Microvascular Reconstructions in Elderly Patients with Oral Squamous Cell Carcinoma—Too Old for Surgical Treatment?
Abstract
Introduction
Patients and Methods
Statistics
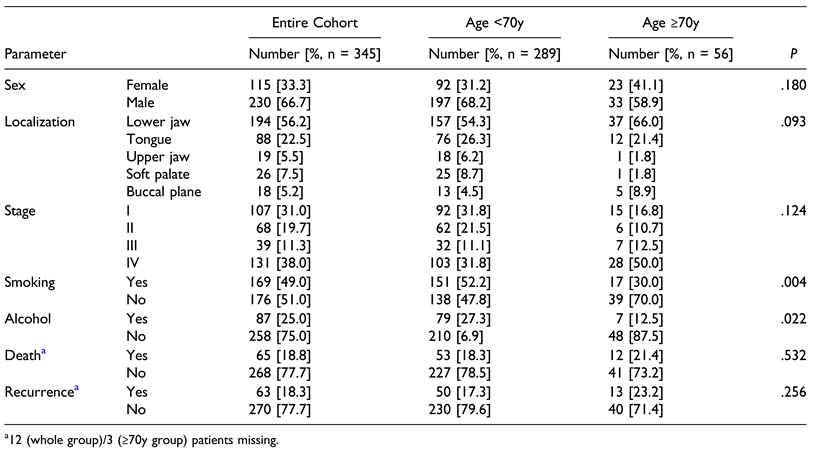
Results
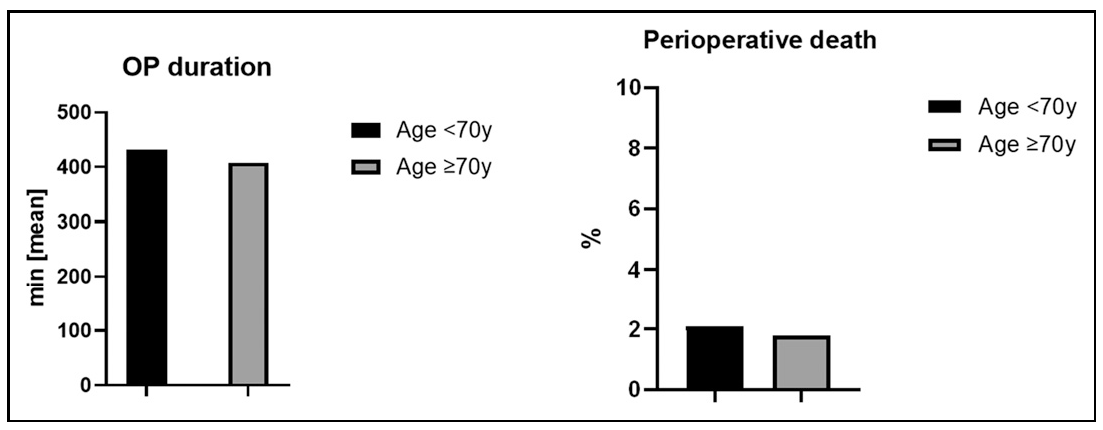
Treatment Comparison of Both Groups
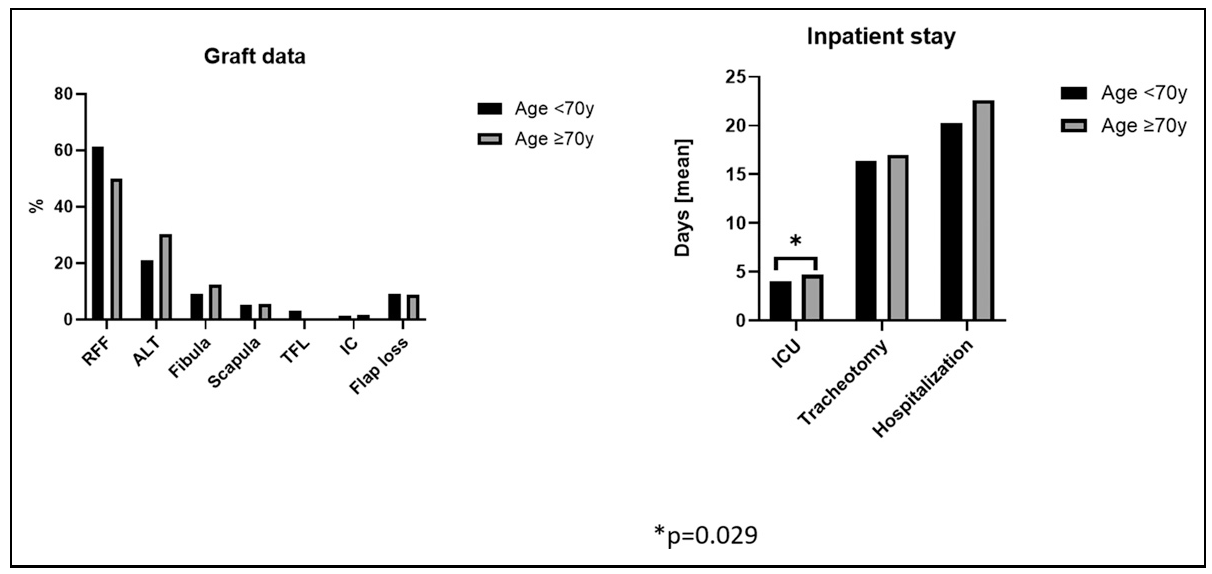
Perioperative Morbidity and Hospital Stay
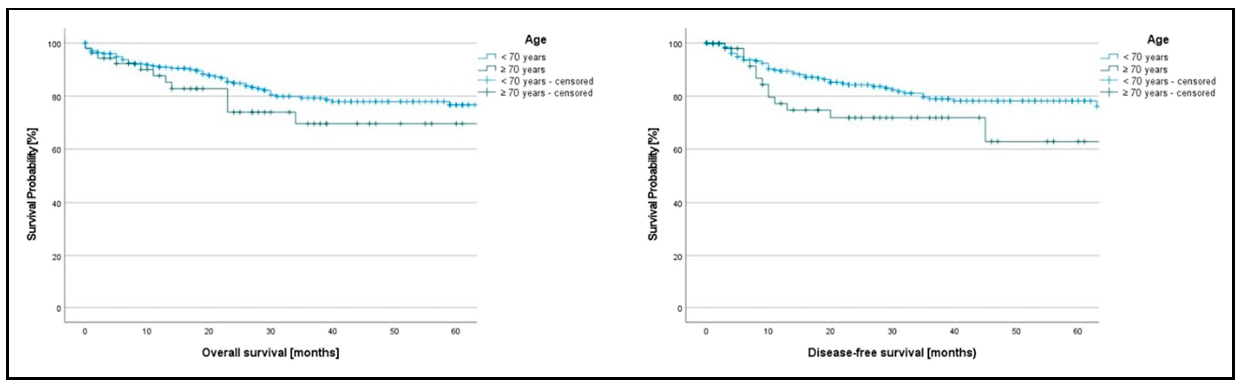
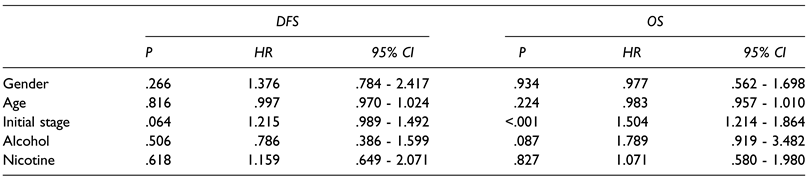
Oncological Follow-Up
Discussion
Conclusion
Funding
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Funk, G.F.; Karnell, L.H.; Robinson, R.A.; Zhen, W.K.; Trask, D.K.; Hoffman, H.T. Presentation, treatment, and outcome of oral cavity cancer: A national cancer data base report. Head Neck 2002, 24, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.D.; Ferlay, J.; Jemal, A.; Sankaranarayanan, R.; Chaturvedi, A.K.; Bray, F.; Soerjomataram, I. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J. Clin. 2017, 67, 51–64. [Google Scholar] [CrossRef]
- Cancer today - International Agency for Research on Cancer (IARC) (https://gco.iarc.who.int) (Data version: GLOBO- CAN 2022). Lip, oral cavity. Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/1-lip-oral-cavity-fact-sheet.pdf (accessed on 14 February 2024).
- Cancer over time - International Agency for Research on Cancer (IARC) (https://gco.iarc.who.int) (Date version 2.0, n.d.). Age-standardized rate (World) per 100 000, mortality, males and females Germany: Lip, oral cavity and pharynx. Available online: https://gco.iarc.fr/overtime/en/dataviz/trends?populations=276&sexes=1_2&types=1&multiple_populations=0&mode=can-cer&multiple_cancers=1&cancers=1 (accessed on 14 February 2024).
