Introduction
Surgical management of head and neck cancer has evolved to utilize microvascular free flap reconstruction following ablative surgery for patients with advanced disease. A major thrust of the contemporary era of microvascular reconstructive surgery is the restoration of patients at the time of the ablative procedure. This stands in stark contrast to the prior era of head and neck reconstructive surgery, in which surgeons relied on time-consuming and often laborious techniques to reconstruct complex defects. Some of these procedures included using flap delay to expand the vascularity of marginal areas and to waltz tissue from distant sites.[
1,
2]
However, there is a subset of advanced head and neck cancer patients who are unsuitable candidates for single-stage reconstructions, and are better served by multi-staged procedures. These patients often present with complex comorbid medical conditions and have had prior surgery and radiation therapy.
In this select group of patients, it has been the senior author’s approach to adopt a staged set of reconstructions. This method allows for closure of the oral cavity and eliminates the risk of a salivary leak in future procedures, while decreasing the number of vascular anastomoses required in any given operation. Additionally, the first stage of the reconstruction can provide potential recipient vessels for a subsequent stage, decreasing the need for vein grafts in the vessel-depleted neck.
This paper aims to present a select group of patients managed with sequential reconstructions to increase awareness of the clinical circumstances and techniques employed. There are no other reported series in the literature that address this management approach.
Methods
We report a case series of three patients treated at our institution who underwent staged reconstructions of major defects following initial treatment for head and neck cancer.
Case 1
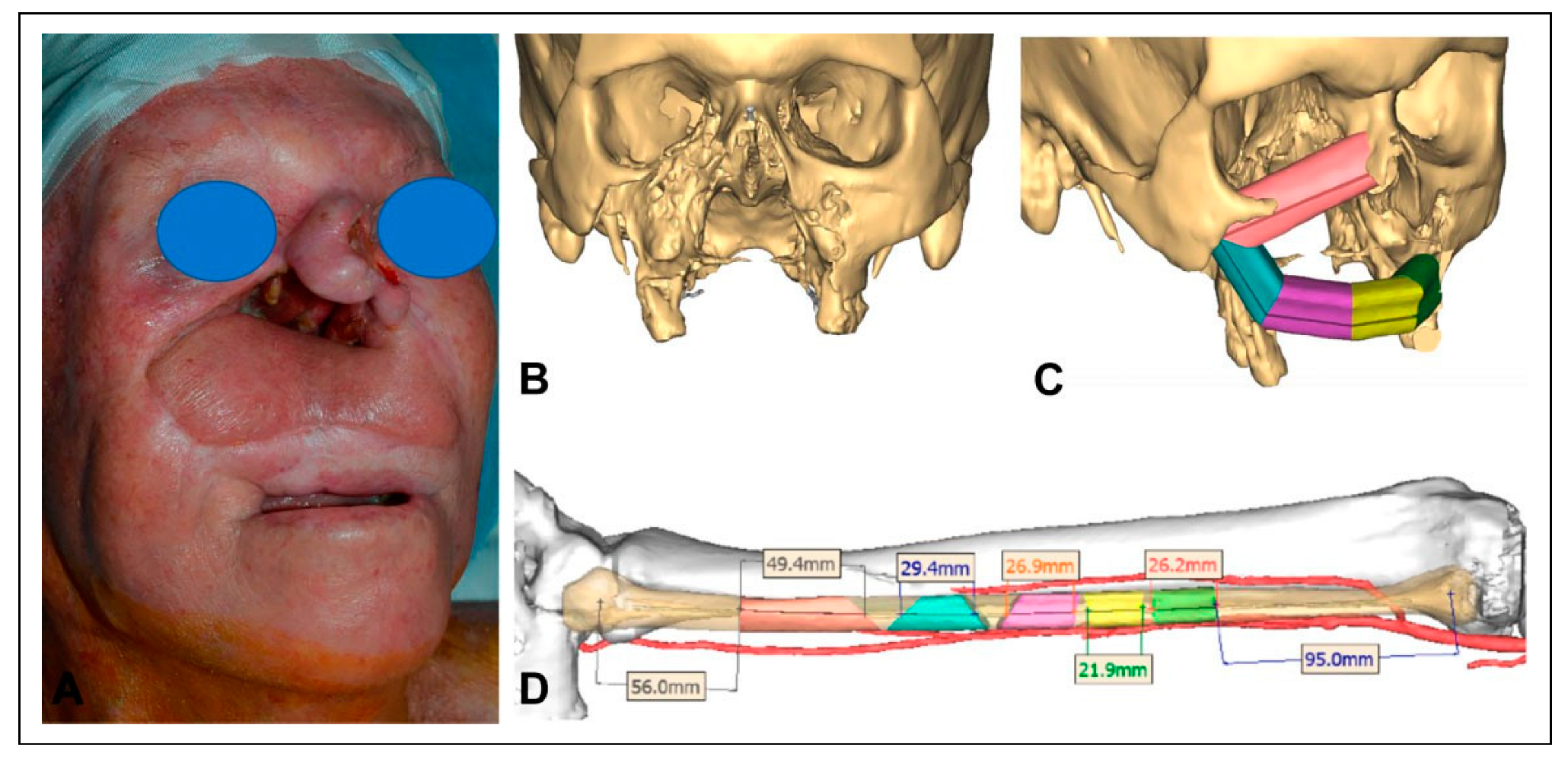
A 65-year-old male with a history of multiply-recurrent basal cell skin cancers of the head and neck region presented with a large palatomaxillary defect, significant distortion of the upper lip, and a severe nasal deformity. His medical history was significant for being immunocompromised secondary to therapy for a lung transplant at age 40.
