Endoscopically Assisted Marginal Mandibulectomy Using an Intraoral Approach Alone for Squamous Cell Carcinoma of the Posterior Mandibular Gingiva: A Technical Note
Abstract
:Introduction
Material and Methods
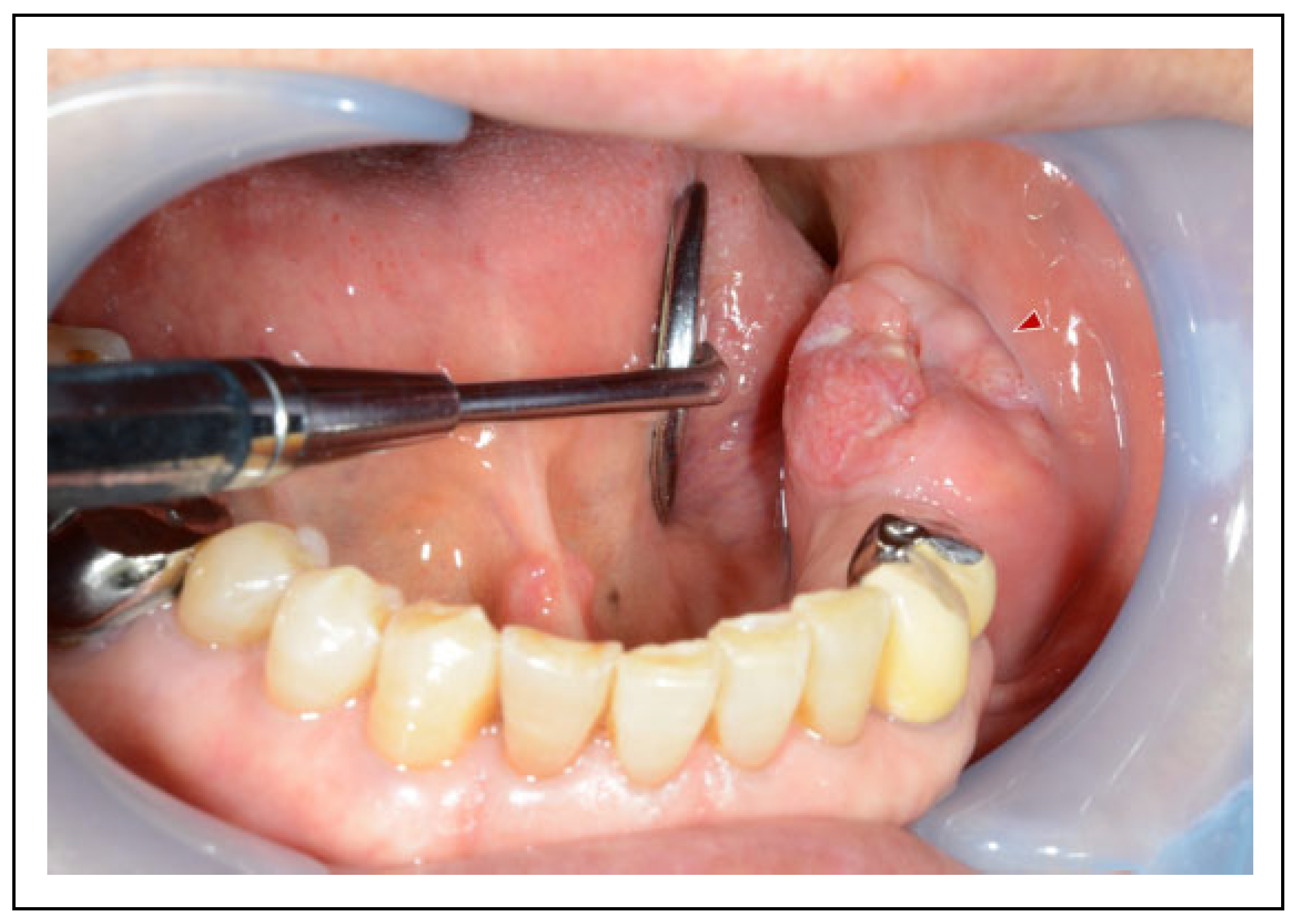
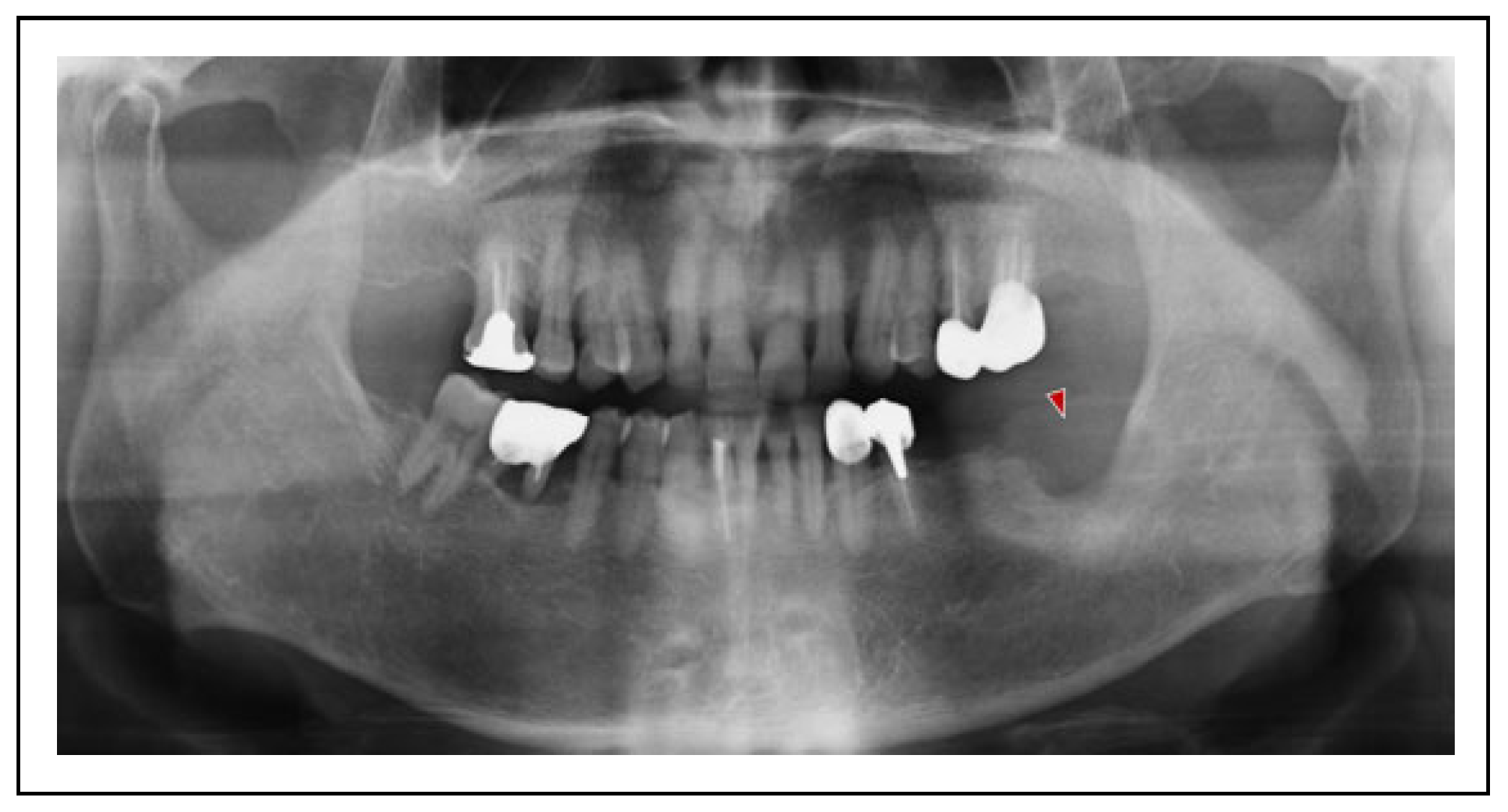
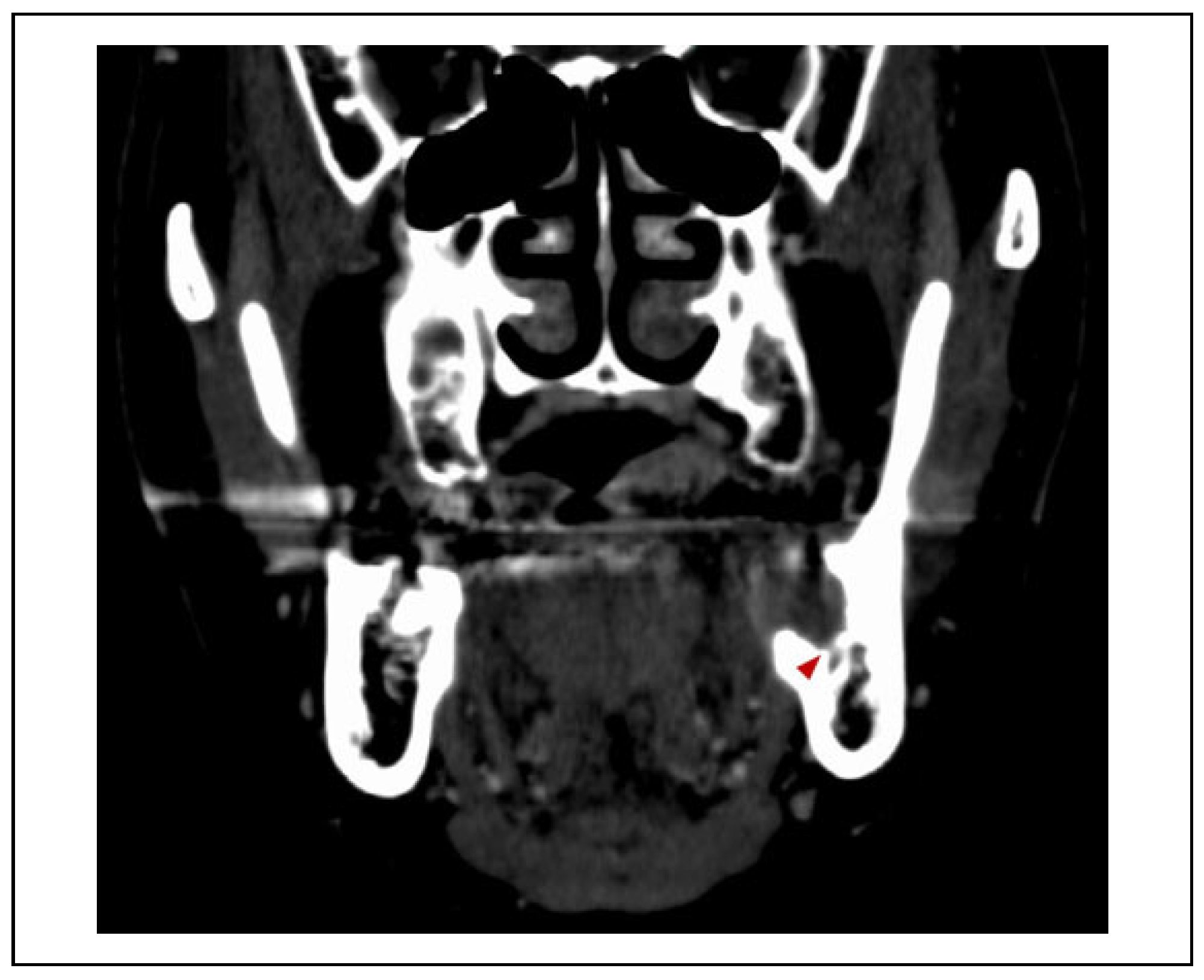
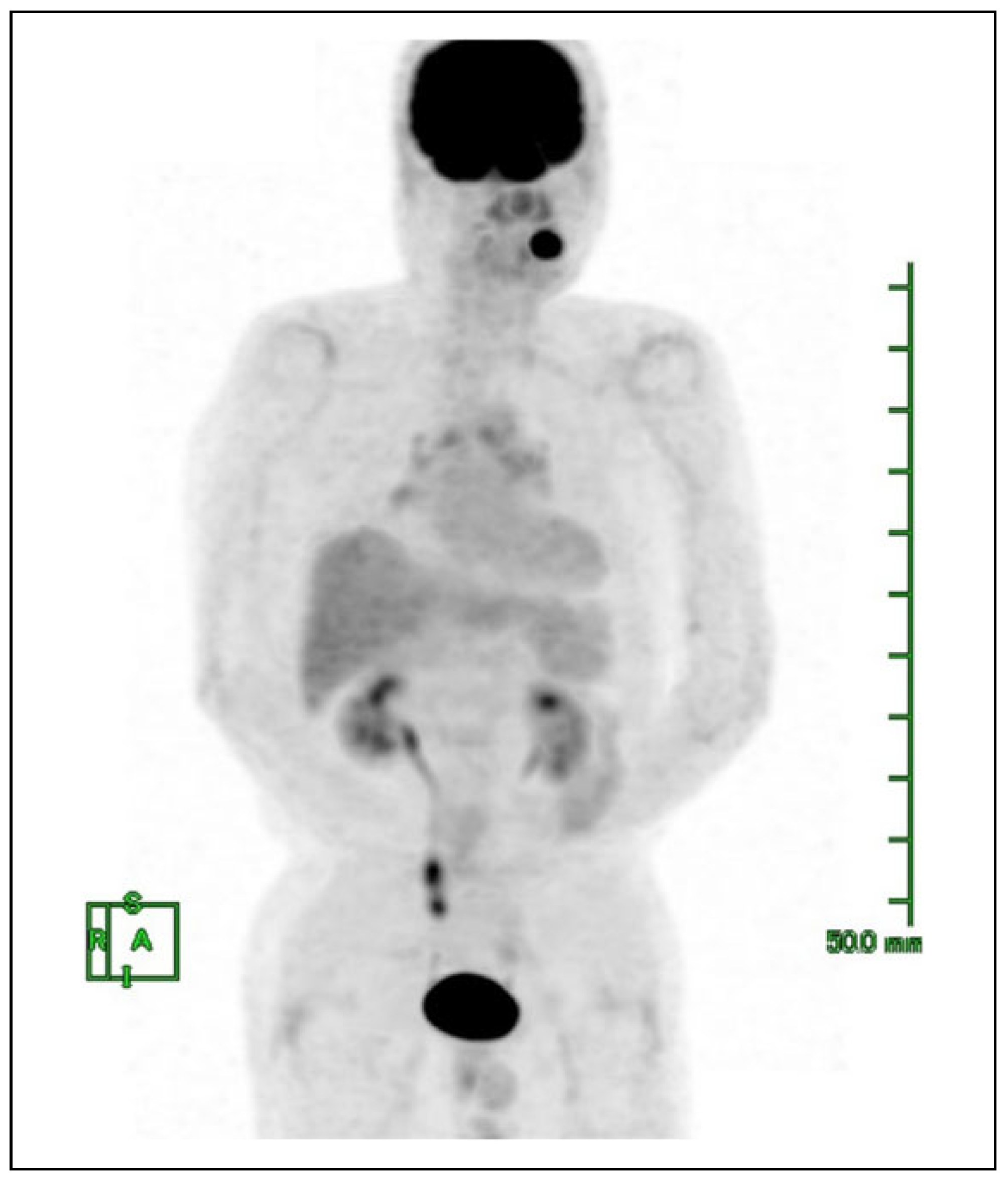
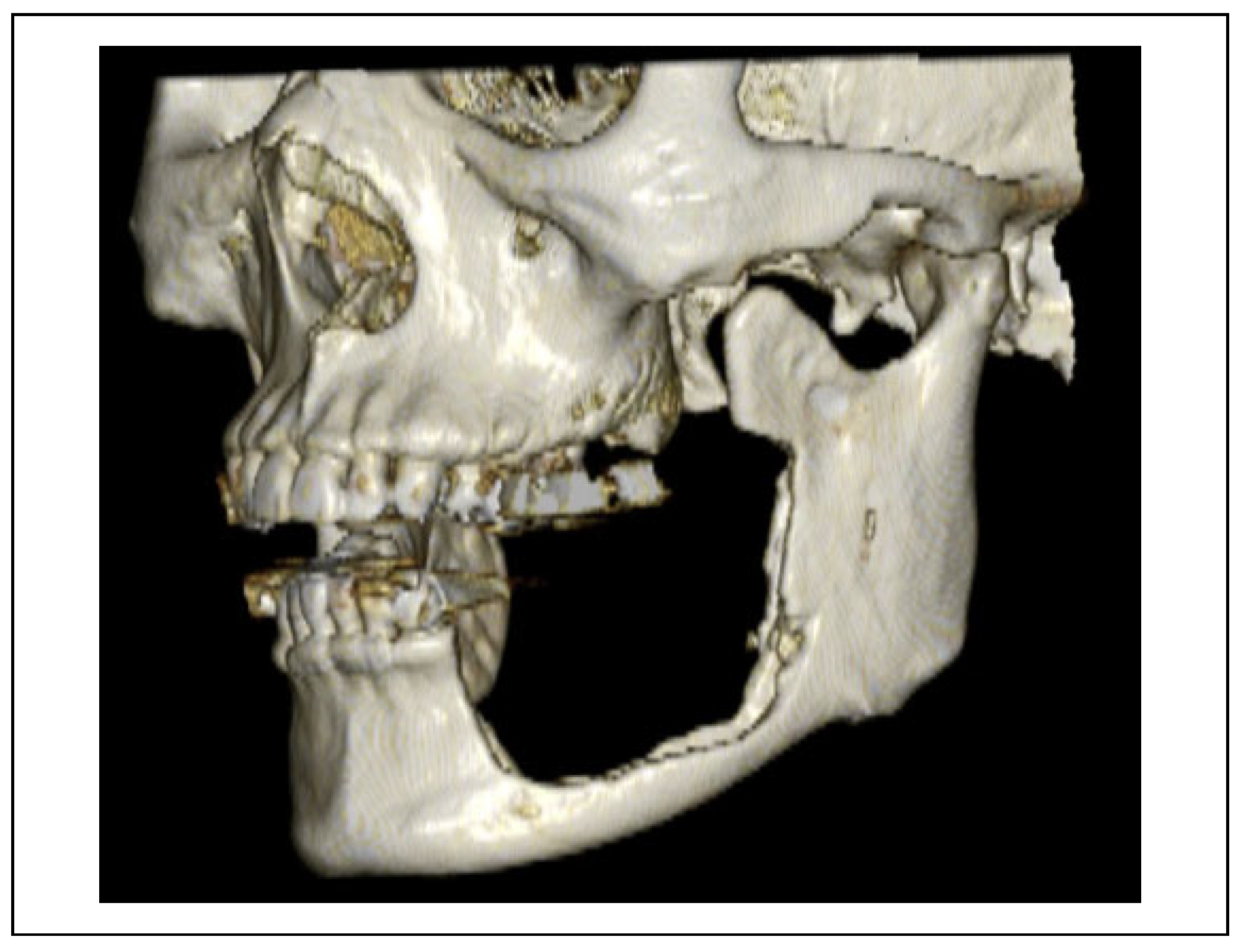
Case Presentation
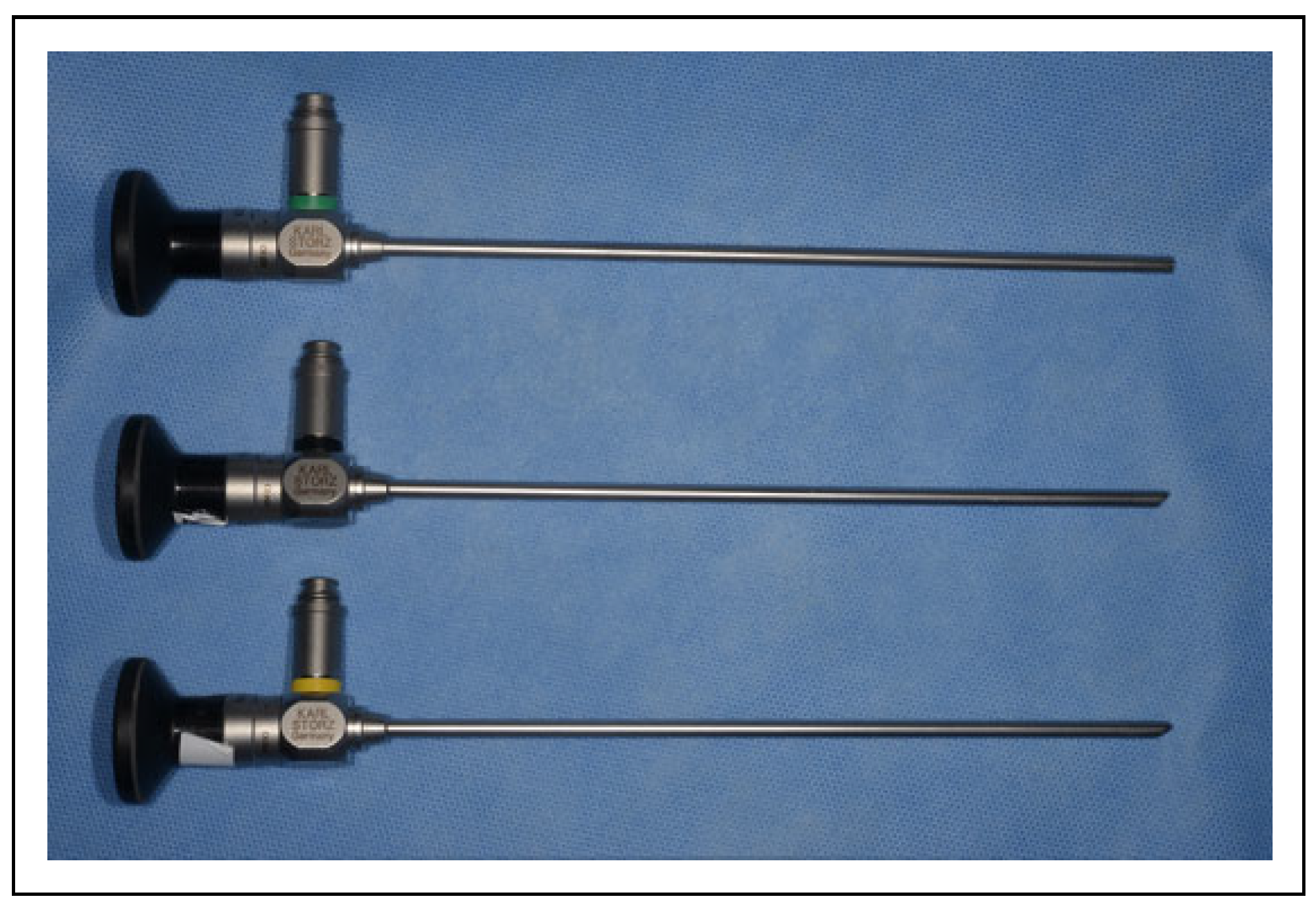
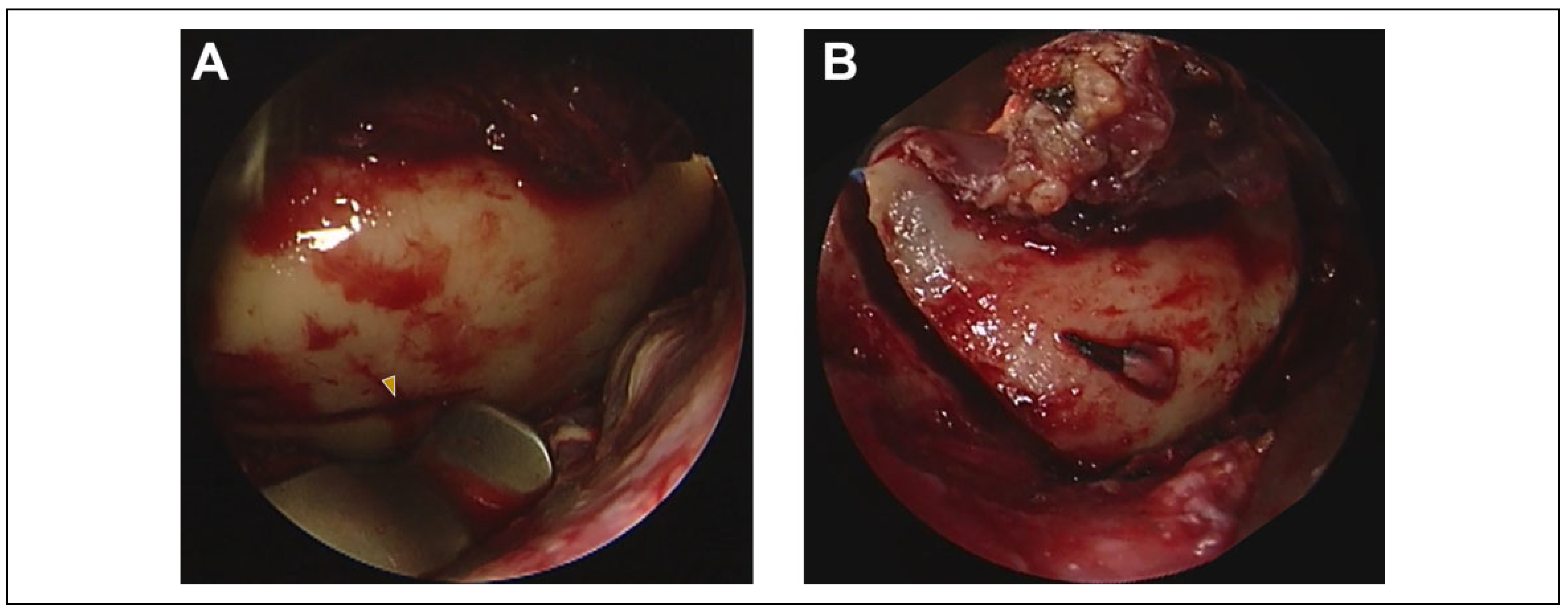
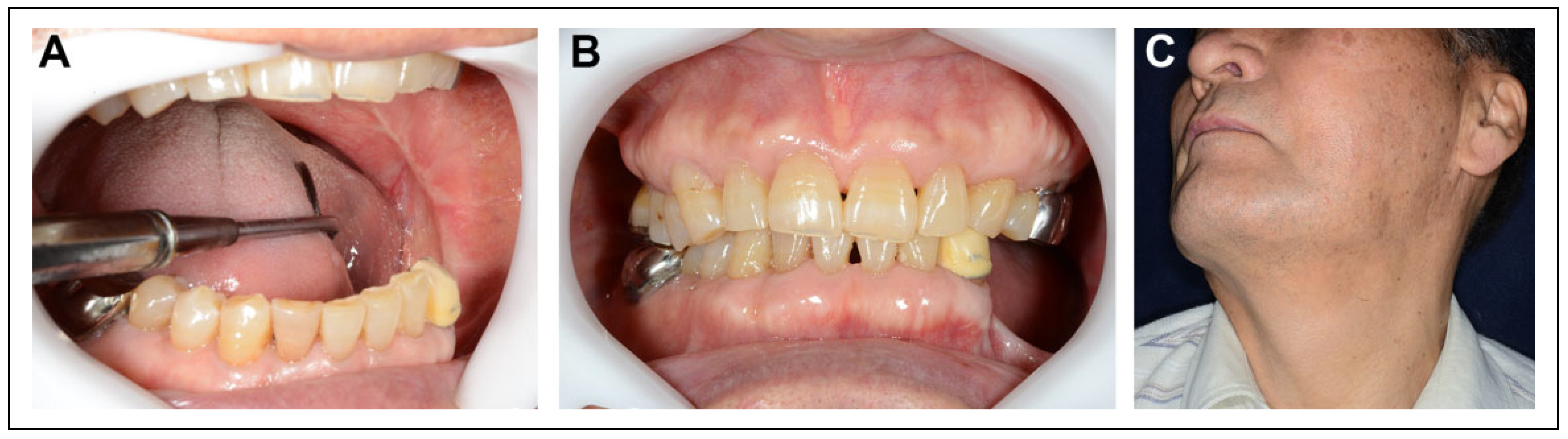
Surgical Procedure
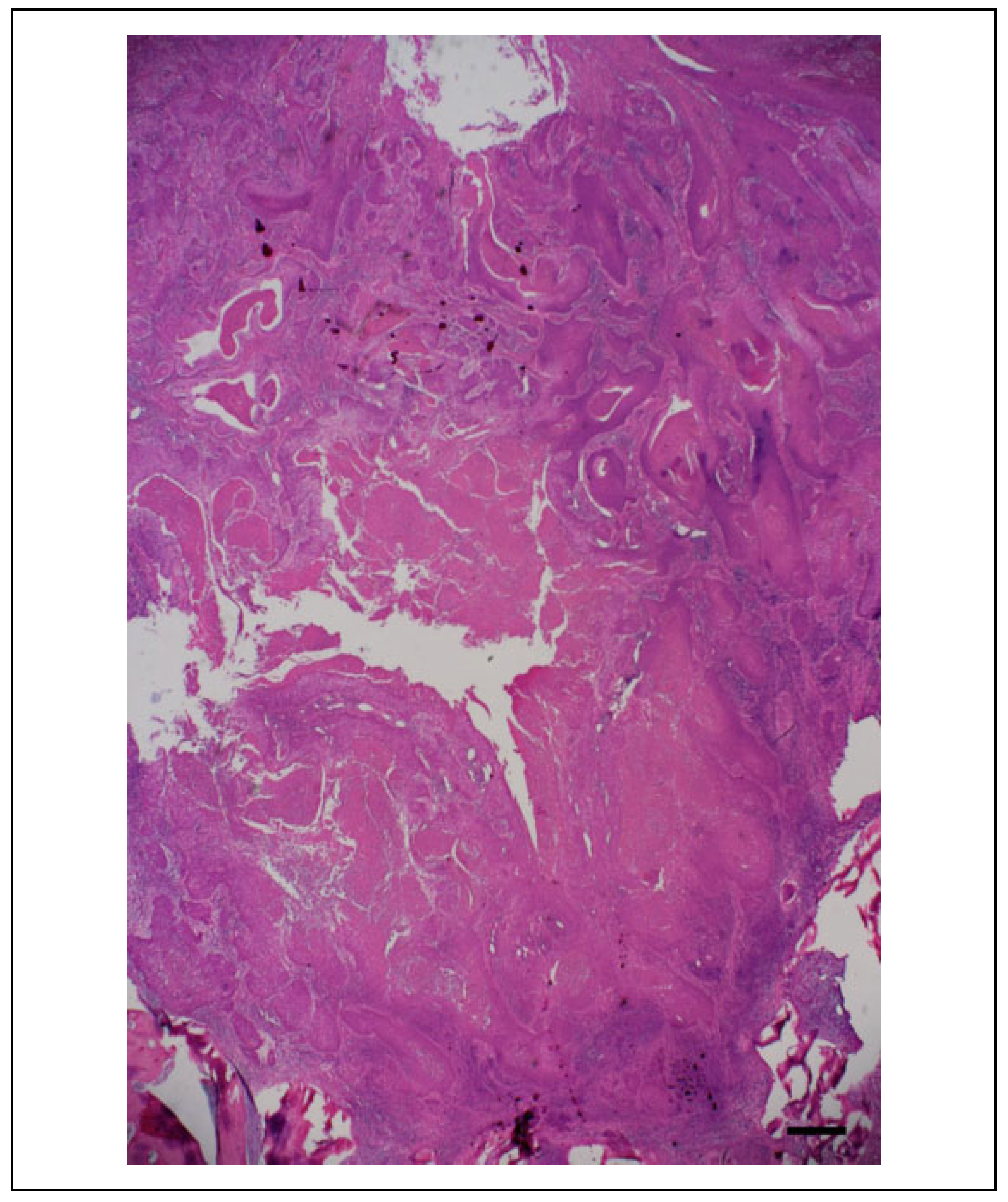
Postoperative Course
Discussion
Conclusion
Funding
Acknowledgments
Conflicts of Interest
References
