Background
Craniosynostosis is a relatively common birth defect affecting up to 1 in 1000 children. Left untreated, craniosynostosis can affect normal brain growth and development, can cause visual impairment, and can have a significant psychosocial impact on the child [
1]. Unicoronal synostosis (UCS) is a common subtype of craniosynostosis that is especially difficult to treat due to the asymmetrical nature of the deformity. Cranial vault reconstruction (CVR) is a primary method to surgically correct UCS, where the goals of surgery are to expand the intracranial volume, reduce the risk of abnormal brain growth, and to normalize head shape and appearance [
2].
The use of virtual surgical planning (VSP) has been increasing for craniosynostosis reconstruction. VSP has been shown to reduce intra-operative procedure length [
3,
4,
5]. Virtual technologies have demonstrated particular utility for complex craniofacial cases [
6,
7]. Time spent deciding where to make osteotomies and in what configuration to plate bone segments is shifted from the operating room to the preplanning virtual environment. Efforts using VSP in CVR have used normal skulls as a template to guide surgical osteotomies and plating [
8,
9,
10]. Having a reference skull reduces the subjectivity of surgical decision making. While studies have produced aesthetic results using this technique, planning times and cost are prohibitive to its use, limiting this technology to few tertiary centers [
7,
11]. Introducing automation in virtual planning is an attractive option to reduce the guesswork in surgical decision making, and to improve time and cost efficiency. Objectivity and accessibility are key design criteria in the development of a CVR workflow. Minimizing surgeon involvement in the surgical decisionmaking process reduces the degree of guesswork. A method for automatic CVR planning that meets these design elements may increase the availability of advanced technologies in the surgical planning environment.
Preliminary attempts at automating this process have demonstrated success in a symmetrical craniosynostosis phenotype [
12,
13,
14]. Porras et al [
12] introduced a transformation model that automatically generated a surgical plan for fronto-orbital advancement (FOA) in metopic craniosynostosis in 4 hours. The algorithm was improved to include overcorrection and planning times reduced to under 30 seconds [
15]. Expert surgeons agreed on the usefulness of the proposed computer-generated surgical plans. The workflow does not allow for bone exchange, limiting its use to the correction of metopic craniosynostosis. The workflow utilizes the software CranioPlan which is available on an open source platform and requires a moderate degree of computer literacy to navigate.
Using computer algorithms to generate surgical plans has the potential to reduce the subjectivity, planning duration, and cost of virtual surgical planning. Previous methods of achieving this are mathematically advanced and have not been applied to asymmetric craniosynostosis. The first objective of this study was to develop a manual virtual surgical workflow for an FOA for UCS using the in-house software at the study institution. The second objective was to automate components of the workflow to reduce surgeon involvement in the planning process, creating a partially automated workflow. The overall goal was to build the conceptual framework for the development of a fully automated computer algorithm for UCS surgery.
Methods
The University of Alberta ethics institution review board approved this study (Pro00085524). An iterative design process was used to develop virtual workflows in this study using Geomagic Freeform Plus software (Version 2017). In-house workflow development adhered to the accessibility and cost-effective design criteria as the costs of outsourcing development were avoided. Based on available imaging, a three-month-old female with right-sided UCS and an age- and sex-matched normal skull were used as the index case pair in this single case feasibility study. In part 1, the initial virtual workflow was developed and did not include automated components. This “manual workflow” served as the template, into which automated steps were introduced in part 2. This study represents the first attempt at virtual planning using in-house software for CVR at the study institution, and therefore the manual workflow was a necessary first step in the development of an automated workflow.
Part I: Manual Workflow
CT scans were retrieved using a local radiology database after ethics review board approval (Pro85524). The UCS skull and matched normal skull were registered using a previously published workflow [
16], and the corresponding stereolithography (STL) files were imported into Geomagic Freeform Plus (3D Systems, Rock Hill, South Carolina, USA). Key steps in the manual workflow are isolating the frontal bones (FBs) and supraorbital bar (SOB) regions of interest (ROI) and manipulating them to approximate a normal head shape. This software allows bending, rotation, and translation of isolated bone segments. The manual workflow steps are outlined in
Figure 1. This represents an example of an FOA using the patient’s existing frontal bones to create the neo-forehead—a method commonly employed at the study institution. Input from a pediatric craniomaxillofacial surgeon was obtained throughout development to assess the fidelity of the virtual surgical workflow.