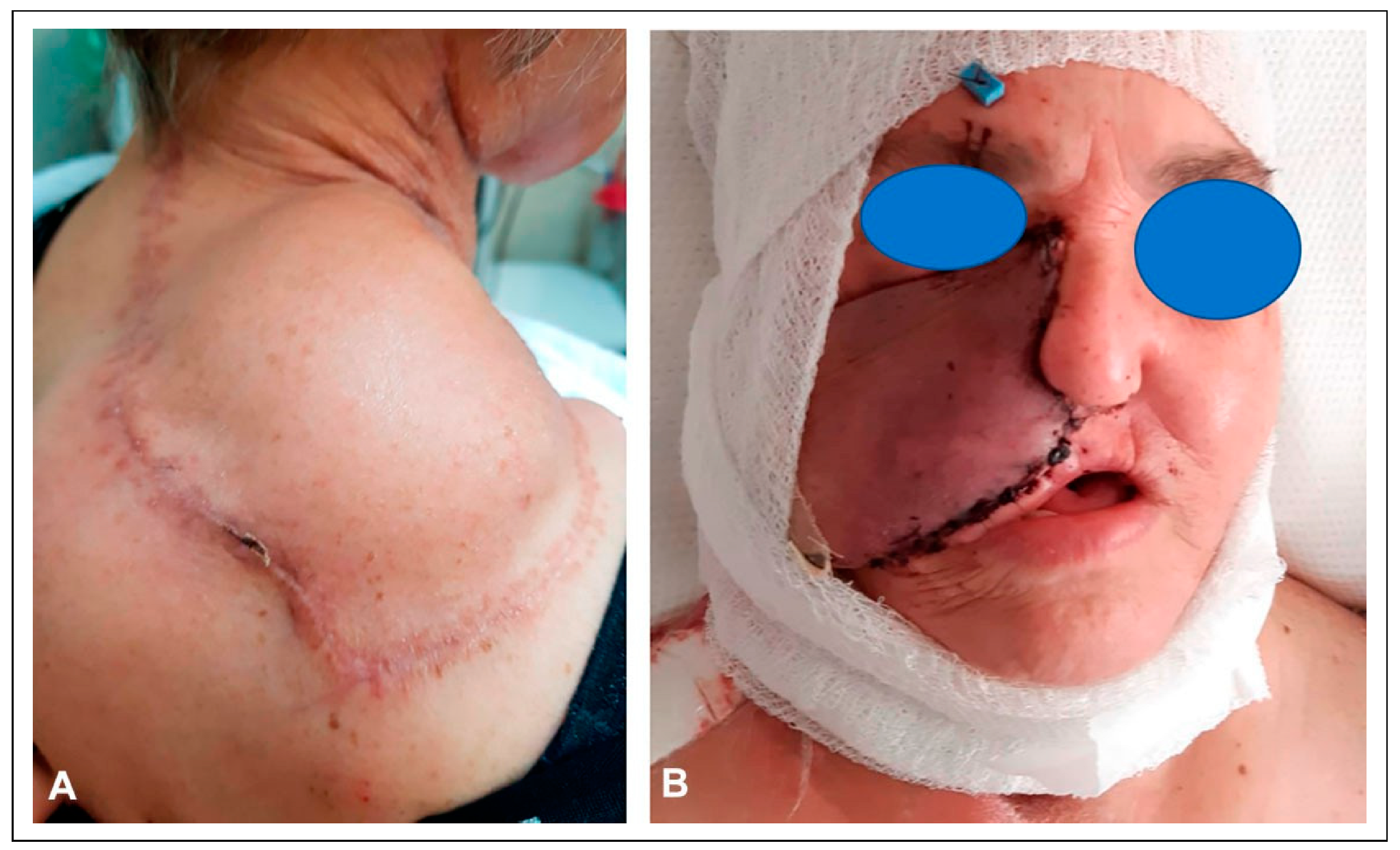
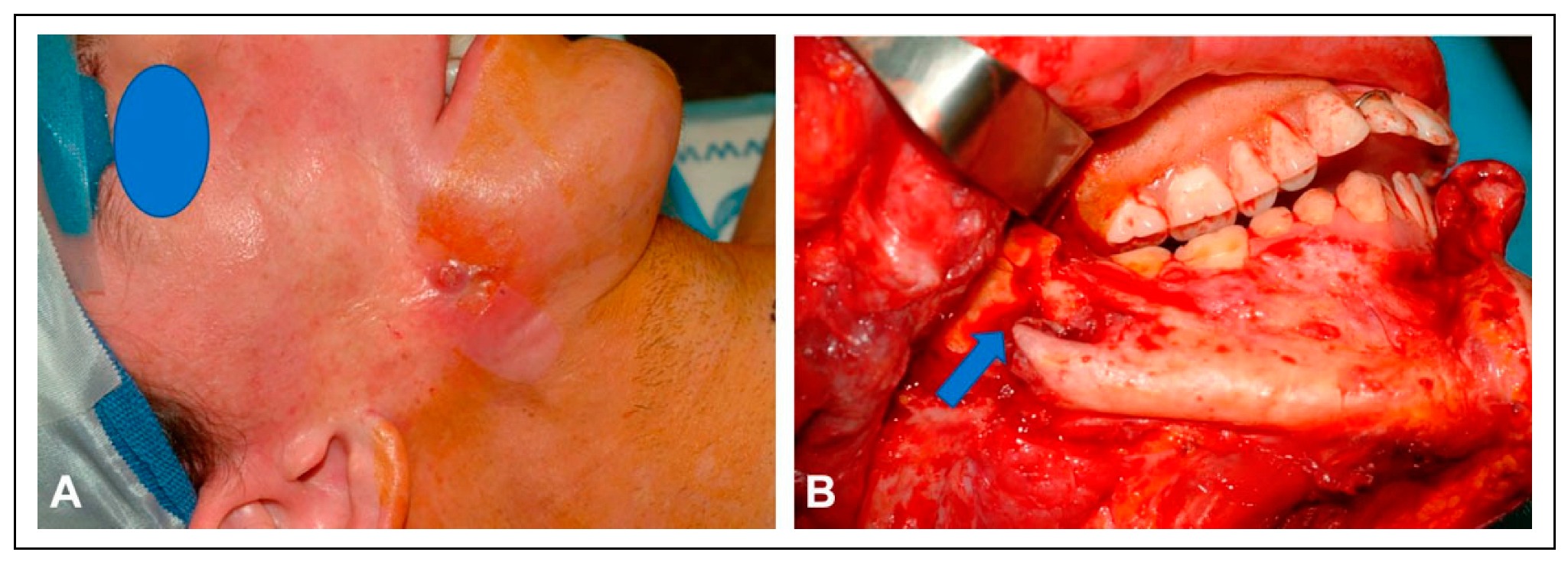
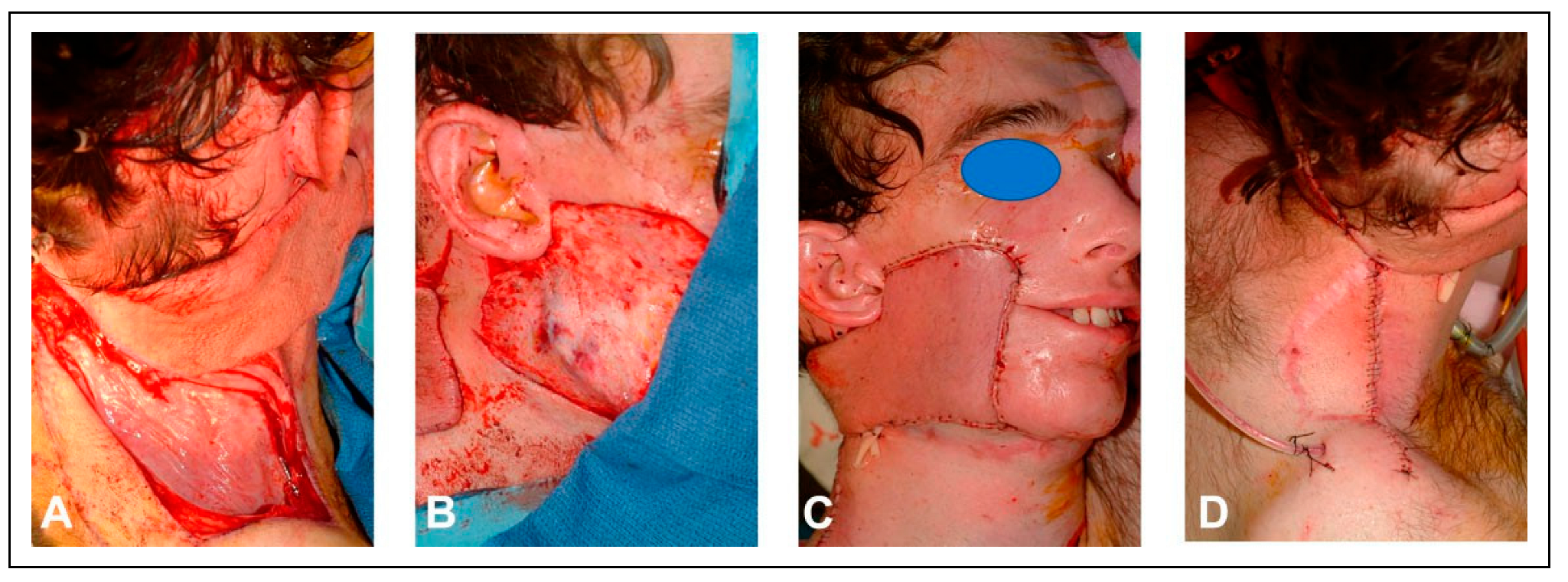
The patient’s skin cancer was initially treated with combined resection and adjuvant radiation of the nose. Four years later, the patient underwent total nasal reconstruction with a forehead flap at an outside institution, during which residual carcinoma was found. This persistent disease was subsequently treated with proton therapy. One year following proton therapy, the patient underwent a palatomaxillectomy resection for a new cancer, leaving a large nasal defect and an opening in the right cheek that directly communicated with the mouth and maxillectomy cavity (
Figure 1).
