Abstract
Study Design: This article is to evaluate the early outcomes of dental implants placed in bone generated with tissueengineering techniques, specifically recombinant human bone morphogenic protein-2 (rhBMP-2), allogeneicbone particulate, and bone marrow aspirate concentrate (BMAC) in patients with resection of benignpathology. Objective: To evaluate the long-term prognosis of dental implants placed in tissue engineered mandibular reconstruction. Methods: We retrospectively evaluated 12 patient records, all of whom underwent segmental mandibular resection of benign pathology and reconstruction with a combination of BMAC, rhBMP-2, and allogeneic bone. Collecteddata points included the patient's age, gender, medical and social histories, implant site and placement date, resection/reconstruction date, final prosthesis, pathology resected, and follow-up dates (average 25 monthsof follow-up). Implant success was defined as clinical osseointegration (immobility), absence of peri-implantradiolucency, and absence of infection. Results: Twelve patients met inclusion criteria with a total of 46 implants. The overall implant survival rate was 91.3%. There were 4 implant failures occurring in two patients: 1 failure in Patient 3 and 3 failures in Patient 8. Neitherpatient had any existing medical comorbidities or social history known to increase the risk for implant failure. The average implant placement occurred 11.6 months after mandibular reconstruction. Conclusions: Preliminary findings of implant placement in bone generated with tissue engineering techniques have shown to be another predictable alternative for orofacial rehabilitation. Practical Implications: Dental rehabilitationusing dental implants is a predictable treatment option for patients that have required reconstruction of largebony defects status post resection of benign pathology using novel tissue engineering techniques.
Introduction
The goal of mandibular and maxillary reconstruction is orofacial rehabilitation of the patient. This includes removal of disease, reconstruction of the maxillomandibular structures, and dental rehabilitation. For many patients, this includes placement of dental implants. Indications for reconstruction and rehabilitation include severe mandibular atrophy, post-traumatic defects, post-resection defects secondary to infection or pathology, or congenital birth defects.
Currently, the gold standard for reconstruction of large mandibular segmental defects is microvascular free tissue transfer such as the fibular free flap. Despite high success rates, these flaps often associated with inadequate bone height, width, or shape for the purposes of dental implant placement. [1,2] Alternative techniques, including prefabricated fibula flaps with dental implants using virtual surgical planning and pre-milled guides, may require staged procedures and can significantly increase treatment costs. [3,4] If adequate bone volume is achieved, success rates for implants placed in transplanted fibula bone range from 81 to 90.1% at 5 years. [3,4] Attia et al found that the success rate of fibula flaps was 97% in their study population and the implant success rate was 81% and 78% at 5 and 11 years, respectively. Fang et al (2014) found that the highest implant failure rates in the fibula occurred in men, patients receiving primary fibula flap reconstruction, and radiotherapy. [5] These limitations may result in challenges for the surgical and restorative teams, and the patient.
The advancement of tissue engineering techniques provides an alternative reconstructive modality that can decrease surgical morbidity and duration of inpatient hospitalization, while maintaining excellent postoperative outcomes and effective bone regeneration. [6] Tissue engineering can be accomplished by the recapitulation of the embryonic process of tissue generation. Mesenchymal stem cells (MSCs), growth factors (such as recombinant human bone morphogenetic protein-2), and cytokines found in platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and bone marrow aspirate concentrate (BMAC) can be combined to engineer tissues for reconstruction. [7] While the generation of bone using these techniques has been documented in the literature, the success rates of dental implants in tissue-engineered bone have not been previously reported.
The purpose of this study was to evaluate preliminary outcomes of dental implants placed in the mandible reconstructed with tissue engineering. Other patient factors, such as uncontrolled chronic conditions and smoking history were also evaluated to determine if other known factors contributed to implant failure.
Methods
Patient charts were retrospectively evaluated at the University of Texas Health Science Center at Houston. This study was approved by the Institutional Review Board via the Committee for the Protection of Human Subjects. Success was defined as clinical osseointegration (immobility), absence of peri-implant radiolucency, and absence of infection. Inclusion criteria for this study included patients reconstructed with a combination of BMAC, rhBMP-2, and allogeneic bone particulate that had also undergone dental implant placement in tissue-engineered bone between 2010 and 2021.