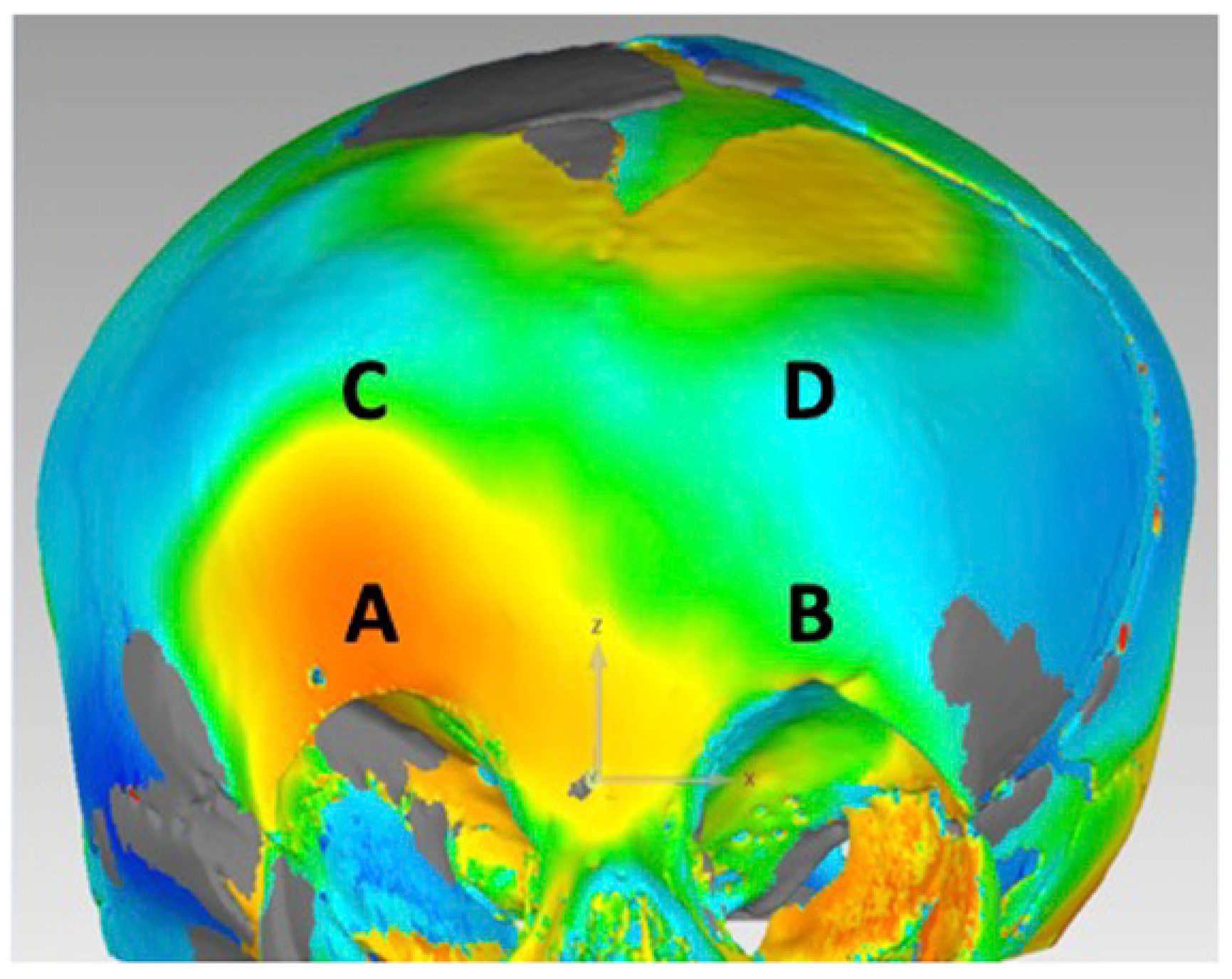
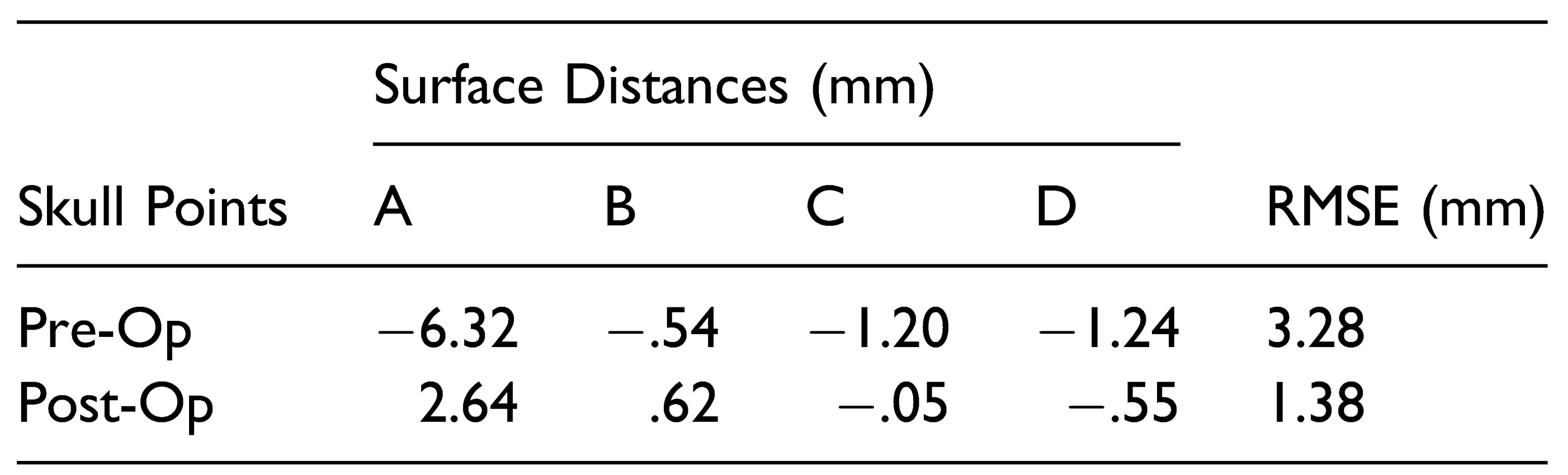
The reconstruction model using the manual VSP workflow was analyzed using Geomagic Control (Version 2015.3.1.0). Maximum Hausdorff surface distances were calculated at 4 points along the frontal bones and supraorbital bar and compared to the control skull model (
Figure 2). The root mean squared error (RMSE) of the maximum surface distances at each point was calculated. The same calculations were made for the pre-operative skull model. A smaller RMSE represents a model that is closer shape to a normal reference skull and therefore a successful reconstruction. In addition, color maps were generated in Geomagic Control to qualitatively assess the improvement in skull morphology at the frontal bones and superior orbital bar.