- Oral cancer - the fight must go on against all odds…. Evid Based Dent; 2022.
- Cancer tomorrow - International Agency for Research on Cancer (IARC) (https://gco.iarc.who.int) (Data version: Globocan 2022 (version 1.1)). Estimated number of new cases from 2022 to 2045: Both sexes, age [65-85+] for Lip and oral cavity. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?types=0&sexes=0&mode=population&group_popula-tions=0&multiple_populations=0&multiple_cancers=1&cancers=1&populations=900&apc=cat_ca20v1.5_ca23v-1.5&group_cancers=1&single_unit=5000&age_start=13 (accessed on 14 February 2024).
- Cancer tomorrow - International Agency for Research on Cancer (IARC) (https://gco.iarc.who.int) (Data version: Globocan 2022 (version 1.1)). Estimated number of new cases from 2022 to 2045, Both sexes, age [0-64], Lip, oral cavity. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/bars?cancers=1&age_end=12 (accessed on 14 February 2024).
- Wolff, K.-D.; Follmann, M.; Nast, A. The Diagnosis and Treatment of Oral Cavity Cancer. Dtsch. Aerzteblatt Online 2012, 109, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Hashim, D.; Boffetta, P. Head and neck cancers. In Occupational Cancers, 2nd ed.; Anttila, S., Boffetta, P., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 57–105. [Google Scholar]
- Mohamad, I.; Glaun, M.D.; Prabhash, K.; Busheri, A.; Lai, S.Y.; Noronha, V.; Hosni, A. Current Treatment Strategies and Risk Stratification for Oral Carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389810. [Google Scholar] [CrossRef]
- Shaw, R.J.; O’connell, J.E.; Bajwa, M. Basic surgical principles and techniques. Textb. Oral Cancer 2020, 10, 253–282. [Google Scholar] [CrossRef]
- Mericske-Stern, R.; Perren, R.; Raveh, J. Life table analysis and clinical evaluation of oral implants supporting prostheses after resection of malignant tumors. Int J Oral Maxillofac Implant. 1999, 14, 673–680. [Google Scholar]
- Juarez, J.E.; Choi, J.; John, M.S.; Abemayor, E.; TenNapel, M.; Chen, A.M. Patterns of Care for Elderly Patients With Locally Advanced Head and Neck Cancer. Int. J. Radiat. Oncol. 2017, 98, 767–774. [Google Scholar] [CrossRef]
- AWMF. S3-Leitlinie Diagnostik und Therapie des Mundho¨ hlenkarzinoms: Leitlinien Onkologie; 2021. Available online: https://regis-ter.awmf.org/assets/guidelines/007-100OLk_S3-Diagnostik-Therapie-Mundhoehlenkarzinom_2021-03.pdf.
- Rosenberg, A.; Van Cann, E.; van der Bilt, A.; Koole, R.; van Es, R. A prospective study on prognostic factors for free-flap reconstructions of head and neck defects. Int. J. Oral Maxillofac. Surg. 2009, 38, 666–670. [Google Scholar] [CrossRef]
- Sittitrai, P.; Ruenmarkkaew, D.; Klibngern, H. Pedicled Flaps versus Free Flaps for Oral Cavity Cancer Reconstruction: A Comparison of Complications, Hospital Costs, and Functional Outcomes. Int. Arch. Otorhinolaryngol. 2022, 27, e32–e42. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Molteni, G.; Valerini, S.; Alicandri-Ciufelli, M.; Sprio, A.E.; Crosetti, E.; Berta, G.N.; Presutti, L.; Succo, G. Unravelling the risk factors that underlie oral and oropharyngeal surgery in elderly. Acta Otorhinolaryngol. Ital. 2018, 38, 409–416. [Google Scholar] [CrossRef]
- Farquharson, S.M.; Gupta, R.; Heald, R.J.; Moran, B.J. Surgical Decisions in the Elderly: The Importance of Biological Age. J. R. Soc. Med. 2001, 94, 232–235. [Google Scholar] [CrossRef]
- Peters, T.T.A.; van Dijk, B.A.C.; Roodenburg, J.L.N.; van der Laan, B.F.A.M.; Halmos, G.B. Relation Between Age, Comorbidity, and Complications in Patients Undergoing Major Surgery for Head and Neck Cancer. Ann. Surg. Oncol. 2014, 21, 963–970. [Google Scholar] [CrossRef]
