Printing in Time for Cranio-Maxillo-Facial Trauma Surgery: Key Parameters to Factor in
Abstract
:Introduction
Methodology
Phases for Obtaining a 3D Printed Model
- 1.
- Virtual Surgical Planning
- 2.
- Printing
- 3.
- Shipping
- 4.
- Implant Contouring and Sterilization
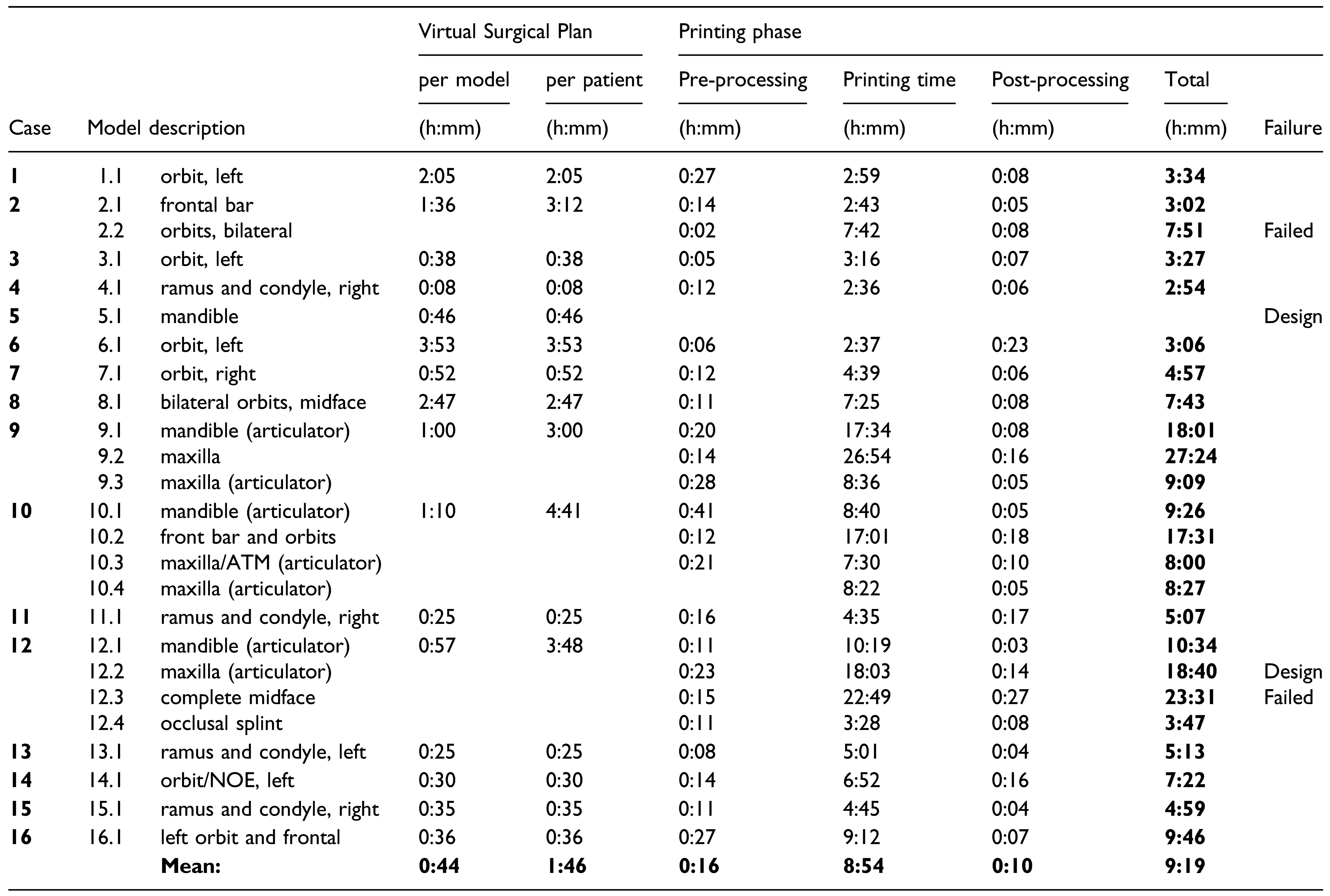
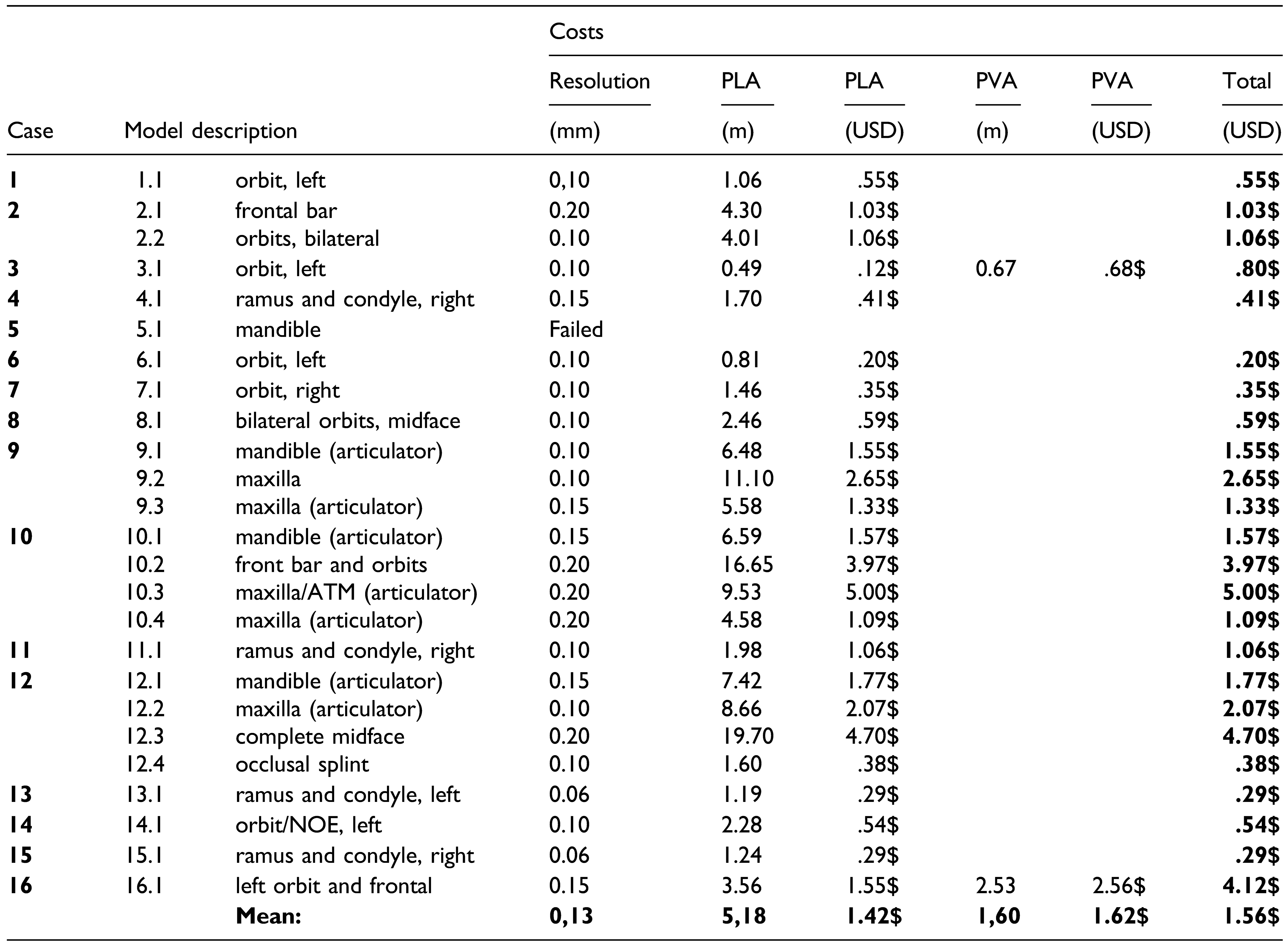
Results
Discussion
Accuracy of the Model
Printing Supply Cost
Printer Cost
Printing Time
Printing Strategy to Decrease Time
Labor Cost and Training
Regulatory Agency Approval
Limitations
Conclusion
Author Contributions
Funding
Ethics
Conflicts of Interest
References
- Hollier, L.H., Jr. The confluence of technique and technology in craniofacial surgery. Plast. Reconstr. Surg. 2021, 147, 1027–1028. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, L.; Bonapace-Potvin, M.; Bergeron, F. In-house 3D model printing for acute Cranio-maxillo-facial trauma surgery: process, time, and costs. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3804. [Google Scholar] [CrossRef]
- Marschall, J.S.; Dutra, V.; Flint, R.L.; et al. In-house digital workflow for the management of acute mandible fractures. J. Oral. Maxillofac. Surg. 2019, 77, 2084.e1–2084.e9. [Google Scholar] [CrossRef]
- Bergeron, L.; Bouchard, S.; Bonapace-Potvin, M.; Bergeron, F. Intraoperative surgical navigation reduces the surgical time required to treat acute major facial fractures. Plast. Reconstr. Surg. 2019, 144, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, L.; Bouchard, S.; Bonapace-Potvin, M.; Bergeron, F. Response to Drs. Chen and Bradley’s recent letter to the editor concerning our paper on the use of navigation in the treatment of major facial fractures. Plast. Reconstr. Surg. 2020, 146, 509e–510e. [Google Scholar] [CrossRef]