- Pedroletti, F.; Johnson, B.S.; McCain, J.P. Endoscopic techniques in oral and maxillofacial surgery. Oral Maxillofac Surg Clin North Am. 2010, 22, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xue, R.; Lai, Q.; et al. Endoscope-assisted resection of nonneoplastic space-occupying lesion in oral and maxillofacial areas. Sci Rep. 2017, 7, 16920. [Google Scholar] [CrossRef] [PubMed]
- Hyppolito, J.O.P.; Araújo, R.A.C.; Souza, M.S.M.; et al. The use of intra-oral endoscopy in oral and maxillofacial surgery: literature review. Int J Oral Maxillofac Surg. 2019, 48, 272. [Google Scholar] [CrossRef]
- Mohamed, A.H.; Joseph, P.M.; David, Y.A.; Maria, J.T. Minimally invasive endoscopic oral and maxillofacial surgery. Oral Maxillofac Surg Clin North Am. 2019, 31, 561–567. [Google Scholar]
- Víctor, B.; Ramon, F.; Wilfried, E. Endoscopic visualization of anatomic structures as a support tool in oral surgery and implantology. J Oral Maxillofac Surg. 2012, 70, e1–e6. [Google Scholar]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; et al. Head and neck cancers—major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef]
- Okura, M.; Yanamoto, S.; Umeda, M.; et al. Prognostic and staging implications of mandibular canal invasion in lower gingival squamous cell carcinoma. Cancer Med. 2016, 5, 3378–3385. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Murali, R.; Gao, K.; Elliott, M.S.; Clark, J.R. The prognostic and staging implications of bone invasion in oral squamous cell carcinoma. Cancer 2011, 117, 4460–4467. [Google Scholar] [CrossRef] [PubMed]
