Does Platelet-Rich Fibrin Enhance Recovery From Neurosensory Disturbance Following Mandibular Fractures? A Double-Blind, Split-Mouth Randomized Clinical Trial
Abstract
Introduction
Methods
Preparation of the PRF
Surgical Procedure
Sensory Neural Evaluation
Study Variables
Statistical Analysis
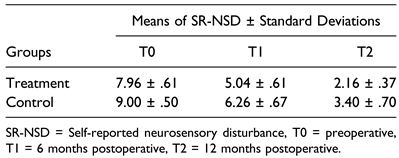
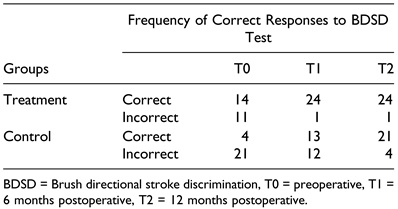
Results
Discussion
Conclusion
Funding
Conflicts of Interest
References
- Abhinav, R.P.; Selvarasu, K.; Maheswari, G.U.; Taltia, A.A. The patterns and etiology of maxillofacial trauma in South India. Ann Maxillofac Surg. 2019, 9, 114–117. [Google Scholar] [CrossRef]
- Alladi, S.; Dhasarathan, P.; Muralidoss, H.O.R.; Murugesan, K.; Vivigdha, V. Incidence neurosensory deficit in mandibular fractures. Bioinformation. 2023, 19, 725–728. [Google Scholar] [CrossRef]
- Singh, R.K.; Pal, U.S.; Singh, P.; Singh, G. Role of fixation in posttraumatic nerve injury recovery in displaced mandibular angle fracture. Natl J Maxillofac Surg. 2016, 7, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, J.S.; Jacobsen, C.; Rostetter, C.; Grätz, K.W.; Rücker, M.; Gander, T. Inferior alveolar nerve function after open reduction and internal fixation of mandibular fractures. J Cranio-Maxillo-Fac Surg. 2016, 44, 743–748. [Google Scholar] [CrossRef]
- Thurmüller, P.; Dodson, T.B.; Kaban, L.B. Nerve injuries associated with facial trauma: natural history, management, and outcomes of repair. Oral Maxillofac Surg Clin. 2001, 13, 283–293. [Google Scholar]
- Tabrizi, R.; Pourdanesh, F.; Khoshnik, P.L.; Centenero, S.A. Does the lag time between injury and treatment play a role in recovery of inferior alveolar nerve neurosensory disturbances following mandibular body fracture? J Craniofac Surg. 2019, 30, 2128–2130. [Google Scholar] [CrossRef]
- Tay, A.B.; Lai, J.B.; Lye, K.W.; et al. Inferior alveolar nerve injury in trauma-induced mandible fractures. J Oral Maxillofac Surg. 2015, 73, 1328–1340. [Google Scholar] [CrossRef]
- Boffano, P.; Roccia, F.; Gallesio, C.; Karagozoglu, K.; Forouzanfar, T. Inferior alveolar nerve injuries associated with mandibular fractures at risk: a two-center retrospective study. Craniomaxillofacial Trauma Reconstr. 2014, 7, 280–283. [Google Scholar] [CrossRef]
