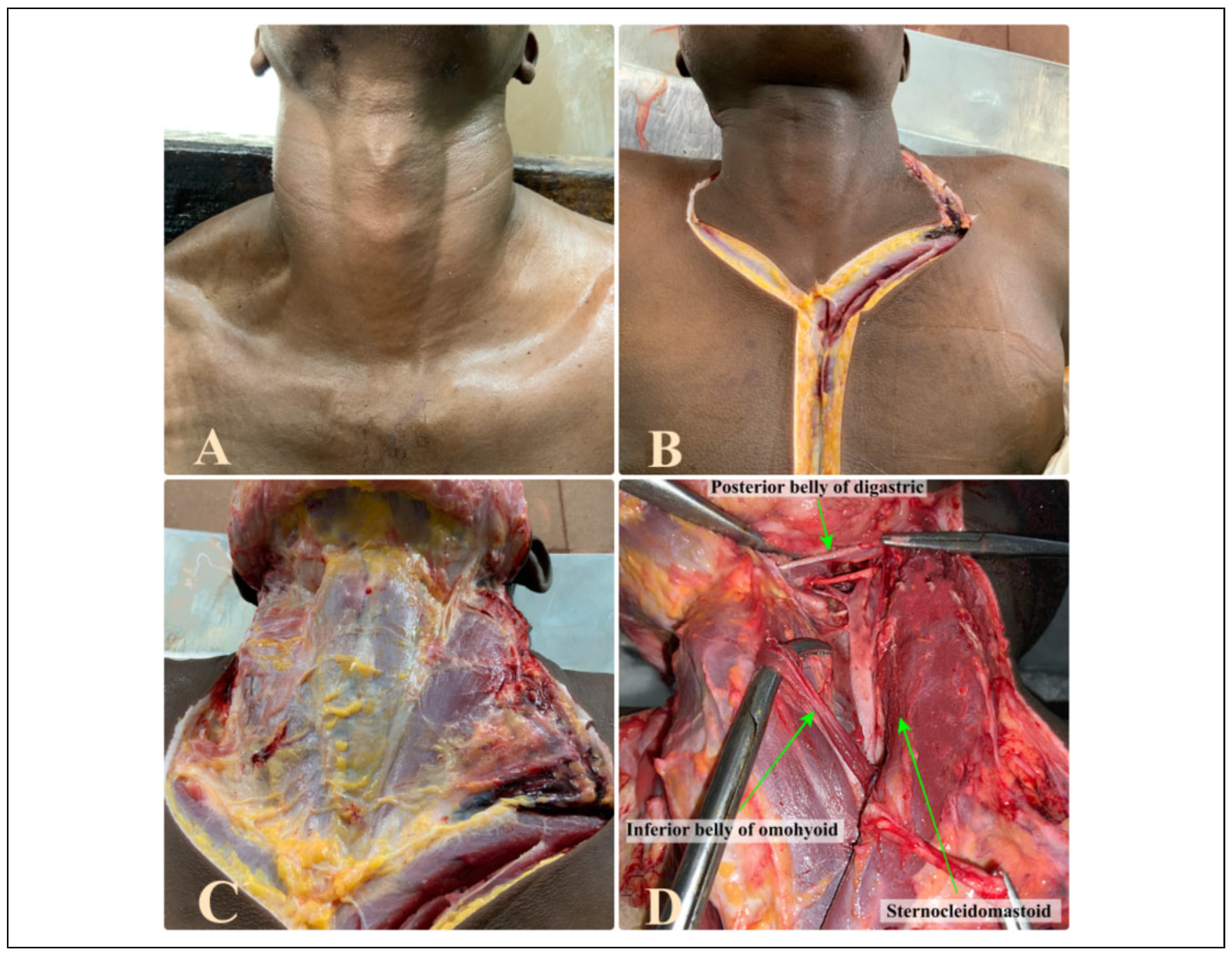