- Sproll, C.K.; Holtmann, H.; Schorn, L.K.; et al. Mandible handling in the surgical treatment of oral squamous cell carcinoma: lessons from clinical results after marginal and segmental mandibulectomy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020, 129, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Tei, K.; Totsuka, Y.; Iizuka, T.; Ohmori, K. Marginal resection for carcinoma of the mandibular alveolus and gingiva where radiologically detected bone defects do not extend beyond the mandibular canal. J Oral Maxillofac Surg. 2004, 62, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.P.; Shukla, M.; Sharma, V.; Pandey, M. Mandibular conservation in oral cancer. Surg Oncol. 2012, 21, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.F.M.; Gias, L.N.; Campo, F.R.; Pérez, J.S. Marginal and segmental mandibulectomy in patients with oral cancer: a statistical analysis of 106 cases. J Oral Maxillofac Surg. 2003, 61, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Lin, L.; Shi, B.; Zhu, X. Does different mandibulectomy (marginal vs segmental) affect the prognosis in patients with oral squamous cell carcinoma? J Oral Maxillofac Surg. 2018, 76, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Kuo, S.W.; Fang, K.H.; Hao, S.P. Prognostic impact of marginal mandibulectomy in the presence of superficial bone invasion and the nononcologic outcome. Head Neck 2011, 33, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.; Costa, F.; Robiony, M.; Rinaldo, A.; Ferlito, A. Review of segmental and marginal resection of the mandible in patients with oral cancer. Acta Otolaryngol. 2000, 120, 569–579. [Google Scholar] [PubMed]