Part II: Automated Workflow
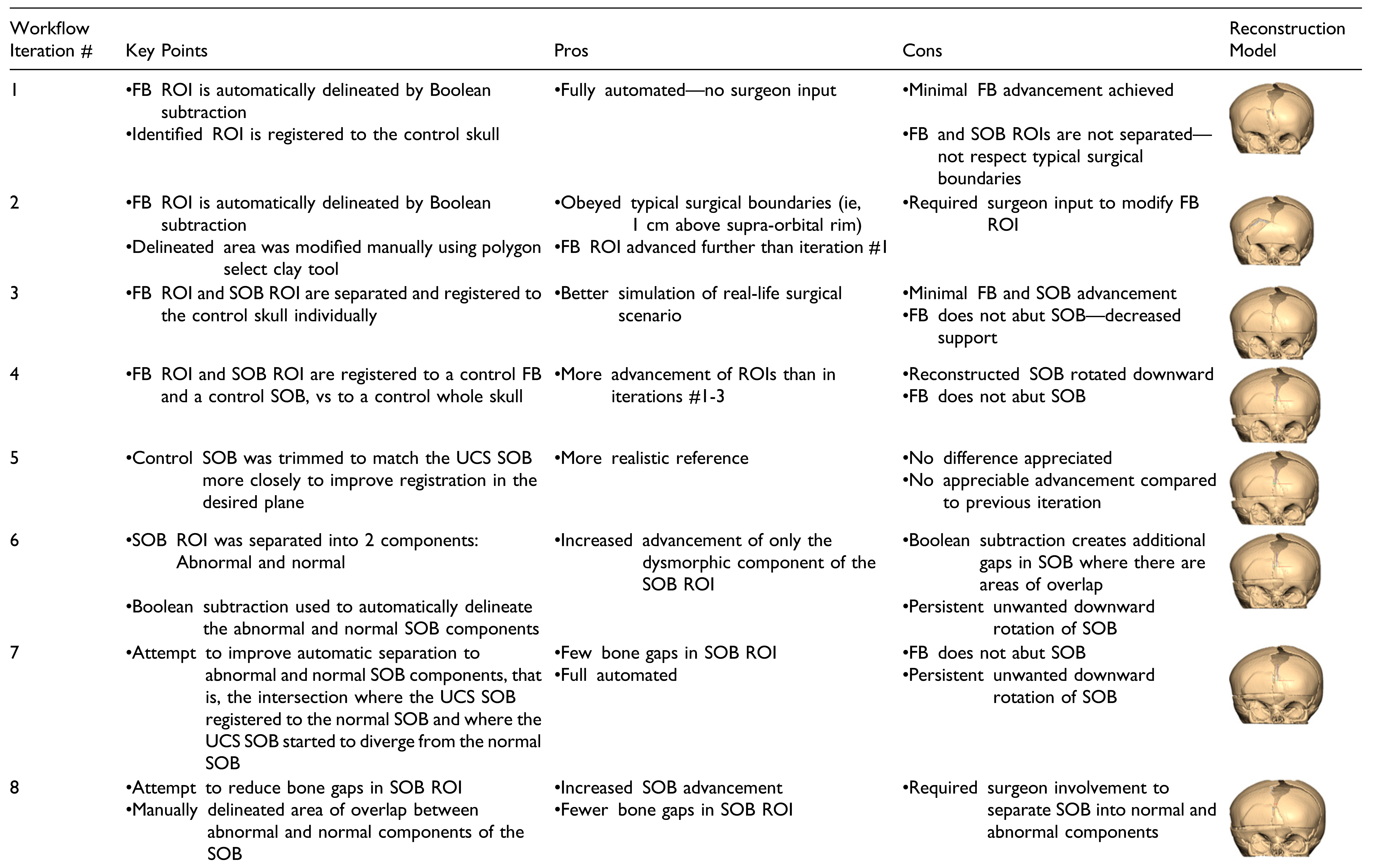
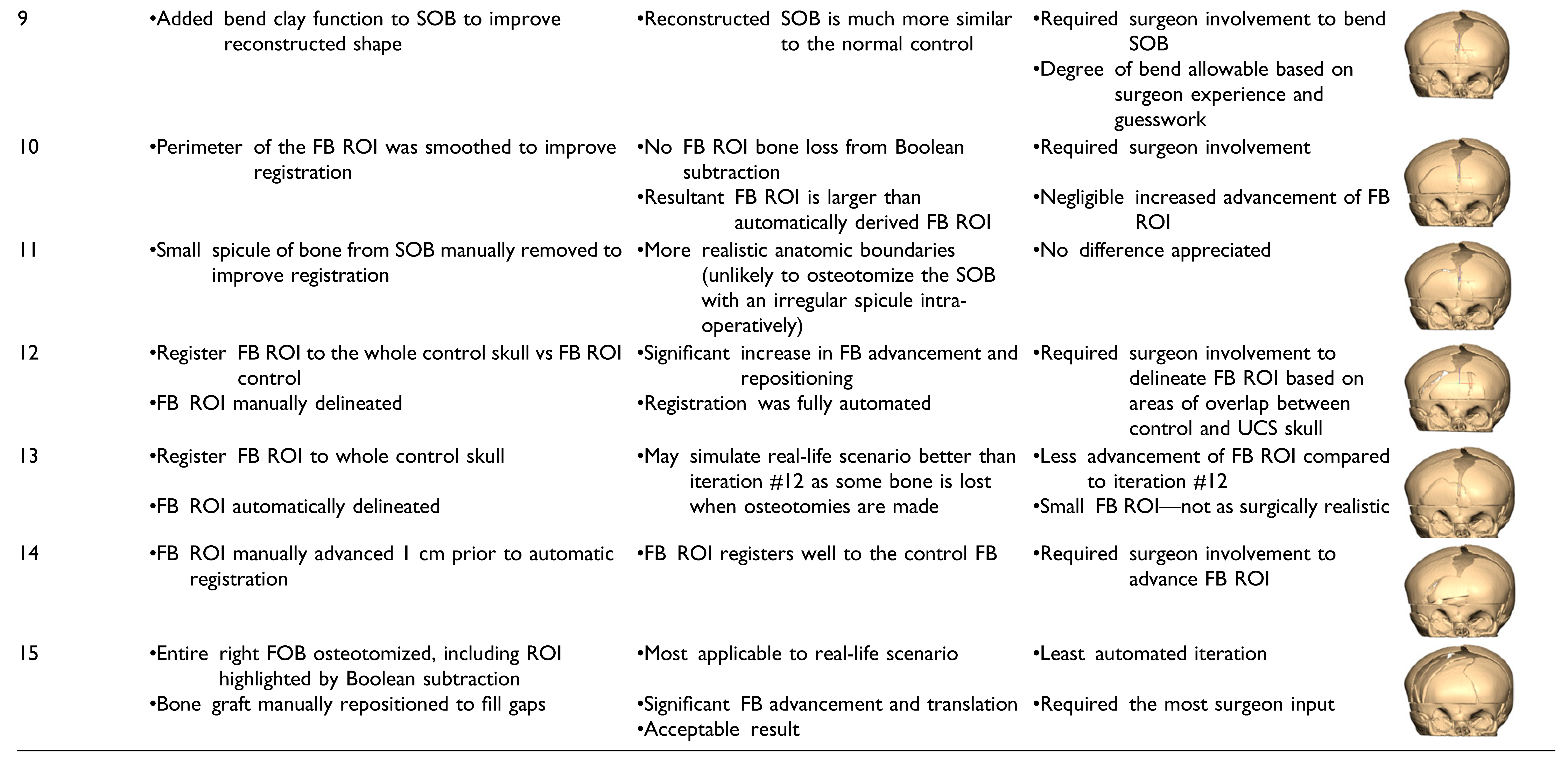
Fifteen iterations of a partially automated workflow were carried out. Automated steps were introduced into the manual workflow by using techniques such as Boolean operations on polygons and object registration. All the fifteen automated workflow it-erations varied in the surgeon involvement required. There are iterations that follow a rigid algorithm design requiring zero input from the surgeon, and others that follow a semi-rigid paradigm and require a degree of surgeon guidance in the planning degree of process. The key changes, pros, and cons of each iteration are summarized in
Table 1.