Since the patient was immunocompromised and heavily radiated, it was decided to stage the reconstructive surgeries to allow for sealing of the oral cavity, before proceeding with an osteocutaneous fibula free flap. In the first stage, the patient underwent reconstruction of the midface region using a combination of a radial forearm flap and a submental island transposition flap. The former was used to reconstruct the soft tissue lining of the oral cavity and the palatal defect, while the latter was used to restore the facial skin with color matched skin.
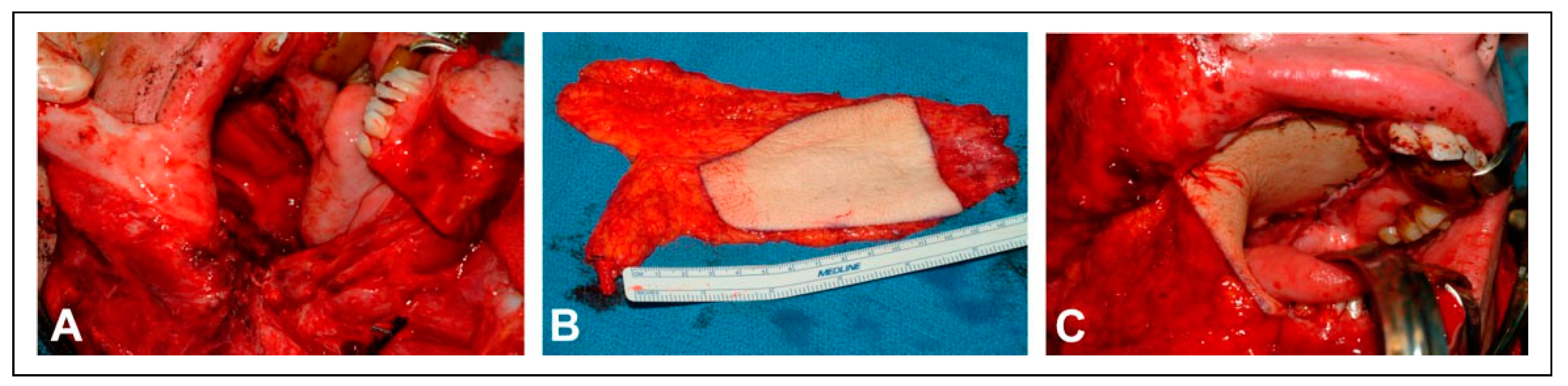
The second stage was performed three months later with reconstruction of the palatomaxillary defect using a left fibular free flap. Preoperative computer planning was performed to achieve two goals: restoration of the palatal arch with vascularized bone (
Figure 2); and the positioning of vascularized bone at the nasion to permit strategic placement of osseointegrated implants to support a nasal prosthesis (
Figure 3).
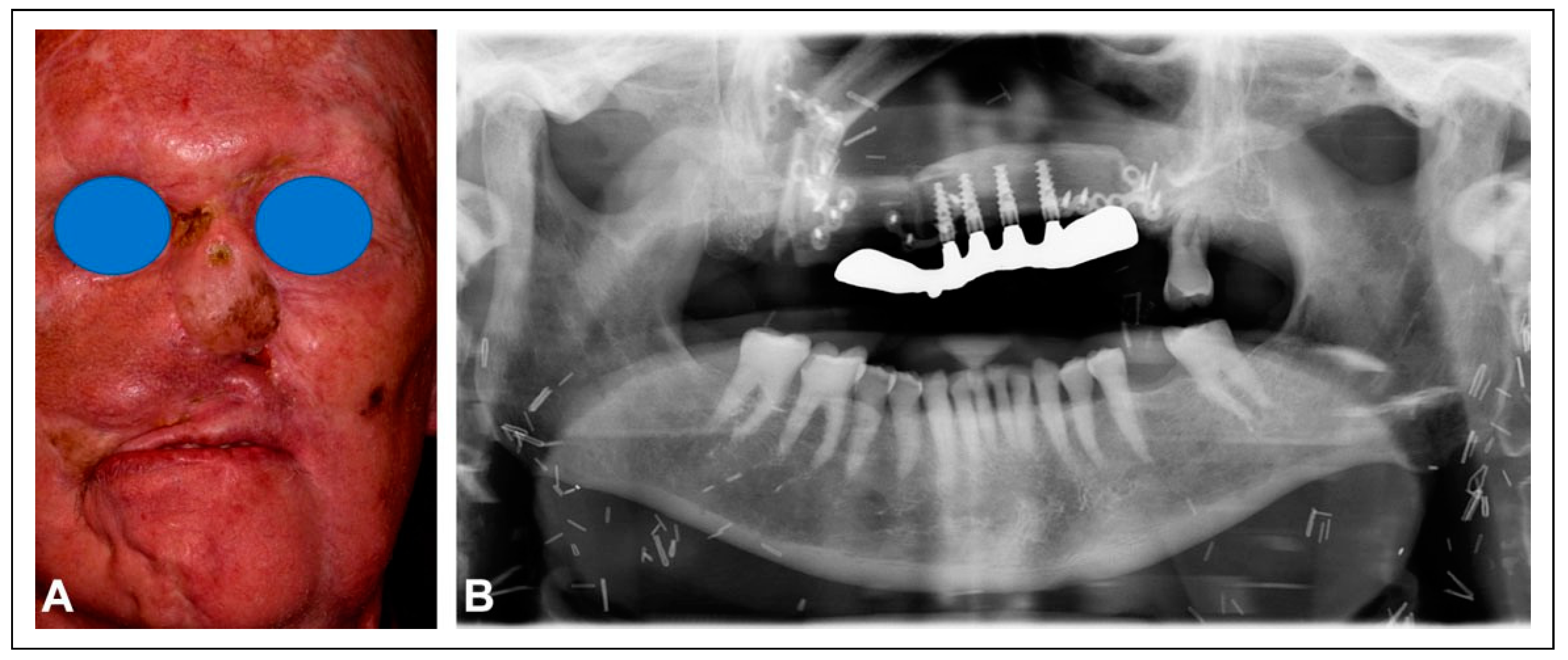
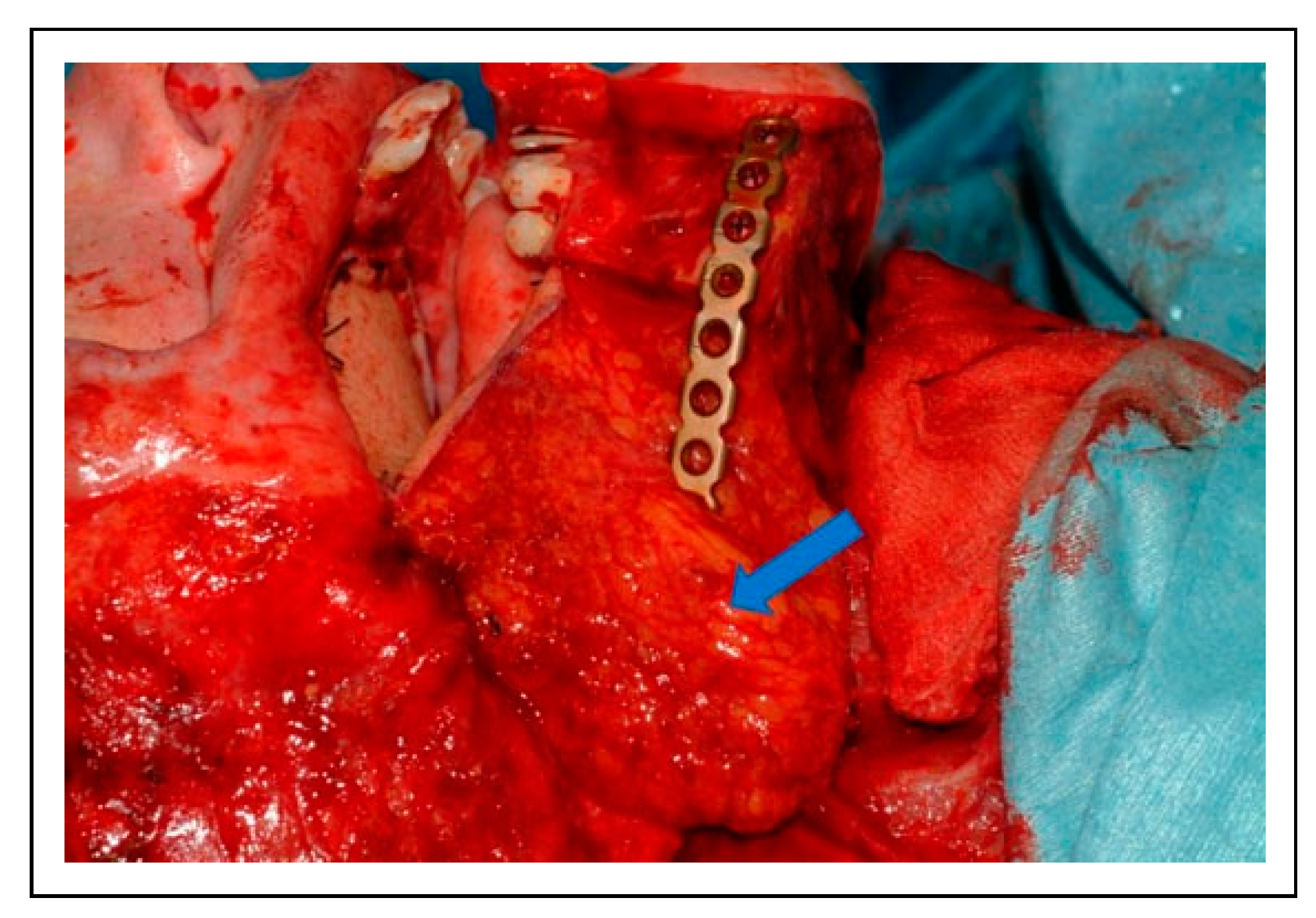
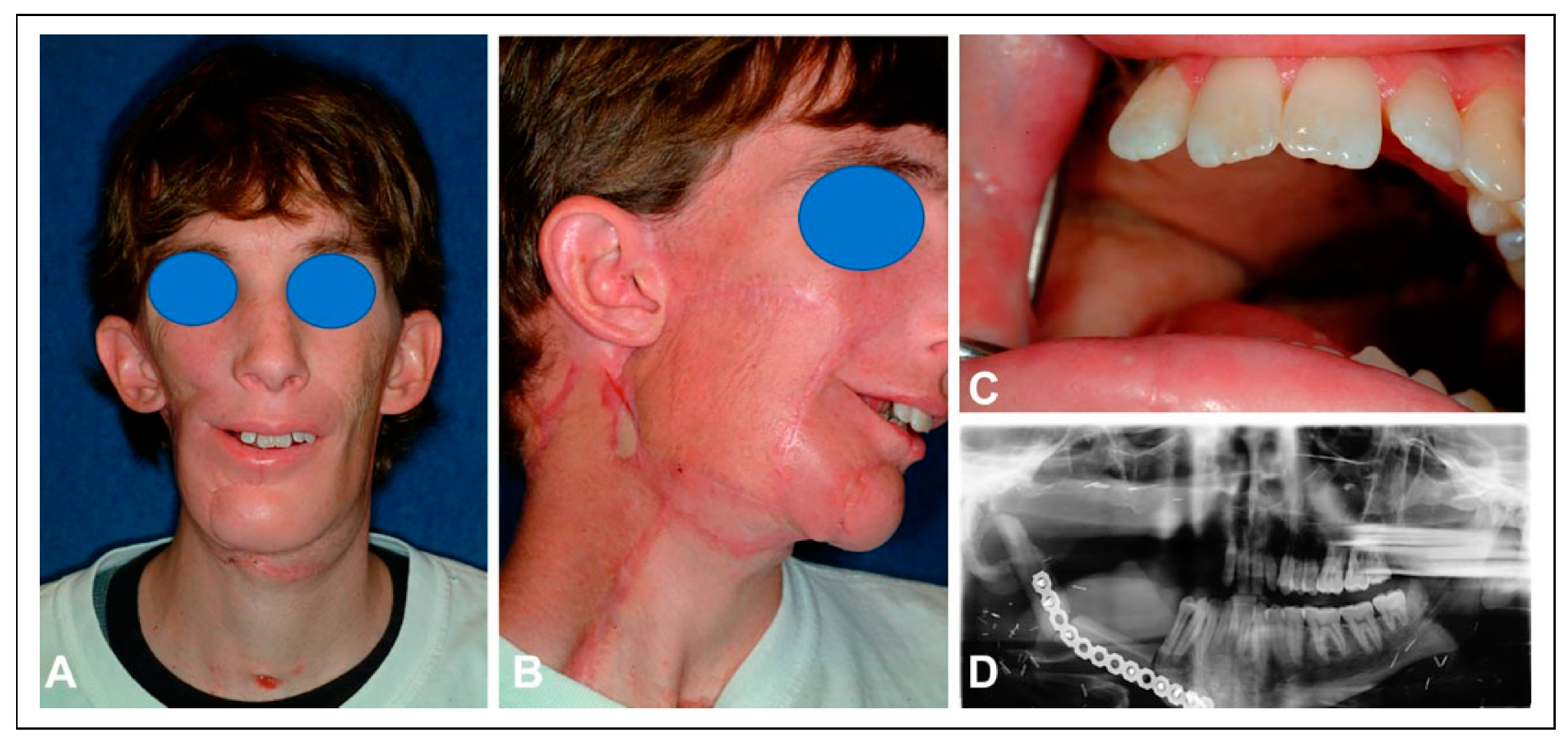
The patient underwent reirradiation for orbital squamous cell carcinoma (SCC) with a second course of proton therapy (
