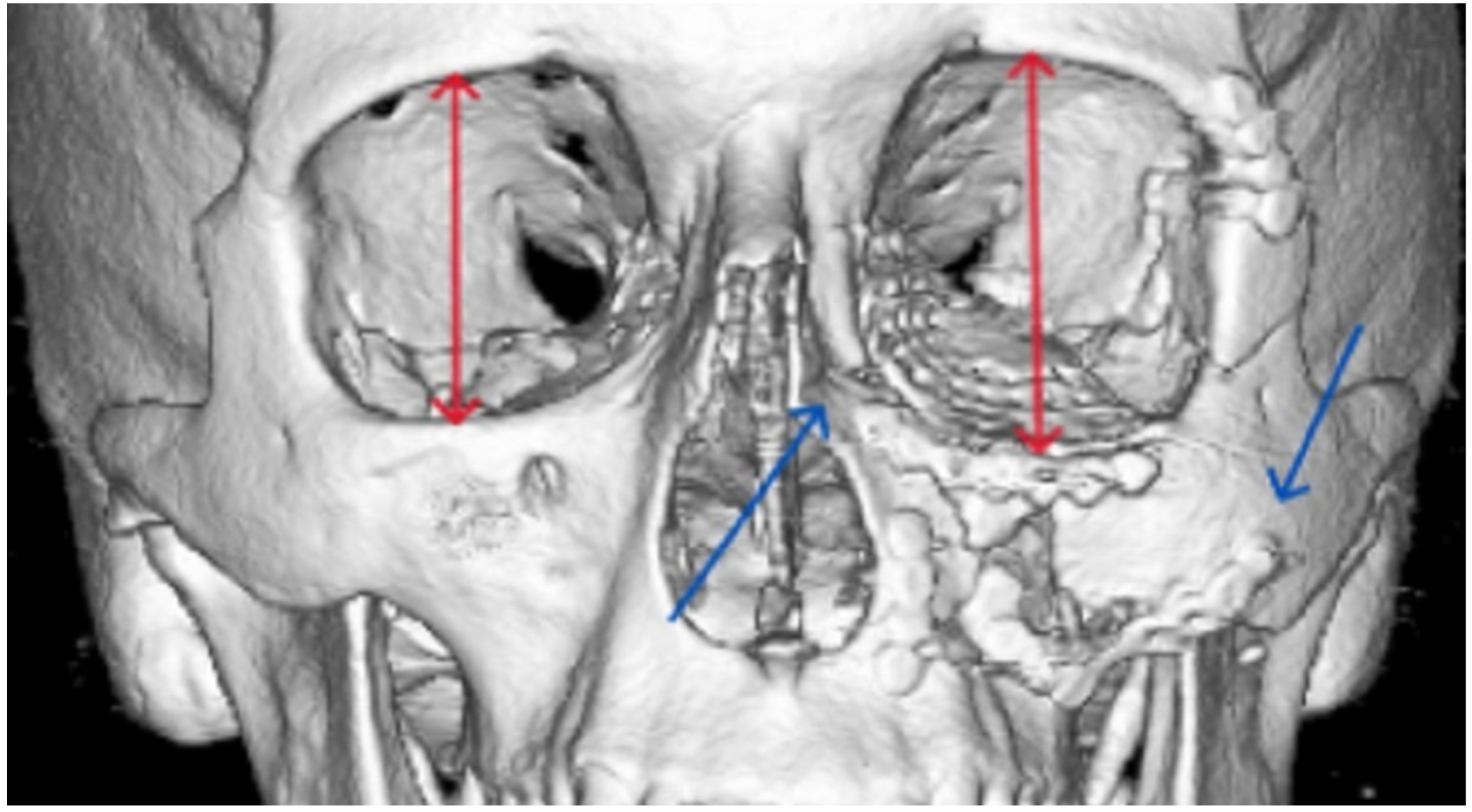
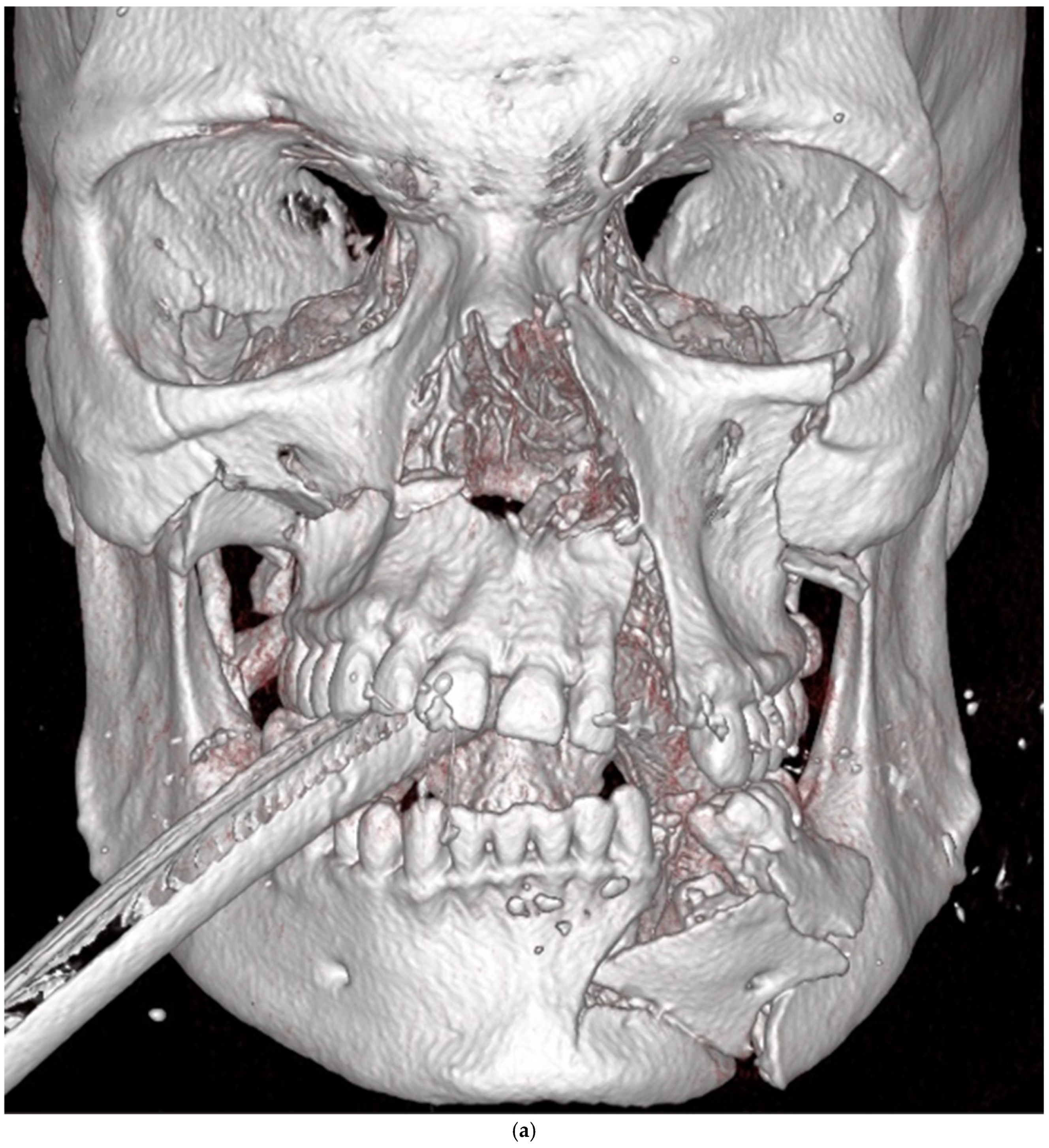
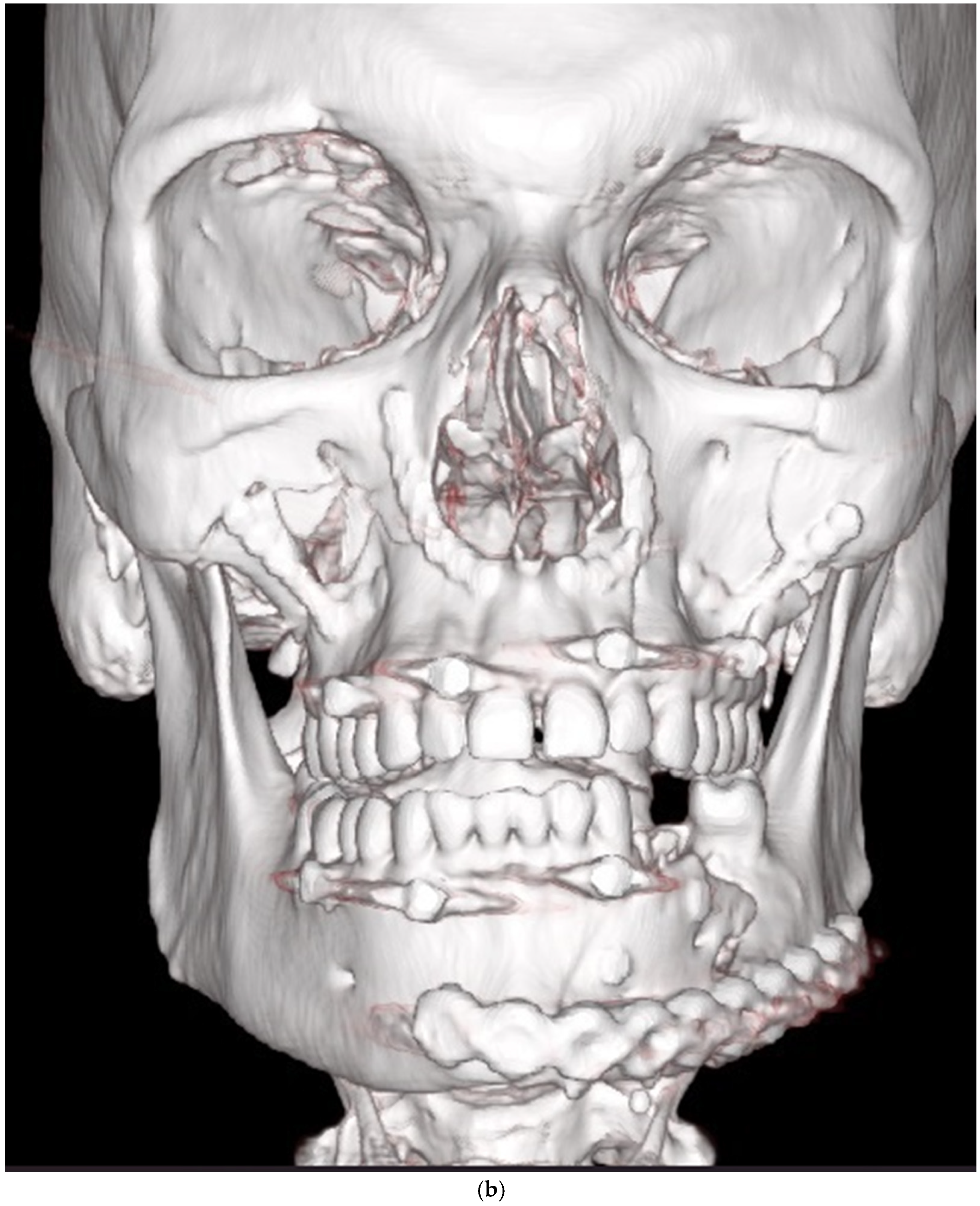
| Zygomatico–orbital complex * | 6 | 4 | 6 inadequate reductions | ||
| Le Fort ** | 4 | 1 (Le Fort III) | 4 inadequate reductions | ||
| Orbit | 4 | 0 | 3 inadequate reconstructions of orbital floor fractures, 1 orbital deformity due to inadequate reduction of the zygomatic bone | ||
| Reason for redo surgery | |||||
| Inadequate fracture reduction | 19 | ||||
| Inadequate orbital reconstruction | 4 | ||||
| Nonunion | 3 | ||||
| Infection | 1 | ||||
| Broken plate | 1 | ||||
| Redo Surgery/Yes | Redo Surgery/No | ||||||
|---|---|---|---|---|---|---|---|
| Number of Patients | % of n | Number of Patients | % of n | p-Value * | |||