All patient had undergone mandibular resection of benign pathology and were reconstructed using tissue engineering techniques. The reconstruction technique for all patients included the use of a BMP-2, BMAC, and allogeneic bone combination covered with PRP. [7] Age, medical and social histories were reviewed to identify if patients had any known risk factors for implant failure (i.e., uncontrolled diabetes mellitus, smoking). Other data points were collected to determine the length of time between resection/ reconstruction and implant placement, the implant location, histopathological diagnosis, and the date of resection. If available, how the implants were restored was also recorded.
Results
12 patients met all inclusion criteria and presented with a total of 46 implants (Table 1). There were 8 males and 4 females with an average age of 46.9 years at the time of implant placement (age range = 21–73). The average number of implants placed per person was 3.8 (8 of 12 patients had 4 mandibular patients; Figure 1). None of the patients had diabetes mellitus or were current smokers. Other medical comorbidities included hypertension (HTN), seizures, gastric reflux (GERD), and depression. Patient 7 presented with a history of radiation to the face for basal cell carcinoma. No patients reported any history of illicit drug use.

Table 1.
Patients, Including the Age at Time of Implant Placement, the Number of Implants Placed, the Number of Months They Presented for Follow-up, Relevant Medical and Smoking History, and if they Experienced Any Implant Failures. (*) indicates which patients experienced an implant failure.
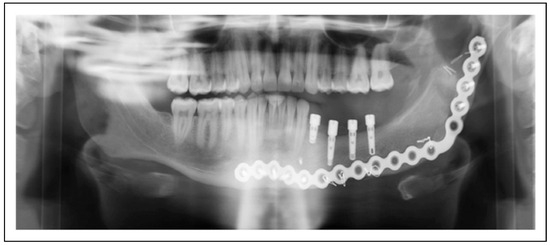
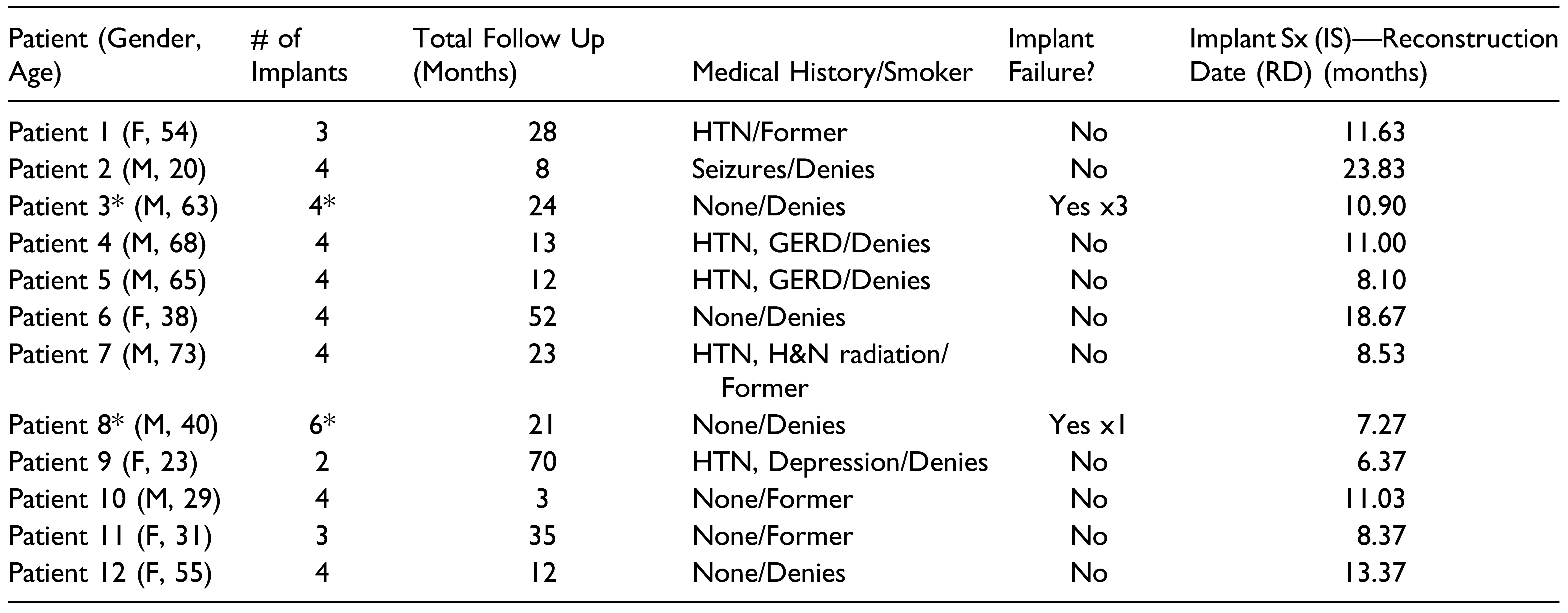
Figure 1.