- Spyropoulou, G.-A.; Jeng, S.-F.; Hsieh, C.-H.; Tsimponis, A.; Shih, H.-S. Microsurgical Reconstruction for Head and Neck Cancer in Elderly Patients. J. Reconstr. Microsurg. 2014, 30, 091–096. [Google Scholar] [CrossRef]
- Teymoortash, A.; Ferlito, A.; Halmos, G.B. Treatment in elderly patients with head and neck cancer : a challenging dilemma. HNO 2016, 64, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Sowder, J.C.; Cannon, R.B.; Buchmann, L.O.; Hunt, J.P.; Hitchcock, Y.; Lloyd, S.; Grossmann, K.F.; Monroe, M.M. Treatment-related determinants of survival in early-stage (T1–2N0M0) oral cavity cancer: A population-based study. Head Neck 2017, 39, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Decroix, Y.; Ghossein, N.A. Experience of the curie institute in treatment of cancer of the mobile tongue. I. Treatment policies and result. Cancer 1981, 47, 496–502. [Google Scholar] [CrossRef]
- Krishnan Nair, M.; Sankaranarayanan, R.; Padmanabhan, T.K. Evaluation of the role of radiotherapy in the management of car-cinoma of the buccal mucosa. Cancer 1988, 61, 1326–1331. [Google Scholar] [CrossRef]
- Turner, S.; Slevin, N.; Gupta, N.; Swindell, R. Radical external beam radiotherapy for 333 squamous carcinomas of the oral cavity — evaluation of late morbidity and a watch policy for the clinically negative neck. Radiother. Oncol. 1996, 41, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Aksu, G.; Karadeniz, A.; Saynak, M.; Fayda, M.; Kadehci, Z.; Kocaelli, H. Treatment results and prognostic factors in oral tongue cancer: analysis of 80 patients. Int. J. Oral Maxillofac. Surg. 2006, 35, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Dobson, G.P. Trauma of major surgery: A global problem that is not going away. Int. J. Surg. 2020, 81, 47–54. [Google Scholar] [CrossRef]
- Vemuri, C.; Wainess, R.M.; Dimick, J.B.; A Cowan, J.; Henke, P.K.; Stanley, J.C.; Upchurch, G.R. Effect of increasing patient age on complication rates following intact abdominal aortic aneurysm repair in the united states1. J. Surg. Res. 2004, 118, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, E.V.; Birkmeyer, J.D. Operative mortality with elective surgery in older adults. Effect Clin Pract 2001, 4, 172–177. [Google Scholar]
- Turrentine, F.E.; Wang, H.; Simpson, V.B.; Jones, R.S. Surgical Risk Factors, Morbidity, and Mortality in Elderly Patients. J. Am. Coll. Surg. 2006, 203, 865–877. [Google Scholar] [CrossRef]
- D’cruz, A.K.; Vaish, R.; Kapre, N.; Dandekar, M.; Gupta, S.; Hawaldar, R.; Agarwal, J.P.; Pantvaidya, G.; Chaukar, D.; Deshmukh, A.; et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. New Engl. J. Med. 2015, 373, 521–529. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.J.; Yang, X.; Peng, H. Diagnostic Efficacy of Sentinel Lymph Node Biopsy in Early Oral Squamous Cell Carcinoma: A Meta-Analysis of 66 Studies. PLOS ONE 2017, 12, e0170322. [Google Scholar] [CrossRef]
- Fan, S.-F.; Zeng, Z.-Y.; Peng, H.-W.; Guo, Z.-M.; Wang, S.-L.; Zhang, Q. Sentinel lymph node biopsy versus elective neck dissection in patients with cT1-2N0 oral tongue squamous cell carcinoma. Oral surgery, oral medicine, oral pathology and oral radiology 2014, 117, 186–190. [Google Scholar] [CrossRef]
- Moya-Plana, A.; Aupérin, A.; Guerlain, J.; Gorphe, P.; Casiraghi, O.; Mamelle, G.; Melkane, A.; Lumbroso, J.; Janot, F.; Temam, S. Sentinel node biopsy in early oral squamous cell carcinomas: Long-term follow-up and nodal failure analysis. Oral Oncol. 2018, 82, 187–194. [Google Scholar] [CrossRef]