- Gleissner, H.; Castrillon-Oberndorfer, G.; Gehrlich, S.T. Introduction of 3D printing in a german municipal hospital - practical guide for CMF surgery. Craniomaxillofacial Trauma. Reconstr. 2021. [Google Scholar] [CrossRef]
- Lim, C.G.; Campbell, D.I.; Clucas, D.M. Rapid prototyping technology in orbital floor reconstruction: application in three patients. Craniomaxillofacial Trauma. Reconstr. 2014, 7, 143–146. [Google Scholar] [CrossRef]
- Jacobs, C.A.; Lin, A.Y. A new classification of three-dimensional printing technologies: systematic review of three-dimensional printing for patient-specific craniomaxillofacial surgery. Plast. Reconstr. Surg. 2017, 139, 1211–1220. [Google Scholar] [CrossRef]
- He, W.; Sun, Y.; Tian, K.; Xie, X.; Wang, X.; Li, Z. Novel arch bar fabricated with a computer-aided design and three-dimensional printing: a feasibility study. J. Oral. Maxillofac. Surg. 2015, 73, 2162–2168. [Google Scholar] [CrossRef]
- Chae, M.P.; Rozen, W.M.; McMenamin, P.G.; Findlay, M.W.; Spychal, R.T.; Hunter-Smith, D.J. Emerging applications of bedside 3D printing in plastic surgery. Front. Surg. 2015, 2, 25. [Google Scholar] [CrossRef]
- Dvoracek, L.A.; Lee, J.Y.; Unadkat, J.V.; et al. Low-cost, three-dimensionally-printed, anatomical models for optimization of orbital wall reconstruction. Plast. Reconstr. Surg. 2021, 147, 162–166. [Google Scholar] [CrossRef]