| Age | p = 0.760 | ||||||
| ≤36.75 (n = 588) | 12 | 2.0 | 576 | 98.0 | |||
| >36.75 (n = 588) | 13 | 2.2 | 575 | 97.8 | |||
| Sex | 1151 | p = 0.537 | |||||
| Male (n = 907) | 18 | 2.0 | 889 | 98.0 | |||
| Female (n = 269) | 7 | 2.6 | 262 | 97.4 | |||
| FISS score | p = 0.760 | ||||||
| ≤2 (n = 600) | 12 | 2.0 | 588 | 98.0 | |||
| ≥3 (n = 576) | 13 | 2.3 | 563 | 97.7 | |||
| Mean | 4.04 | 2.89 | |||||
| Delay from injury to primary surgery (days) | p = 0.873 | ||||||
| <3 (n = 536) | 11 | 2.1 | 525 | 97.9 | |||
| ≥3 (n = 640) | 14 | 2.2 | 626 | 97.8 | |||
| Mean | 4.52 | 4.17 | |||||
| Site of primary surgery | p = 0.200 | ||||||
| Only mandible (n = 648) | 12 | 1.9 | 636 | 98.1 | |||
| Only midface (n = 498) | 11 | 2.2 | 487 | 97.8 | |||
| Combined mandible + midface (n = 30) | 2 | 6.7 | 28 | 93.3 |
| OR (95% CI) | p Value | |
|---|---|---|
| Sex | ||
| Male | 1.0 | 0.488 |
| Female | 1.37 (0.56–3.37) | |
| Age | ||
| ≤36.75 | 1.0 | 0.982 |
| >36.75 | 0.99 (0.44–2.25) | |
| FISS | ||
| ≤2 | 1.0 | 0.794 |
| ≥3 | 1.13 (0.46–2.75) | |
| Delay from injury to primary surgery (days) | ||
| <3 | 1.0 | 0.876 |
| ≥3 | 1.08 (0.43–2.69) | |
| Site of primary surgery | ||
| Only mandible | 1.0 | 0.249 |
| Only midface | 1.19 (0.45–3.12) | |
| Combined mandible + midface | 3.82 (0.80–18.42) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorén, H.; Suojanen, S.; Suominen, A.L.; Puolakkainen, T.; Toivari, M.; Snäll, J. Need for Redo Surgery of Maxillofacial Fractures. Craniomaxillofac. Trauma Reconstr. 2025, 18, 19. https://doi.org/10.3390/cmtr18010019
Thorén H, Suojanen S, Suominen AL, Puolakkainen T, Toivari M, Snäll J. Need for Redo Surgery of Maxillofacial Fractures. Craniomaxillofacial Trauma & Reconstruction. 2025; 18(1):19. https://doi.org/10.3390/cmtr18010019
Chicago/Turabian StyleThorén, Hanna, Sami Suojanen, Anna Liisa Suominen, Tero Puolakkainen, Miika Toivari, and Johanna Snäll. 2025. "Need for Redo Surgery of Maxillofacial Fractures" Craniomaxillofacial Trauma & Reconstruction 18, no. 1: 19. https://doi.org/10.3390/cmtr18010019
APA StyleThorén, H., Suojanen, S., Suominen, A. L., Puolakkainen, T., Toivari, M., & Snäll, J. (2025). Need for Redo Surgery of Maxillofacial Fractures. Craniomaxillofacial Trauma & Reconstruction, 18(1), 19. https://doi.org/10.3390/cmtr18010019