- Canellas, J.; Medeiros, P.J.D.; Figueredo, C.; Fischer, R.G.; Ritto, F.G. Platelet-rich fibrin in oral surgical procedures: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2019, 48, 395–414. [Google Scholar] [CrossRef]
- Bielecki, T.; Dohan Ehrenfest, D.M.; Everts, P.A.; Wiczkowski, A. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: new perspectives. Curr Pharmaceut Biotechnol. 2012, 13, 1153–1162. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006, 101, e51–e55. [Google Scholar] [CrossRef] [PubMed]
- Zumarán, C.C.; Parra, M.V.; Olate, S.A.; Fernández, E.G.; Muñoz, F.T.; Haidar, Z.S. The 3 R’s for platelet-rich fibrin: a “super” tridimensional biomaterial for contemporary naturally-guided oro-maxillo-facial soft and hard tissue repair, reconstruction and regeneration. Materials 2018, 11, 1293. [Google Scholar] [CrossRef]
- Simonpieri, A.; Del Corso, M.; Vervelle, A.; et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: bone graft, implant and reconstructive surgery. Curr Pharmaceut Biotechnol. 2012, 13, 1231–1256. [Google Scholar] [CrossRef]
- Tabrizi, R.; Pourdanesh, F.; Jafari, S.; Behnia, P. Can platelet-rich fibrin accelerate neurosensory recovery following sagittal split osteotomy? A double-blind, split-mouth, randomized clinical trial. Int J Oral Maxillofac Surg. 2018, 47, 1011–1014. [Google Scholar] [CrossRef]
- Khojasteh, A.; Hosseinpour, S.; Nazeman, P.; Dehghan, M.M. The effect of a platelet-rich fibrin conduit on neurosensory recovery following inferior alveolar nerve lateralization: a preliminary clinical study. Int J Oral Maxillofac Surg. 2016, 45, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Behnia, P.; Behnia, H.; Ghanbari, A.M.; Tabrizi, R. Does leucocyte-and platelet-rich fibrin enhance neurosensory recovery after genioplasty? A double-blind, split-mouth, randomized clinical trial. Br J Oral Maxillofac Surg 2023, 61, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Genú, P.R.; Vasconcelos, B.C. Influence of the tooth section technique in alveolar nerve damage after surgery of impacted lower third molars. Int J Oral Maxillofac Surg. 2008, 37, 923–928. [Google Scholar] [CrossRef]
- Şenses, F.; Önder, M.E.; Koçyiğit, I.D.; et al. Effect of platelet-rich fibrin on peripheral nerve regeneration. J Craniofac Surg. 2016, 27, 1759–1764. [Google Scholar] [CrossRef]