Patient 2 is restored with 4 mandibular implants status post resection and reconstruction using tissue engineering techniques. These implants are without radiolucency or other signs of pathology 8 months post-operatively.
The follow-up times for each patient are also shown in Table 1. From the time of implant placement to last follow up with imaging, average follow up was 25.08 months. Median follow up time was 12 months (range from 3 months to 70 months). Implants were placed in tissueengineered mandibles on average 11.59 months after the reconstruction date (range = 6.37–23.83 months).
Patients 3 and 8 had three and one dental implant failures, respectively. Of the 4 implants placed for Patient 3, #21, 23, and 25 failed sequentially due to peri-implantitis at approximately months 4, 4.5, and 8. Patient 8 demonstrated implant failure at site #23 after approximately 6 months. Both patients presented with significant granulation tissue and failure of osseointegration on examination. Neither patient had frank purulence and only Patient 8 reported significant pain with #25.
Discussion
Osseous reconstruction of the mandible is indicated for patients with large osseous defects that require resection; these patients ultimately require dental rehabilitation to regain quality of life. While reconstruction of the mandible using tissue-engineering has been shown in prior studies to decrease donor site morbidity, decrease length of hospitalization, and provide sufficient bone for implant placement, studies evaluating the success of dental implants in tissue-engineered bone have not been completed. [6,7] In this study, we retrospectively evaluated 12 patients with a total of 46 implants placed in tissue-engineered bone. The overall success rate for the 46 implants was 91.3%.
Results from this study support tissue-engineered osseous grafts as a suitable recipient site for the placement and integration of dental implants. Implant survival in tissue-engineered graft, displayed an implant success rate of 91.3% (failure rate of 8.7%) from a total of 46 implants, and mean follow-up time of 2.1 years. In comparison, a study on long-term implant survival in a free-fibular graft has been shown to produce a failure rate of 18%, with a population size of 112, and a mean follow-up time of 4.9 years. [5] The early findings of this case series continue to support the use of tissue engineering as a viable alternative to autologous grafting.
Regarding the failed implants, all occurred in male patients who denied any medical comorbidities or smoking/ substance abuse history. Both patients had a primary pathology of ameloblastoma that was treated with resection and immediate reconstruction using BMAC, rhBMP-2, and allogeneic bone. All implants failures in this study were related to peri-implantitis and were subsequently removed. Patient 3 had four implants placed at 10.9 months after reconstruction. Patient 8 had 4 implants placed at 7.3 months after reconstruction. The average time of implant placement after reconstruction was ∼11.6 months. While both these patients had implants placed before this average time, the limited sample size does not allow for inference that implants are more likely to fail if placed prior to 11.6 months. Patients 4, 5, 7, 9, 10, and 11 also had implants placed prior to 11.6 months and did not experience any complications.
Of our study population, patients 2, 4, 5, 9, 10, and 12 were successfully restored using a lingual bar or other implant supported prosthesis, including bridges and complete dentures (Figure 2, Figure 3A and 3B). Patients 1 and 11 were unable to have their implants restored due to limited finances. Patients 3 and 8 continue to undergo surgical management prior to completing dental rehabilitation. Of note, close coordination with a restorative dentist or prosthodontist is important for successful orofacial rehabilitation of the patients.