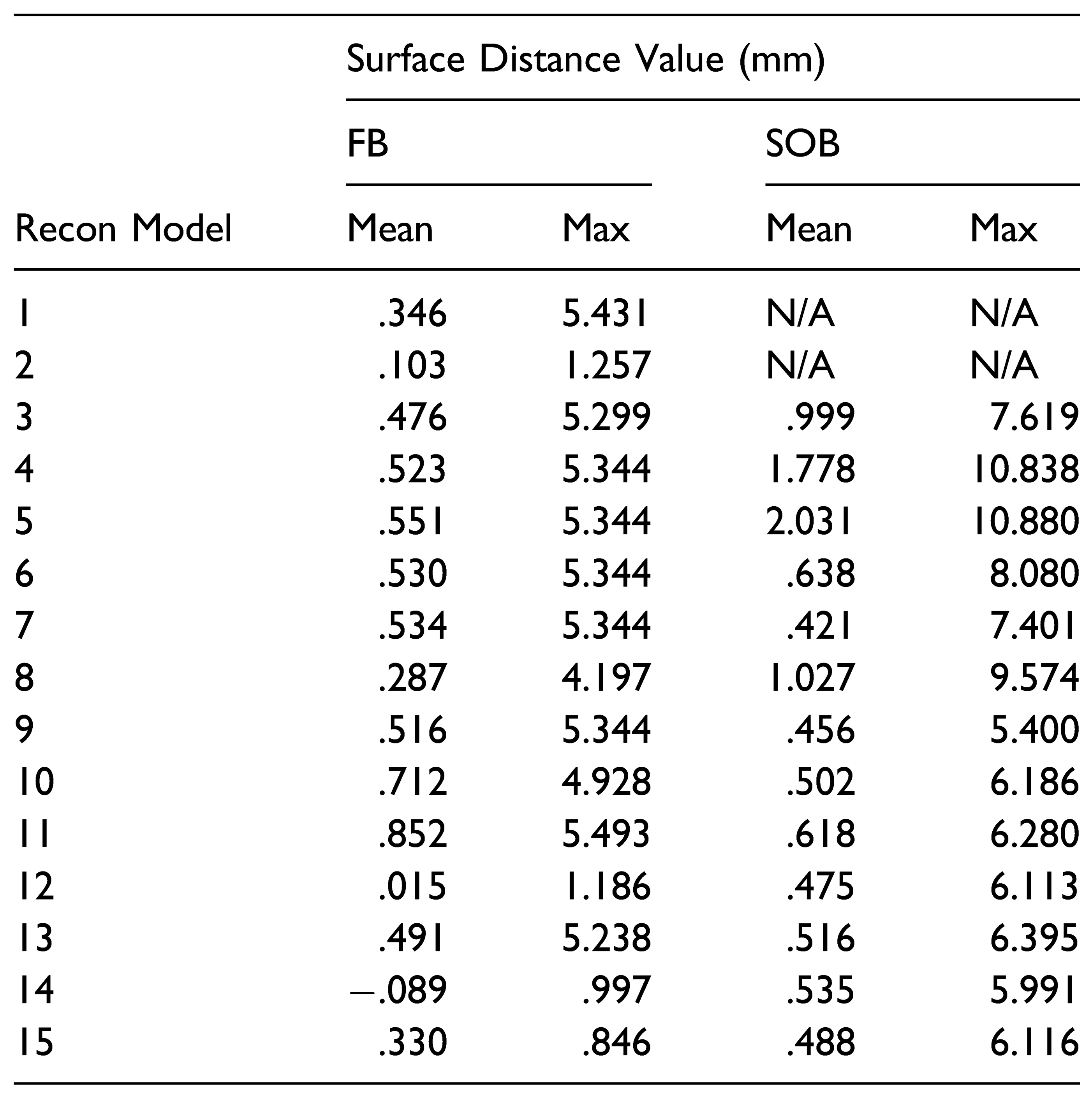
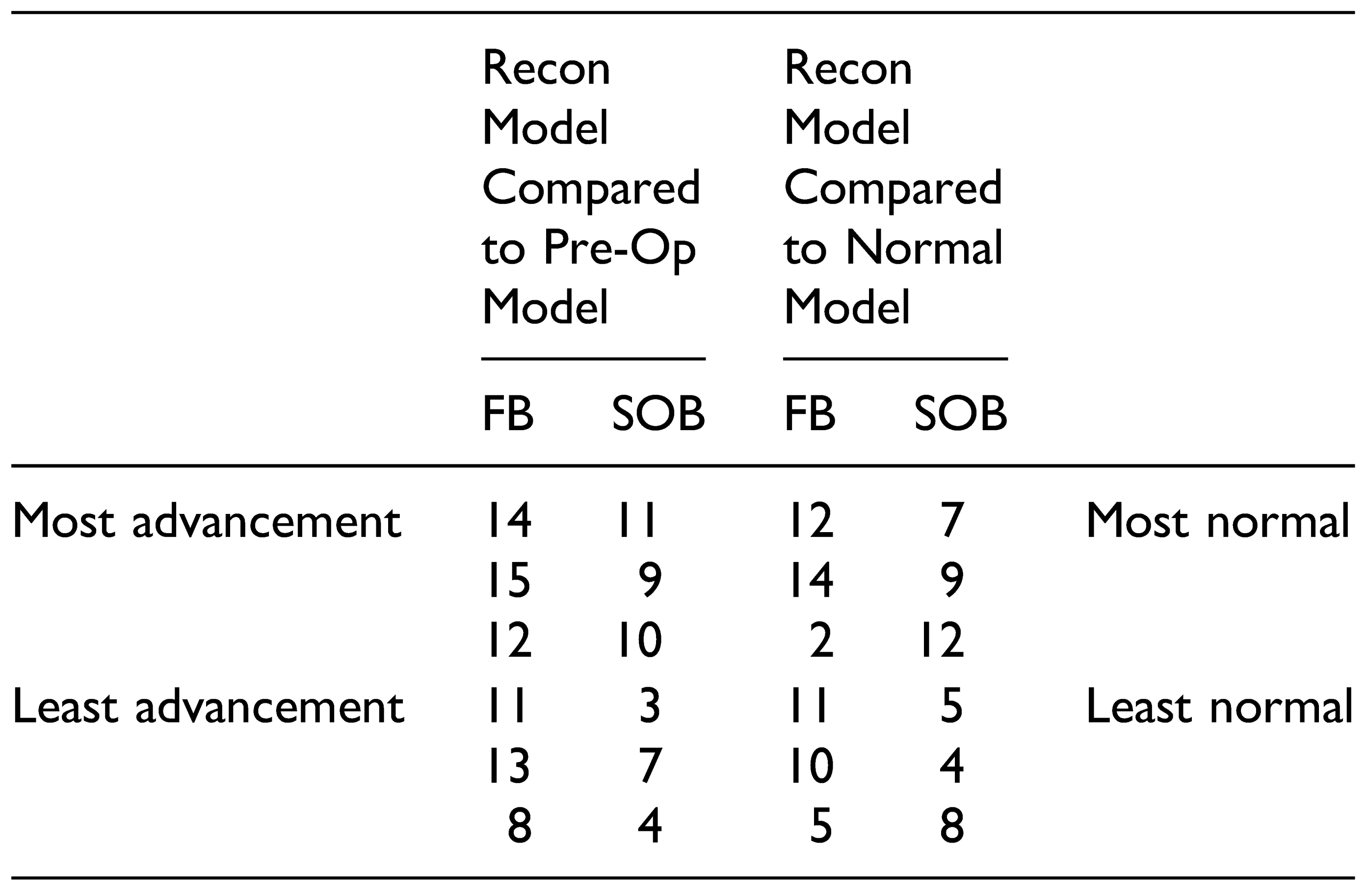
To assess the amount of advancement of the SOB and FB using the partially automated workflows, Hausdorff surface distances were automatically calculated in 3D Slicer (Version 10.2) between the reconstruction models and the pre-operative UCS skull. The same method was used to calculate distances between the reconstruction models and the normal control skull, to assess the similarity between the reconstruction model and normal skull morphology. Skulls that had a large surface distance value in comparison to the pre-operative skull and small surface distance values in comparison to the normal skull were considered successful reconstructions. In addition, color maps for all skull reconstruction models were generated using the Shape Population Viewer module to qualitatively assess the skull morphology.
Regions of Interest
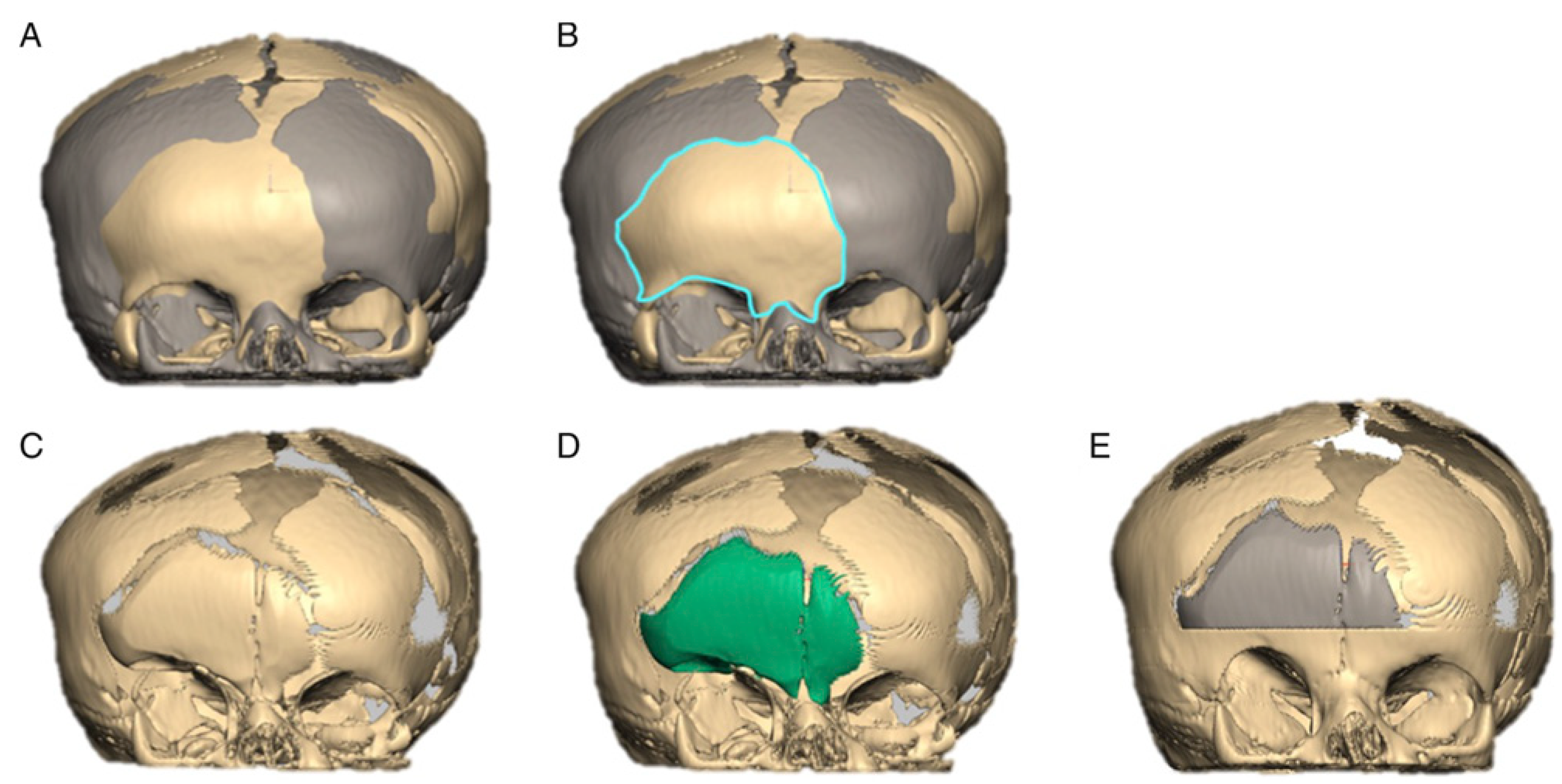
The key concept behind introducing automation into the virtual workflow for FOA is the use of Boolean functions and registration of key ROIs to reshape a UCS skull. The ROIs for an FOA are the SOB and the frontal bones (FBs). A Boolean