- Smith, A.C.; Barry, S.E.; Chiong, A.Y.; et al. Inferior alveolar nerve damage following removal of mandibular third molar teeth. A prospective study using panoramic radiography. Aust Dent J. 1997, 42, 149–152. [Google Scholar] [CrossRef]
- Antony, P.G.; Sebastian, A.; Varghese, K.G.; et al. Neurosensory evaluation of inferior alveolar nerve after bilateral sagittal split ramus osteotomy of mandible. J Oral Biol Craniofac Res. 2017, 7, 81–88. [Google Scholar] [CrossRef]
- Ghali, G.; Epker, B. Clinical neurosensory testing: practical applications. J Oral Maxillofac Surg. 1989, 47, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Mohajerani, S.H.; Tabeie, F.; Bemanali, M.; Tabrizi, R. Effect of low-level laser and light-emitting diode on inferior alveolar nerve recovery after sagittal split osteotomy of the mandible: a randomized clinical trial study. J Craniofac Surg. 2017, 28, e408–e411. [Google Scholar] [CrossRef] [PubMed]

 |
 |
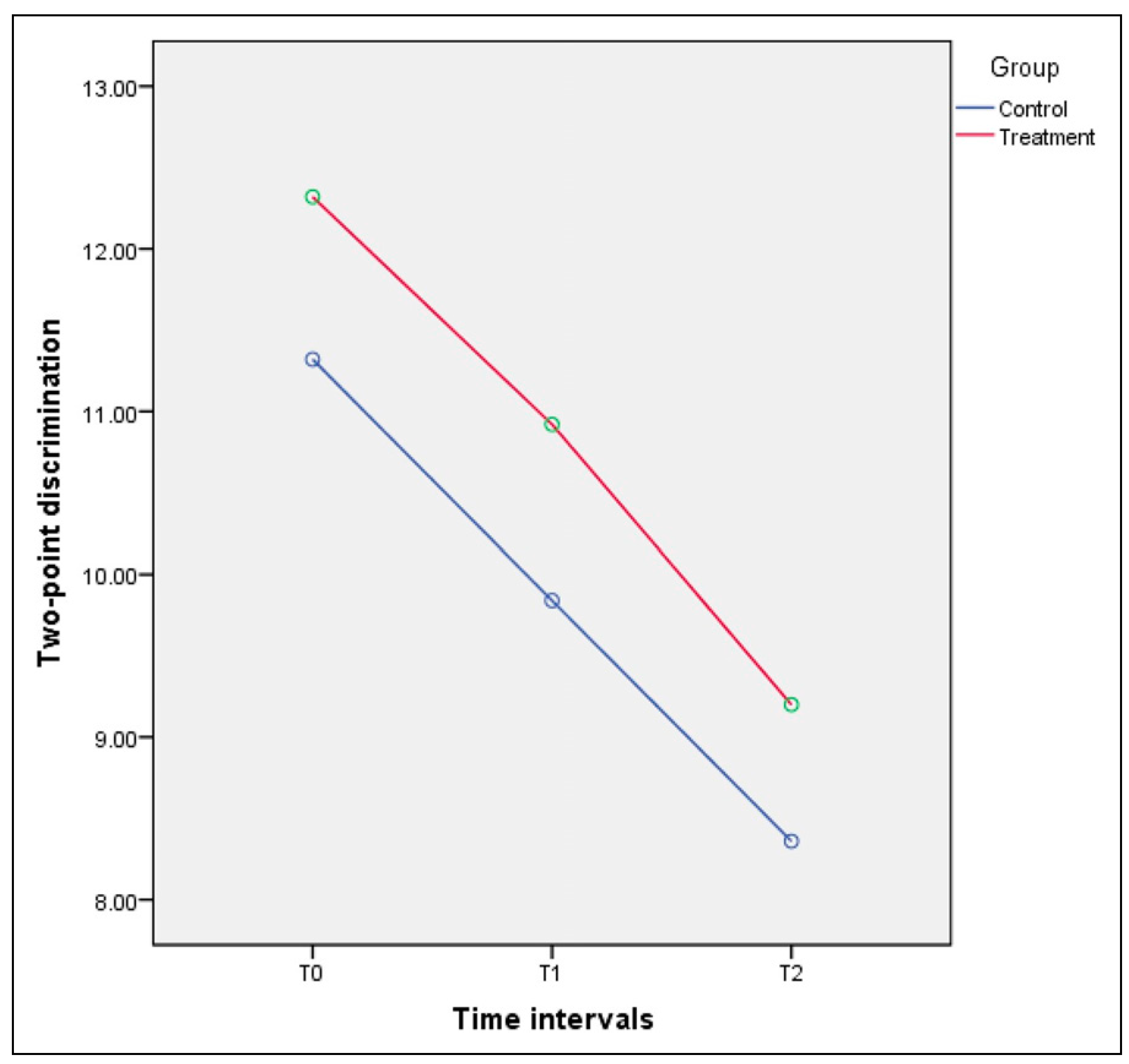
 |
 |
© 2024 by the authors. The Author(s) 2024.
Share and Cite
Tabrizi, R.; Moslemi, H.; Shafiei, S.; Dastgir, R.; Peacock, Z.S. Does Platelet-Rich Fibrin Enhance Recovery From Neurosensory Disturbance Following Mandibular Fractures? A Double-Blind, Split-Mouth Randomized Clinical Trial. Craniomaxillofac. Trauma Reconstr. 2024, 17, 75. https://doi.org/10.1177/19433875241257737
Tabrizi R, Moslemi H, Shafiei S, Dastgir R, Peacock ZS. Does Platelet-Rich Fibrin Enhance Recovery From Neurosensory Disturbance Following Mandibular Fractures? A Double-Blind, Split-Mouth Randomized Clinical Trial. Craniomaxillofacial Trauma & Reconstruction. 2024; 17(4):75. https://doi.org/10.1177/19433875241257737
Chicago/Turabian StyleTabrizi, Reza, Hamidreza Moslemi, Shervin Shafiei, Ramtin Dastgir, and Zachary S. Peacock. 2024. "Does Platelet-Rich Fibrin Enhance Recovery From Neurosensory Disturbance Following Mandibular Fractures? A Double-Blind, Split-Mouth Randomized Clinical Trial" Craniomaxillofacial Trauma & Reconstruction 17, no. 4: 75. https://doi.org/10.1177/19433875241257737
APA StyleTabrizi, R., Moslemi, H., Shafiei, S., Dastgir, R., & Peacock, Z. S. (2024). Does Platelet-Rich Fibrin Enhance Recovery From Neurosensory Disturbance Following Mandibular Fractures? A Double-Blind, Split-Mouth Randomized Clinical Trial. Craniomaxillofacial Trauma & Reconstruction, 17(4), 75. https://doi.org/10.1177/19433875241257737