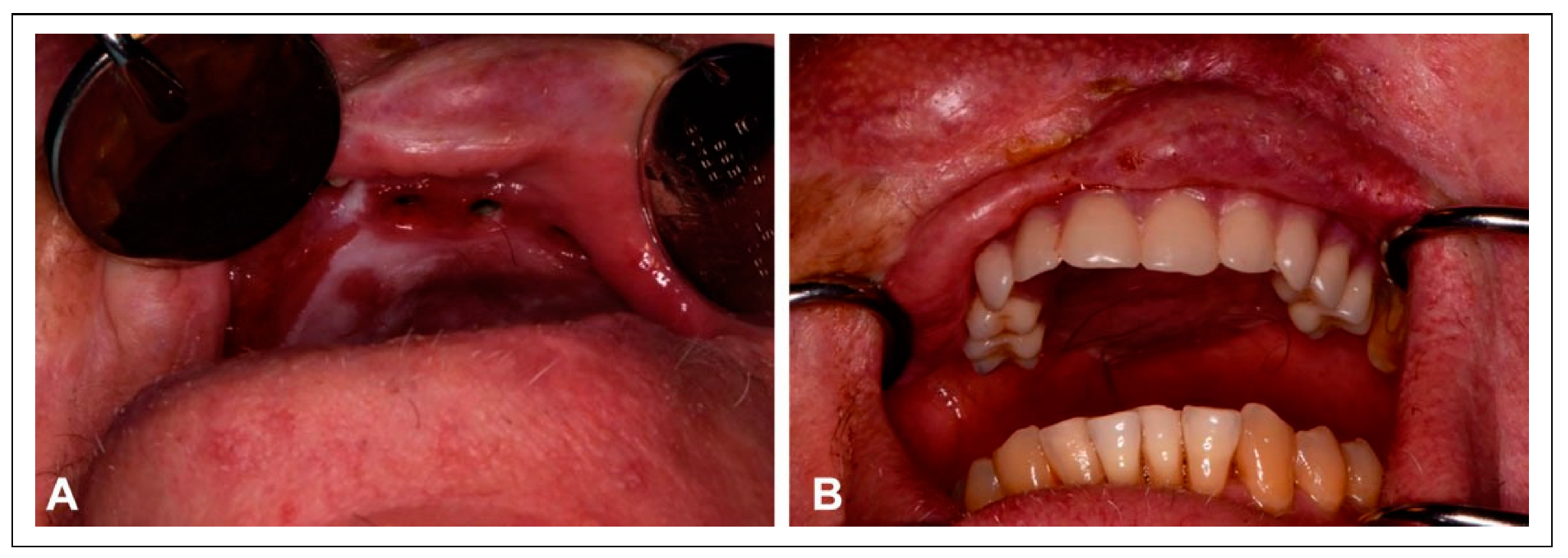
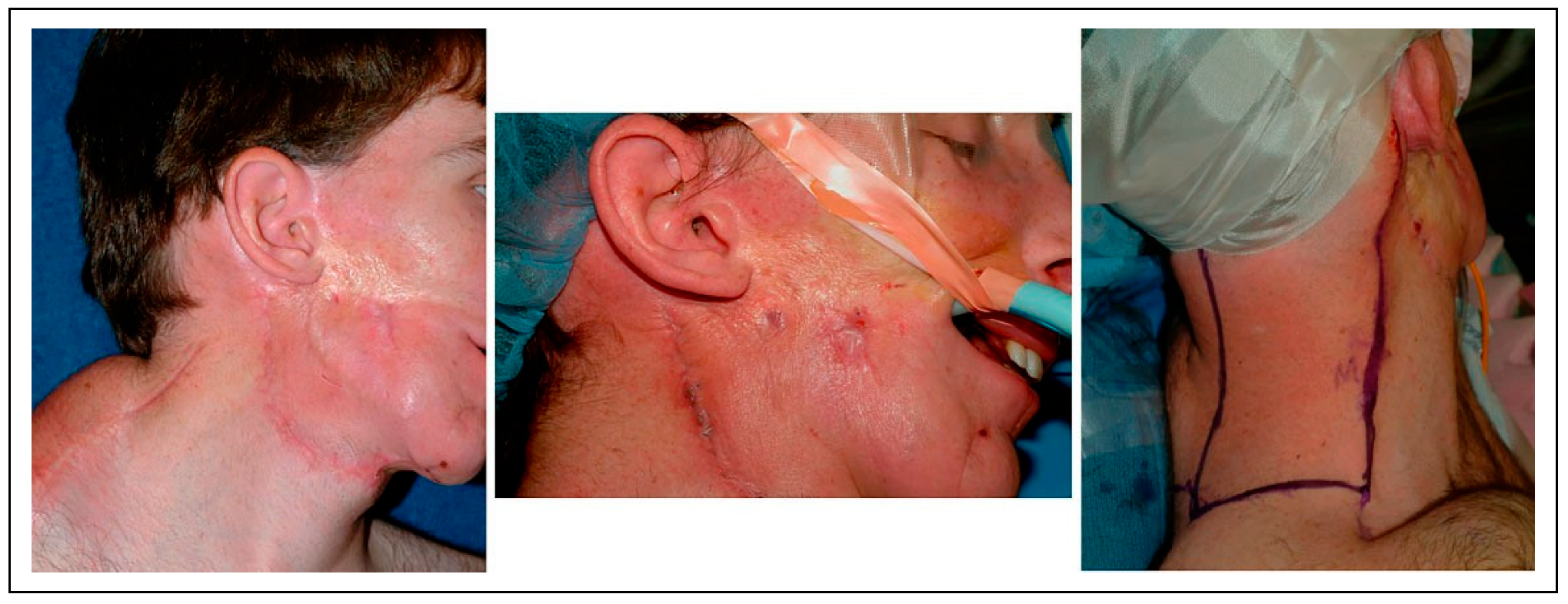
Figure 4). Despite the intervening radiation therapy, dental rehabilitation was successfully achieved with dental implants placed into the fibula flap (
Figure 5).
Case 2
A 55-year-old female with a history of SCC of the maxilla presented with a composite defect involving her right cheek, nose, and palatomaxillary complex. The patient’s maxillary SCC was initially treated at an outside institution with resection and reconstruction using an iliac crest free flap, which was unsuccessful due to vascular compromise in the postoperative period. She was not a candidate for a fibular transfer due to unfavorable arterial anatomy of the lower extremity. A left radial forearm free flap was performed to reconstruct this extensive defect, followed by concurrent chemoradiation (
Figure 6). Following adjuvant therapy, the patient was left with a large defect in her cheek, which communicated directly with the oral cavity through the maxillectomy cavity.
The initial approach was to perform a left scapular free flap, which was complicated by early postoperative flap ischemia that could not be corrected despite salvage efforts. A hematologic workup revealed a factor V Leiden (FVL) mutation.
It was determined that proceeding with free tissue transfer would be best accomplished in a staged fashion with perioperative prophylactic heparinization. Bone reconstruction was felt to be vital for a successful outcome, and a staged approach to reconstruction with the remaining scapular flap was the preferred course of action.
At the first stage, the patient underwent a right radial forearm free flap to reconstruct the palate and seal the oral cavity. Performing only the radial forearm free flap during the first stage of the reconstruction necessitated only 1 vascular anastomosis and permitted a potential arterial graft and venous graft that could then be used in the second phase for the scapular flap transfer.
The second stage was performed three months later with the reconstruction of the right maxilla and right facial skin using a right scapular free flap. Unfortunately, the radial artery was thrombosed and could not be utilized for the new scapular free flap. As a result, the decision was made to harvest the transverse cervical artery and the internal jugular vein, along with a cephalic vein graft. The patient’s post-operative course was uneventful, and she remained on heparin for two weeks before being transitioned to coumadin.