subtraction function was used in this study by taking advantage of the fact that a UCS skull and a normal skull that are registered to each other will overlap in areas of less deformity. Intuitively, areas of the UCS skull that are less dysmorphic will line up well with a normal skull reference. On the other hand, in areas like the flattened frontal bone on the synostotic side of the UCS skull, the skulls will only overlap in areas surrounding the dysmorphic bone, forming a perimeter around the area of flattening. This perimeter is used to automatically delineate the area that requires surgical advancement. An example of how a Boolean subtraction function was used to automate the identification of a frontal bone ROI is shown in
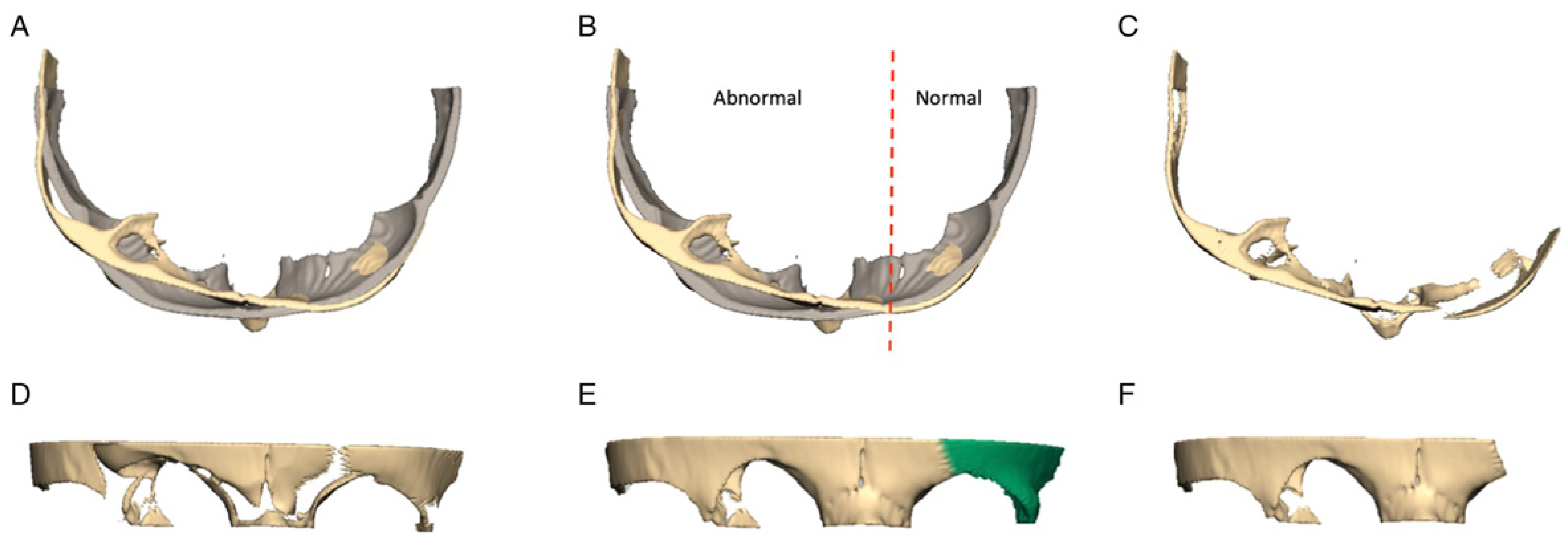
Figure 3. Once identified, this piece is registered to the control skull to determine its optimal position. The registration function in Freeform Plus (Version 2017) automatically positions an ROI in relation to the reference skull by minimizing the surface distances between each model. The piece is automatically translated and rotated in all planes to find the best fit to the normal skull. The same Boolean subtraction function can be applied to the SOB ROI—automatically highlighting the portion that requires advancement and reshaping and to what degree (
Figure 4).
Discussion
An in-house manual VSP workflow was developed using Geomagic Freeform Plus. The workflow was used to simulate an FOA in a three-month-old female with UCS. The skull model reconstructed using the workflow had a forehead that was closer in shape to a normal skull, demonstrated by smaller RMSE values, and was more symmetrical based on a qualitative color map assessment. The benefit of the manual workflow is that a surgical team can simulate an operation multiple times virtually without any consequence to the patient. The downsides are that all the steps are still dictated by subjective decisions made by a surgeon or surgical designer. This guesswork has been attributed to unreliable results and a large variation between surgeons regarding reconstruction outcomes in CVR [
11]. To reduce this subjectivity, Boolean operations on polygons and 3D model registration were introduced to create a partially automated workflow for an FOA procedure that had a reduced the need for surgeon involvement to guide the planning process.