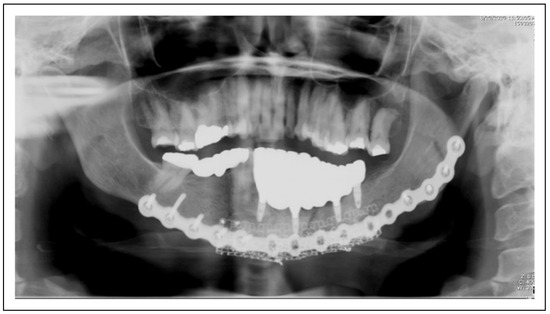
Figure 2.
Patient 12 is restored with 4 mandibular implants that support the dental prosthesis. The implants remain stable and without pathology after 12 months.
Figure 3.
(A) Patient 12 is restored with an implant supported prostheses after reconstruction and rehabilitation with tissue engineering and dental implants. (B) Intraoral view of Patient 12; implant supported prostheses after reconstruction and rehabilitation with tissue engineering and dental implants.
The mean follow-up time was ∼25 months. In this study population, factors that impacted the follow-up attrition rate included financial instability (large patient debts), patient moving to another city, the multidisciplinary nature of treating these patients (lack of access to physician notes), or declining to seek medical evaluation from fear of COVID-19. The authors of this study aim to continue following this patient population to determine if these implants will continue to be successful long-term. Other limitations of the study include the population size, the number of implants placed, and determining if other factors contributed to implant success/failure (i.e., oral hygiene, etc.). Future areas of interest include obtaining a larger sample size (perhaps, collaboration with other sites for a multi-center study), measurements of crestal bone loss and soft tissue evaluation.
Conclusion
Tissue engineering has emerged as a reliable and consistent method for reconstruction of the mandible after resection of osseous defects. In this study, the authors retrospectively evaluated 12 patient charts with 46 implants, and report a clinical success rate of 91.3%. Our early findings indicate that implant placement in tissue-engineered bone may also result in long-term success for complete orofacial rehabilitation. Further studies are indicated.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Kim, D.D.; Ghali, G.E. Dental implants in oral cancer recon- struction. Oral Maxillofac Surg Clin North Am. 2011, 23, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.Y.; Kim, D.D.; Ghali, G.E. Maxillofacial Reconstruction Using Vascularized Fibula Free Flaps and Endosseous Im- plants. Oral Maxillofac Surg Clin North Am. 2019, 31, 259–284. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Harrison, P.; Cheng, A.; Bray, B.; Bell, R.B. Fibular Re- construction of the Maxilla and Mandible with Immediate Implant-Supported Prosthetic Rehabilitation: Jaw in a Day. Oral Maxillofac Surg Clin North Am. 2019, 31, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.; Wiltfang, J.; Pons-Kühnemann, J.; et al. Survival of dental implants placed in vascularised fibula free flaps after jaw reconstruction. J Craniomaxillofac Surg. 2018, 46, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Liu, Y.P.; Ma, Q.; Liu, B.L.; Zhao, Y. Long-term results of mandibular reconstruction of continuity defects with fibula free flap and implant-borne dental rehabilitation. Int J Oral Max- illofac Implants. 2015, 30, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Melville, J.C.; Nassari, N.N.; Hanna, I.A.; Shum, J.W.; Wong, M.E.; Young, S. Immediate transoral allogeneic bone grafting for large mandibular defects. Less morbidity, more bone. A par- adigm in benign tumor mandibular reconstruction? J Oral Maxillofac Surg. 2017, 75, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Melville, J.C.; Tran, H.Q.; Bhatti, A.K.; Manon, V.; Young, S.; Wong, M.E. Is Reconstruction of Large Mandibular Defects Using Bioengineering Materials Effective? J Oral Maxillofac Surg. 2020, 78, 661.e29. [Google Scholar] [CrossRef] [PubMed]
© 2022 by the author. The Author(s) 2022.