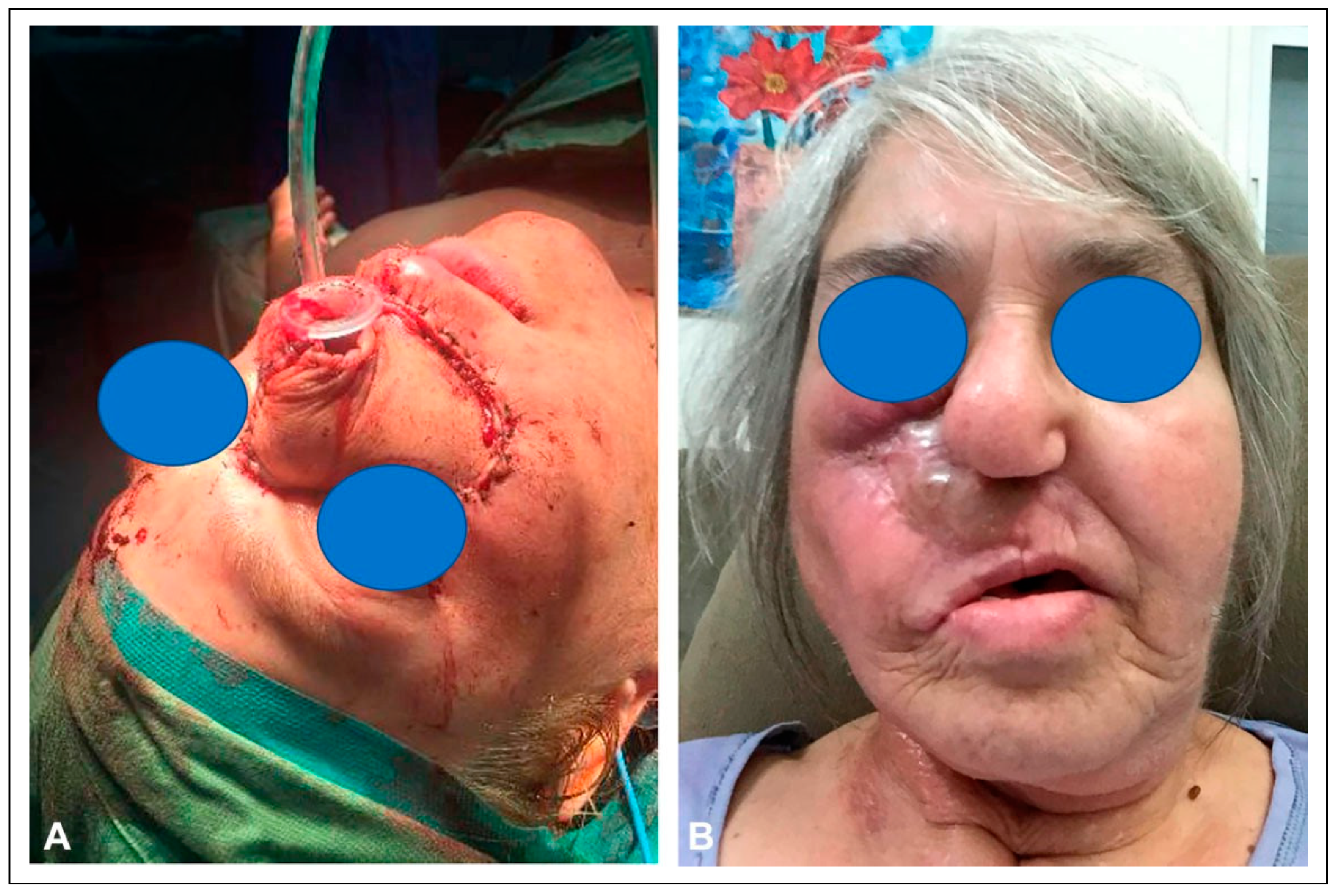
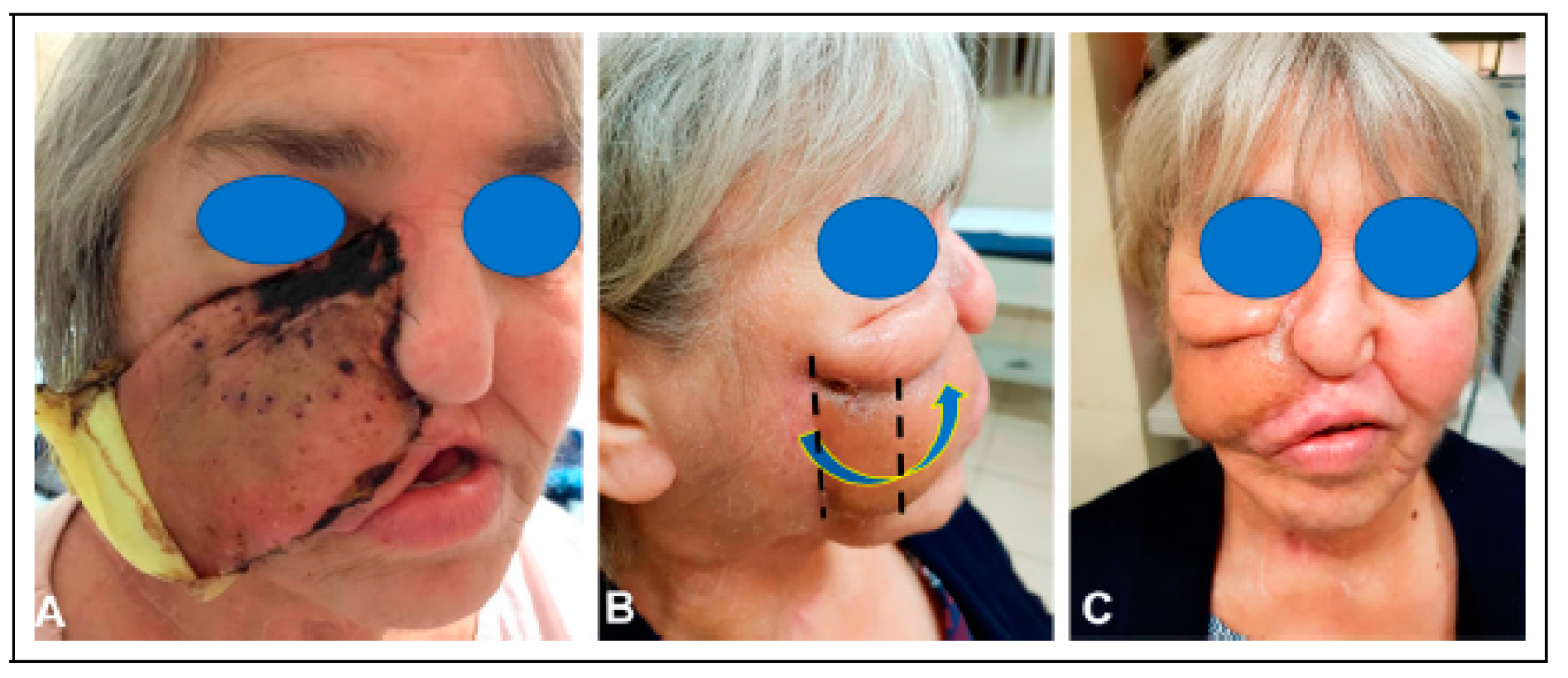
The patient had significant scarring of the right facial skin, as well as a severe ectropion. To overcome this deformity, she then underwent an ipsilateral posterior scalp flap, measuring 8 cm × 6 cm. A tissue expander was placed and a delay phenomenon was performed by incising the boundaries of the flap (
Figure 7). Once the 6 cm × 4 cm tissue expander was in place, four endosseous maxillary dental implants were placed into the scapula.