- Schiefke, F.; Akdemir, M.; Weber, A.; Akdemir, D.; Singer, S.; Frerich, B. Function, postoperative morbidity, and quality of life after cervical sentinel node biopsy and after selective neck dissection. Head Neck 2009, 31, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Kligerman, J.; Lima, R.A.; Soares, J.R.; et al. Supraomohyoid neck dissection in the treatment of T1/T2 squamous cell carcinoma of oral cavity. Am J Surg. 1994, 168, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Iype, E.M.; Sebastian, P.; Mathew, A.; Balagopal, P.; Varghese, B.T.; Thomas, S. The role of selective neck dissection (I–III) in the treatment of node negative (N0) neck in oral cancer. Oral Oncol. 2008, 44, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Menick, F.J. Facial Reconstruction with Local and Distant Tissue: The Interface of Aesthetic and Reconstructive Surgery. Plast. Reconstr. Surg. 1998, 102, 1424–1433. [Google Scholar] [CrossRef]
- Burget, G.C.; Menick, F.J. The Subunit Principle in Nasal Reconstruction. Plast. Reconstr. Surg. 1985, 76, 239–247. [Google Scholar] [CrossRef]
- Thiele, O.C.; Seeberger, R.; Flechtenmacher, C.; Hofele, C.; Freier, K. The role of elective supraomohyoidal neck dissection in the treatment of early, node-negative oral squamous cell carcinoma (OSCC): A retrospective analysis of 122 cases. J. Cranio-Maxillo-Facial Surg. 2011, 40, 67–70. [Google Scholar] [CrossRef]
- Nao, E.E.M.; Dassonville, O.; Poissonnet, G.; Chamorey, E.; Pierre, C.-S.; Riss, J.-C.; Vincent, N.; Peyrade, F.; Benezery, K.; Chemaly, L.; et al. Ablative surgery and free flap reconstruction for elderly patients with oral or oropharyngeal cancer: Oncologic and functional outcomes. Acta Oto-Laryngologica 2011, 131, 1104–1109. [Google Scholar] [CrossRef]
- Audisio, R.A.; Ramesh, H.; Longo, W.E.; Zbar, A.P.; Pope, D. Preoperative Assessment of Surgical Risk in Oncogeriatric Patients. Oncol. 2005, 10, 262–268. [Google Scholar] [CrossRef]
- Lubin, M.F. Is age a risk factor for surgery? Med Clin. North Am. 1993, 77, 327–333. [Google Scholar] [CrossRef]
- Takasaki, R.; Yamagata, K.; Fukuzawa, S.; Uchida, F.; Ishibashi-Kanno, N.; Bukawa, H. Convenient Decision Criteria for Surgery in Elderly Patients with Oral Squamous Cell Carcinoma. Dent. J. 2022, 11, 6. [Google Scholar] [CrossRef]

© 2024 by the author. The Author(s) 2024.
Share and Cite
Radermacher, A.; Horn, D.; Fehrenz, M.; Semmelmayer, K.; Ristow, O.; Engel, M.; Hoffmann, J.; Freier, K.; Moratin, J. Microvascular Reconstructions in Elderly Patients with Oral Squamous Cell Carcinoma—Too Old for Surgical Treatment? Craniomaxillofac. Trauma Reconstr. 2024, 17, 48. https://doi.org/10.1177/19433875241272437
Radermacher A, Horn D, Fehrenz M, Semmelmayer K, Ristow O, Engel M, Hoffmann J, Freier K, Moratin J. Microvascular Reconstructions in Elderly Patients with Oral Squamous Cell Carcinoma—Too Old for Surgical Treatment? Craniomaxillofacial Trauma & Reconstruction. 2024; 17(4):48. https://doi.org/10.1177/19433875241272437
Chicago/Turabian StyleRadermacher, Anne, Dominik Horn, Michael Fehrenz, Karl Semmelmayer, Oliver Ristow, Michael Engel, Jürgen Hoffmann, Kolja Freier, and Julius Moratin. 2024. "Microvascular Reconstructions in Elderly Patients with Oral Squamous Cell Carcinoma—Too Old for Surgical Treatment?" Craniomaxillofacial Trauma & Reconstruction 17, no. 4: 48. https://doi.org/10.1177/19433875241272437
APA StyleRadermacher, A., Horn, D., Fehrenz, M., Semmelmayer, K., Ristow, O., Engel, M., Hoffmann, J., Freier, K., & Moratin, J. (2024). Microvascular Reconstructions in Elderly Patients with Oral Squamous Cell Carcinoma—Too Old for Surgical Treatment? Craniomaxillofacial Trauma & Reconstruction, 17(4), 48. https://doi.org/10.1177/19433875241272437