- Rogers-Vizena, C.R.; Weinstock, P.; Livingston, K.; Prabhu, S.P. Letter to the editor concerning the article “The current role of three-dimensional printing in plastic surgery” by Kamali, P. et al. Plast. Reconstr. Surg. 2017, 139, 811e–812e. [Google Scholar] [CrossRef]
- Kamali, P.; Dean, D.; Skoracki, R.; et al. The current role of three-dimensional printing in plastic surgery. Plast. Reconstr. Surg. 2016, 137, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Goodson, A.M.C.; Parmar, S.; Ganesh, S.; et al. Printed titanium implants in UK craniomaxillofacial surgery. Part I: access to digital planning and perceived scope for use in common procedures. Br. J. Oral. Maxillofac. Surg. 2021, 59, 312–319. [Google Scholar] [CrossRef]
- Lozova, L. Bringing the Ultimaker 3 to Market with Bridge Manufacturing, 2016 ed.; Ultimaker: Utrecht, The Netherlands.
- Naftulin, J.S.; Kimchi, E.Y.; Cash, S.S. Streamlined, Inexpensive 3D Printing of the Brain and Skull. PLoS ONE 2015, 10, e0136198. [Google Scholar] [CrossRef]
- Longcac, M.; Depeyre, A.; Pereira, B.; Barthelemy, I.; Pham Dang, N. Virtual surgical planning and three-dimensional printing for the treatment of comminuted zygomaticomaxillary complex fracture. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 386–390. [Google Scholar] [CrossRef]
- Gerstle, T.L.; Ibrahim, A.M.; Kim, P.S.; Lee, B.T.; Lin, S.J. A plastic surgery application in evolution: three-dimensional printing. Plast. Reconstr. Surg. 2014, 133, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Msallem, B.; Beiglboeck, F.; Honigmann, P.; Jaquiery, C.; Thieringer, F. Craniofacial reconstruction by a cost-efficient template-based process using 3D printing. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1582. [Google Scholar] [CrossRef]