After six weeks of expansion, the patient underwent transfer of the posterior scalp flap, which resurfaced the entire right half of the midface and extended to the inferior orbital rim. The patient still needed additional skin in the cheek below the lower eyelid, so a superiorly based cheek flap (8 x 3 cm) was transferred to restore this region (
Figure 8). The patient subsequently underwent placement of a provisional dental prosthesis using the previously placed endosseous maxillary implants (
Figure 9). Her final nasal reconstruction involved transfer of a paramedian forehead flap to reconstruct the persistent deformity on the right side of the nose (
Figure 10). The patient healed well and is scheduled to have a definitive dental prosthesis fabricated as well as refinement of the facial soft tissues.
Case 3
A 29-year-old man presented for reconstruction of a combined mandibular-maxillary defect. He had a complex 12-year history of treatment for adenoid cystic carcinoma of the right maxillary sinus. The patient had initially been treated with external beam radiation; nine years later, he underwent an extensive resection of the right maxillary sinus and base of skull for a recurrence. Rehabilitation of the palatal defect was achieved with a palatal prosthesis.
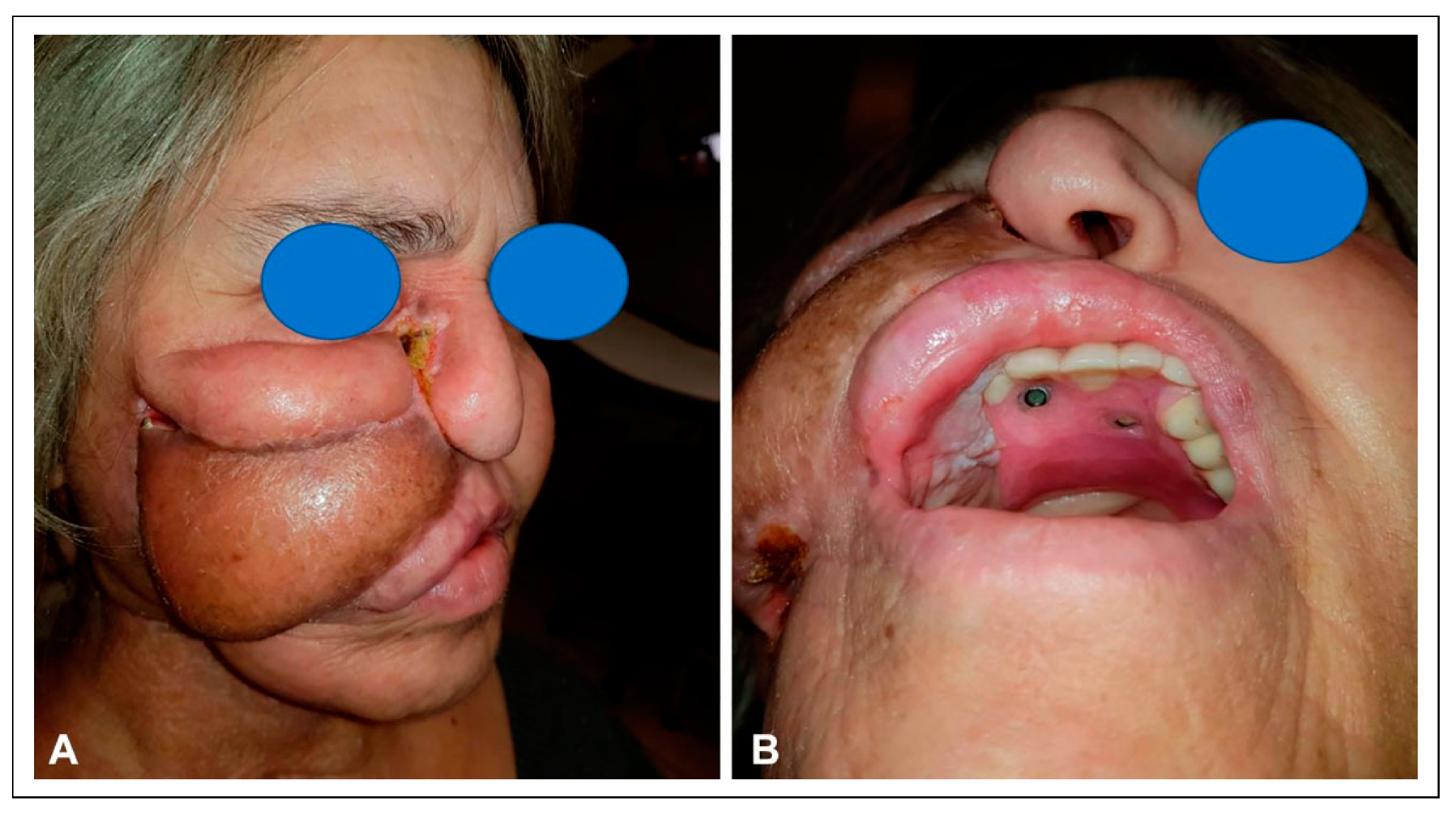
The patient then presented to our clinic one year later with a central palatal defect, as well as mandibular osteoradionecrosis that had resulted in a pathologic fracture. The patient had severe trismus that prevented him from removing his palatal prosthesis. In addition, the skin of the right cheek showed signs of thinning over the fracture and severe radiation fibrosis (
Figure 11).
A staged approach was adopted in order to address the patient’s multitude of problems. At the initial surgery, a segmental mandibular resection and coronoidectomy were performed. The bony mandibular defect was bridged with a 3D reconstruction plate. During this procedure, the central palatal defect was reconstructed with a radial forearm flap that sealed the palate and restored the mucosa of the right cheek and floor of mouth (
Figure 12). A subcutaneous fascial extension of the radial forearm flap was placed over the reconstruction plate to protect the thinned, radiated facial skin (
Figure 13).