Boolean operations on polygons are popular methods for designing solid objects. Typically primitive shapes are introduced, and new objects and 3D models can be created by using the 3 types of Boolean functions: union, subtraction, and intersection [
17]. Boolean operations have been used previously in VSP, particularly for a surgical guide and splint design [
18,
19,
20,
21]. Similar to this study, Wang et al [
22] used Boolean subtraction to outline the surgical region of interest to create an aesthetically pleasing toe-to-thumb transfer. This is the first study that uses Boolean operations to outline surgical ROIs in virtual cranial vault remodeling. A subtraction function worked well to identify the frontal bone area requiring advancement. This might be of particular benefit in surgical planning when deciding whether to perform a unilateral vs a bilateral FOA. If the frontal bone ROI did not cross midline, the argument could be made to attempt a unilateral FOA.
The same automation techniques were applied to the SOB. However, the results were less consistent and of limited utility. The superior and inferior rotation of the SOB was not controlled in the automated workflow when registering it to a normal reference. As a result, a large distance could refer to a large downward rotation, limited anterior translation, and therefore not correlate to a clinically improved SOB. Limiting planes of motion for specific surgical maneuvers such as SOB advancement for a virtual FOA would be an important component of an automated workflow.
Iterations of the automated workflow that followed a partially automated paradigm produced better results. Fully automated iterations produced reconstruction models that had a suboptimal forehead shape and did not simulate typical surgical boundaries, such as the cranial edge of the supra-orbital bar. However, several iterations that resulted in successfully advanced ROIs and improved forehead shape had a combination of manual and automated steps. In iteration #15, for example, the FB ROI was automatically outlined using a Boolean operation. Then, the FB osteotomy was manually designed to incorporate this area but adhered to a triangular configuration that mimics how frontal bones are typically osteotomized at the study institution. The result was a reconstruction model that had significant advancement of the ROIs and approximated a normal skull shape, while suggesting steps that are surgically realistic. This contrasts with iteration #12 that produced a high performing model based on the outlined criteria. While the reconstruction model in this iteration produced a skull that approximated a normal reference and achieved a large degree of advancement of the ROIs, the design of the osteotomy would likely result in noticeable contour abnormalities on the patient’s forehead. The benefits of a procedure like the one described for iteration #15 are that the surgeon has additional control over the design of the osteotomy with the benefit of automatically derived suggestions on where to position the bone fragments. This highlights the important concept that automated surgical planning can successfully reduce the subjectivity of surgical decision making and result in reconstruction models that are mathematically superior to the preoperative models. However, clinician input is still crucial to refine a plan and ensure its feasibility. In addition, involvement in the planning process is an important component of surgical training. Computer automation can be viewed as a helpful adjunct to traditional surgeonbased planning. Using automation to reduce the guesswork in surgical planning in conjunction with human input may increase the uptake of new technology, especially in initial phases. Even in highly sophisticated computation models, there are steps that require human input [
14].
The presented workflows can be carried out using fundamental computing functions that are widely available in many software programs. In addition, these functions are easily automated, and the demonstration of their potential in surgical planning is optimistic for the development of a fully automated planning algorithm. The workflow uses comparative normal skulls as the reference model, a resource that most institutions have access to. While statistical shape models allow for accurate registration and analysis, their development requires expertise in medical imaging and analysis [
23]. Although there are downsides to a simplified approach to automating surgical planning, it affords a degree of flexibility that can be applied to surgical planning for asymmetric craniosynostosis. Automatically outlining ROIs has the potential to reduce the guesswork associated with CVR and may be valuable to surgical teams.
The automatic delineation of an ROI can have a number of applications. This information can be used simply to refine or confirm a pre-determined surgical FOA plan. It can also be used to virtually plan an entire operation. In the latter scenario, it would be possible to print surgical cutting and plating guides to translate the virtual plan to the operating room with increased precision—a technique that has demonstrated to be beneficial [
3,
4,
5,
6,
7,
8,
9,
10,
11].
Limitations
There are limitations of the presented workflows. Firstly, proprietary software was used and may be a deterrent to their widespread use. However, Boolean operations are common and available in open-source software programs, such as 3D Slicer (Version 4.10.2). It is possible that the concept can be applied in programs that are more accessible.
There is also no biomechanical information regarding the properties of infant bone in either the manual or automated orkflow. The challenge of introducing bone bending in automated CVR algorithms has only recently been introduced [
14]. In the proposed workflow, the degree to which bones can bend before fracturing is based on visual judgment and surgeon experience. Additional components key to the planning process for an FOA, such as bone growth and the potential for relapse, also rely on surgeon’s judgment and are not automated in the outlined paradigm.
One limitation with pre-operative imaging in infants relates to the rapid growth of the craniofacial skeleton at this age. Due to this, imaging used for surgical planning should ideally be obtained within 1 month of surgery to best match the size of the calvarium at the time of surgery.
Lastly, the automated workflow relies on the successful registration of a UCS and a normal skull. Using age- and sex-matched skulls to achieve this has been done frequently with success [
11,
24,
25]. This relies on the availability of a library of normal skull CT scans. Some institutions may not have such a repository of CT information available, or some skulls may be difficult to match for other reasons such as the degree of deformity. In these circumstances, the presented workflow may be of limited use. One method to improve the registration accuracy in these scenarios would be to use statistical shape modeling as opposed to matched skulls [
26,
27].