- Hirsch, D.L.; Dierks, E.J. Use of a transbuccal technique for marginal mandibulectomy: a novel approach. J Oral Maxillofac Surg. 2007, 65, 1849–1851. [Google Scholar] [CrossRef] [PubMed]
- Ohba, S.; Yamashita, H.; Takashi, I.; Asahina, I. Marginal mandibulectomy for lower gingival carcinoma with a cheeksplitting transbuccal approach and reconstruction by buccal fat pad flap: a case report. J Oral Maxillofac Surg. 2013, 71, e143–e146. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Kurita, K.; Hayashi, H.; Ito, Y. Cheek-splitting technique for marginal mandibulectomy: a novel approach. J Clin Exp Dent. 2019, 11, e675–e678. [Google Scholar] [CrossRef] [PubMed]
- Deganello, A.; Ferrari, M.; Paderno, A.; et al. Endoscopicassisted maxillectomy: operative technique and control of surgical margins. Oral Oncol. 2019, 93, 29–38. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Bakst, R.L.; Guo, T.; Sun, J. A combination of modified transnasal endoscopic maxillectomy via transnasal prelacrimal recess approach with or without radiotherapy for selected sinonasal malignancies. Eur Arch Otorhinolaryngol. 2015, 272, 2933–2938. [Google Scholar] [CrossRef] [PubMed]






© 2021 by the author. The Author(s) 2021.
Share and Cite
Shudo, A. Endoscopically Assisted Marginal Mandibulectomy Using an Intraoral Approach Alone for Squamous Cell Carcinoma of the Posterior Mandibular Gingiva: A Technical Note. Craniomaxillofac. Trauma Reconstr. 2022, 15, 175-183. https://doi.org/10.1177/19433875211015045
Shudo A. Endoscopically Assisted Marginal Mandibulectomy Using an Intraoral Approach Alone for Squamous Cell Carcinoma of the Posterior Mandibular Gingiva: A Technical Note. Craniomaxillofacial Trauma & Reconstruction. 2022; 15(2):175-183. https://doi.org/10.1177/19433875211015045
Chicago/Turabian StyleShudo, Atsushi. 2022. "Endoscopically Assisted Marginal Mandibulectomy Using an Intraoral Approach Alone for Squamous Cell Carcinoma of the Posterior Mandibular Gingiva: A Technical Note" Craniomaxillofacial Trauma & Reconstruction 15, no. 2: 175-183. https://doi.org/10.1177/19433875211015045
APA StyleShudo, A. (2022). Endoscopically Assisted Marginal Mandibulectomy Using an Intraoral Approach Alone for Squamous Cell Carcinoma of the Posterior Mandibular Gingiva: A Technical Note. Craniomaxillofacial Trauma & Reconstruction, 15(2), 175-183. https://doi.org/10.1177/19433875211015045