- Coase, R. The problem of social cost. J. Law. Econ. 1960, 3, 1–44. [Google Scholar] [CrossRef]
- Williamson, O.E. The theory of the firm as governance structure: from choice to contract. J. Econ. Perspect. 2002, 16, 171–195. [Google Scholar] [CrossRef]
- Dahlman, C. The problem of externality. J. Law. Econ. 1979, 22, 141–162. [Google Scholar] [CrossRef]
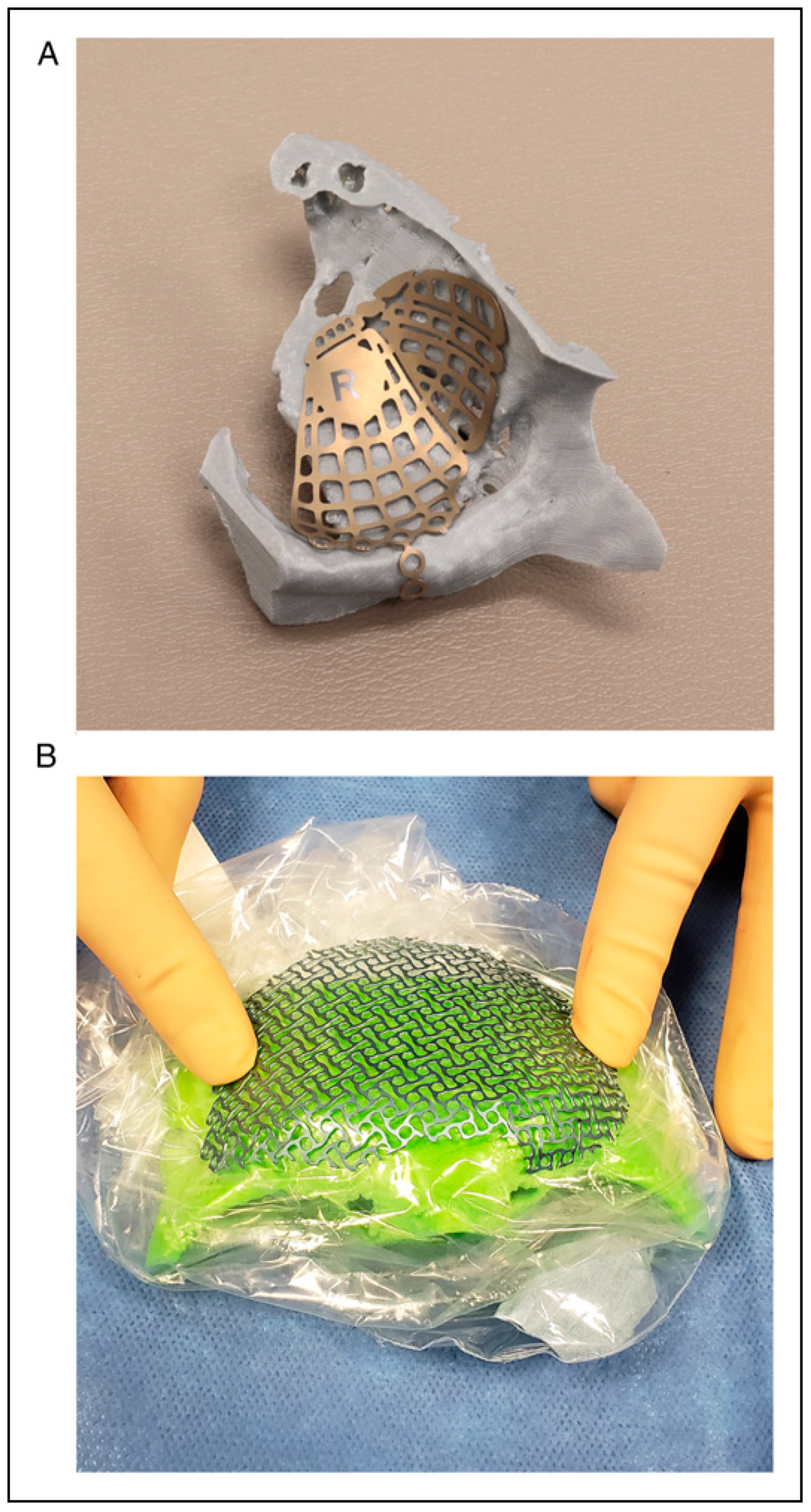


 |
 |
 |
© 2022 by the author. Multimed Inc.
Share and Cite
Bergeron, L.; Bonapace-Potvin, M.; Bergeron, F. Printing in Time for Cranio-Maxillo-Facial Trauma Surgery: Key Parameters to Factor in. Craniomaxillofac. Trauma Reconstr. 2023, 16, 121-129. https://doi.org/10.1177/19433875221083231
Bergeron L, Bonapace-Potvin M, Bergeron F. Printing in Time for Cranio-Maxillo-Facial Trauma Surgery: Key Parameters to Factor in. Craniomaxillofacial Trauma & Reconstruction. 2023; 16(2):121-129. https://doi.org/10.1177/19433875221083231
Chicago/Turabian StyleBergeron, Léonard, Michelle Bonapace-Potvin, and François Bergeron. 2023. "Printing in Time for Cranio-Maxillo-Facial Trauma Surgery: Key Parameters to Factor in" Craniomaxillofacial Trauma & Reconstruction 16, no. 2: 121-129. https://doi.org/10.1177/19433875221083231
APA StyleBergeron, L., Bonapace-Potvin, M., & Bergeron, F. (2023). Printing in Time for Cranio-Maxillo-Facial Trauma Surgery: Key Parameters to Factor in. Craniomaxillofacial Trauma & Reconstruction, 16(2), 121-129. https://doi.org/10.1177/19433875221083231