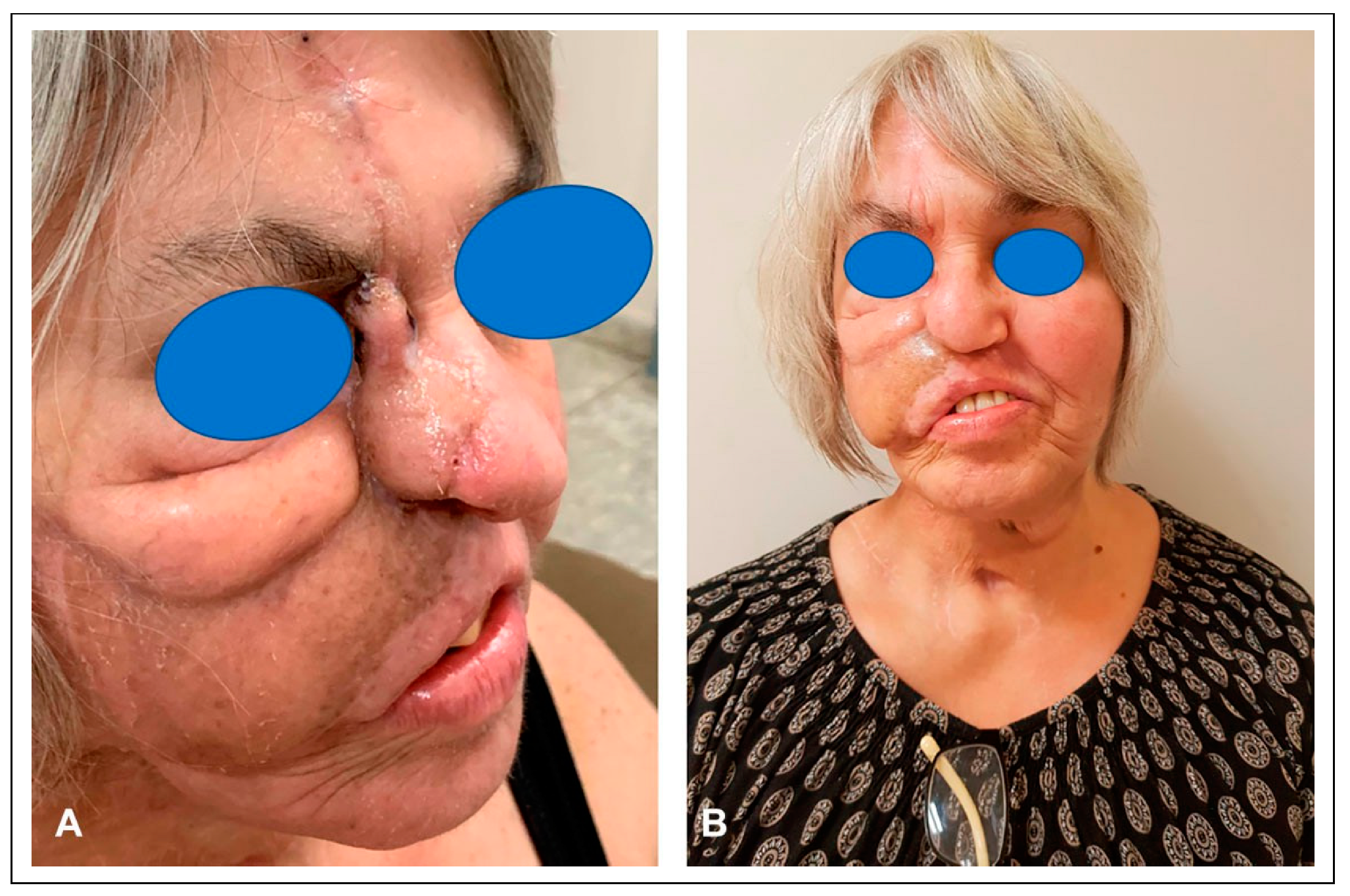
Despite this maneuver, it became evident that the damaged skin would not tolerate the mandibular fixation hardware. To overcome this problem, a tissue expander was placed into the donor site of the right posterior scalp flap (
Figure 14). Following pre-expansion of the posterior scalp flap for six weeks, a fibular free flap was transferred to reconstruct the mandibular defect, and the posterior scalp flap was placed over the posterior, inferior cheek (
Figure 15). After three weeks of uneventful healing, the posterior scalp flap was transected and returned (
Figure 16).
Discussion
In the early era of head and neck reconstruction, surgeons attempted complex and often time-consuming procedures to successfully transfer tissue from donor sites. The “waltzing” tubed pedicled flap, described by Sir Harold Gillies involved a tube of skin and soft tissue that was swung or “waltzed” to the recipient site in a series of surgical procedures that relied upon neovascularization of the skin, allowing it to be moved to the desired recipient site in a step wise fashion.[
1] Tagliacozzi described a method to reconstruct the nose, in which a skin flap from the arm was sutured to the nasal stump while retaining its blood supply in the arm until a new blood supply was established in the face.[
3] After a period of time, the flap was released from the arm and based solely on the vascular supply in the face.[
3]
One of the major advances associated with the advent of microvascular surgery was the reliable, single-stage transfer of tissue of varying types to the head and neck region.[
4] The ability to achieve reconstruction in a single operative setting saved time, allowed for a more rapid preparedness to undergo adjuvant therapy, and provided a quicker return to a favorable quality of life. However, select cases may require multiple flaps transferred from more than 1 donor site in order to achieve the desired outcome.
Difficulties in free tissue reconstruction can occur due to the availability of recipient vessels, impaired wound healing in previously radiated recipient sites, presence of thrombotic disorders, infection, and poor local tissue vascularity. These factors make reconstruction in a single operation particularly risky and place valuable donor tissue at risk.
The three patients described in this series had extensive previous treatment and underlying conditions that complicated reconstructive efforts. They each had a composite defect in the setting of prior radiation therapy involving multiple facial subunits. In two patients, this defect was addressed with a pre-expanded posterior scalp flap, while a submental flap was used in the third to restore facial skin with skin of favorably matched color and texture. In all three cases there was a need to import vascularized bone and soft tissue, which was achieved with a fibular flap in two patients and a scapular flap in the third.
In Case 1, the patient was immunocompromised due to his previous lung transplant. Immunosuppressive medications have been shown to impair wound healing and to increase the risk of flap loss, anastomotic thrombosis, and operative morbidity.[
5] In addition, extensive radiation therapy led to a recipient bed with impaired wound healing.
After the transfer of the radial forearm flap to seal the oral cavity and the submental flap to reconstruct the cutaneous defect of the cheek, the stage was set for the transfer of vascularized bone. The goal of the bone transfer was three-fold: 1. to establish midface architectural support and upper lip projection; 2. to provide a platform of vascularized bone for implant placement to support dental rehabilitation; 3. to strategically introduce vascularized bone for implant placement to retain a nasal prosthesis. Dental rehabilitation was readily accomplished along with a secondary vestibuloplasty in order to promote oral competence.
In Case 2, the patient suffered from an underlying hypercoagulable state that resulted in two free flap failures. This led to the decision to stage the procedures in order to ensure the safe and successful transfer of a soft tissue free flap before putting another bone flap at risk. In addition, an attempt was made to avoid a vein graft in the subsequent scapular transfer by placing the radial artery in a strategic location for use in that second free flap. However, that radial artery could not be repurposed for arterial inflow to the scapular flap due to subsequent thrombosis. Anticoagulation was utilized throughout the perioperative period for the scapular flap. Perioperative therapeutic heparinization was employed with trepidation for the transfer of a microvascular flap as large as a scapular free flap.
In Case 3, the patient presented with a twice radiated recipient site. Reconstructive procedures performed in previously radiated settings are associated with higher complication rates, due in part to the creation of an environment of chronic ischemia in the recipient bed caused by destruction of the microvasculature.[
6,
7,
8] These risks are magnified in head and neck reconstructions due to the impact on recipient vessels, which are prone to more advanced atherosclerotic changes, thereby impacting the safety and reliability of microsurgical procedures.[
9,
10]
Staging of the reconstruction achieves several benefits. The first stage can serve as documentation of wound healing and proof that the patient can undergo a successful free flap. Staged reconstruction also allows for the importation of additional recipient vessels that can be utilized for subsequent flap transfers. This strategy was adopted in Case 2 in this series, but the radial artery thrombosed after cessation of anticoagulation following the first procedure, which eliminated this option in the subsequent flap transfer.
In planning a staged procedure, there is no absolute rule that determines how much time should pass following the first stage before the second stage of the reconstruction can be performed. In general, we give patients at least three to four weeks after the first procedure to allow them to fully recover. If the second procedure involves pre-expansion of a skin flap, the time between procedures will need to be prolonged to allow for adequate expansion. Successful wound healing after each stage must be documented in order to proceed to the subsequent stage.