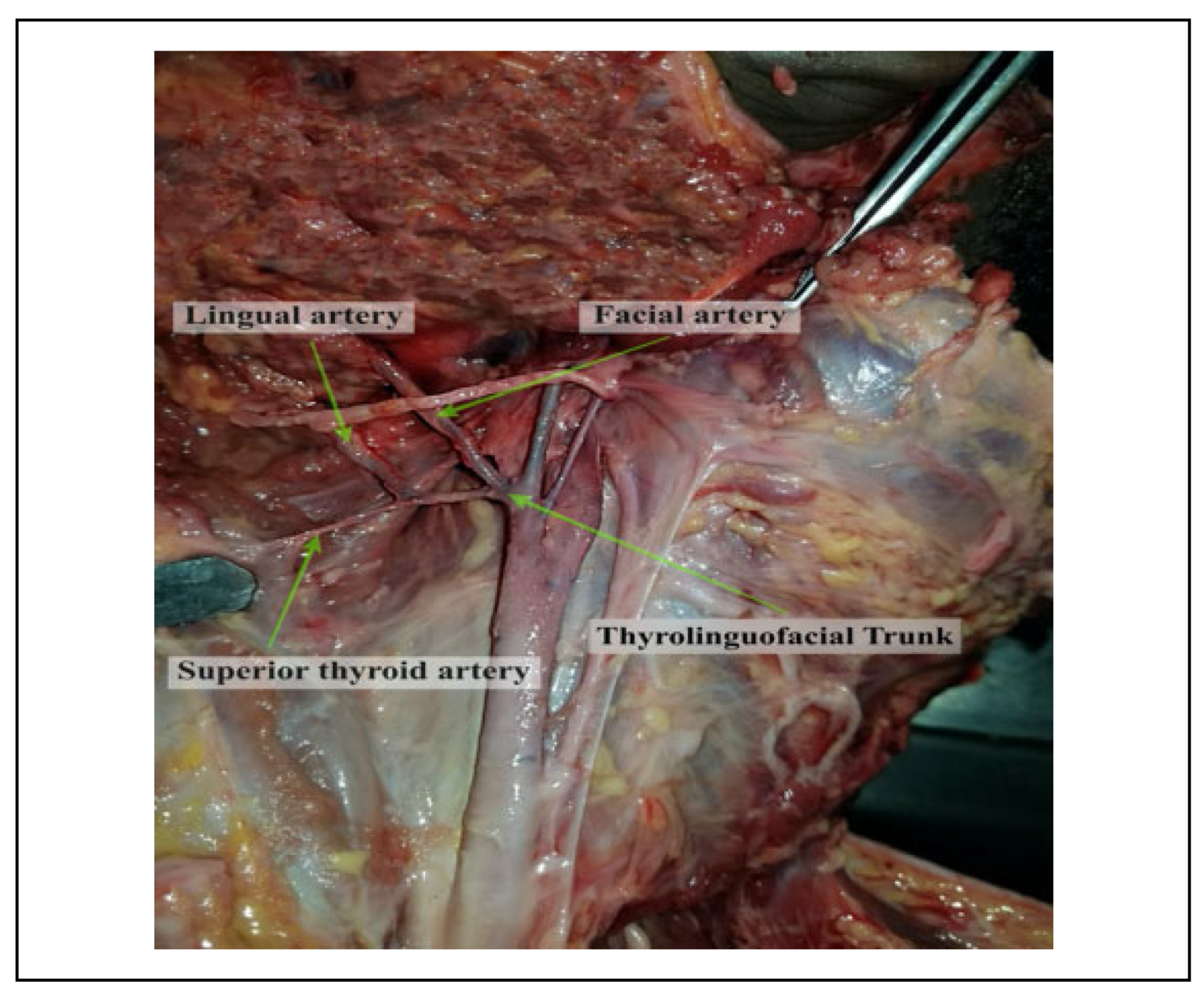
Introduction
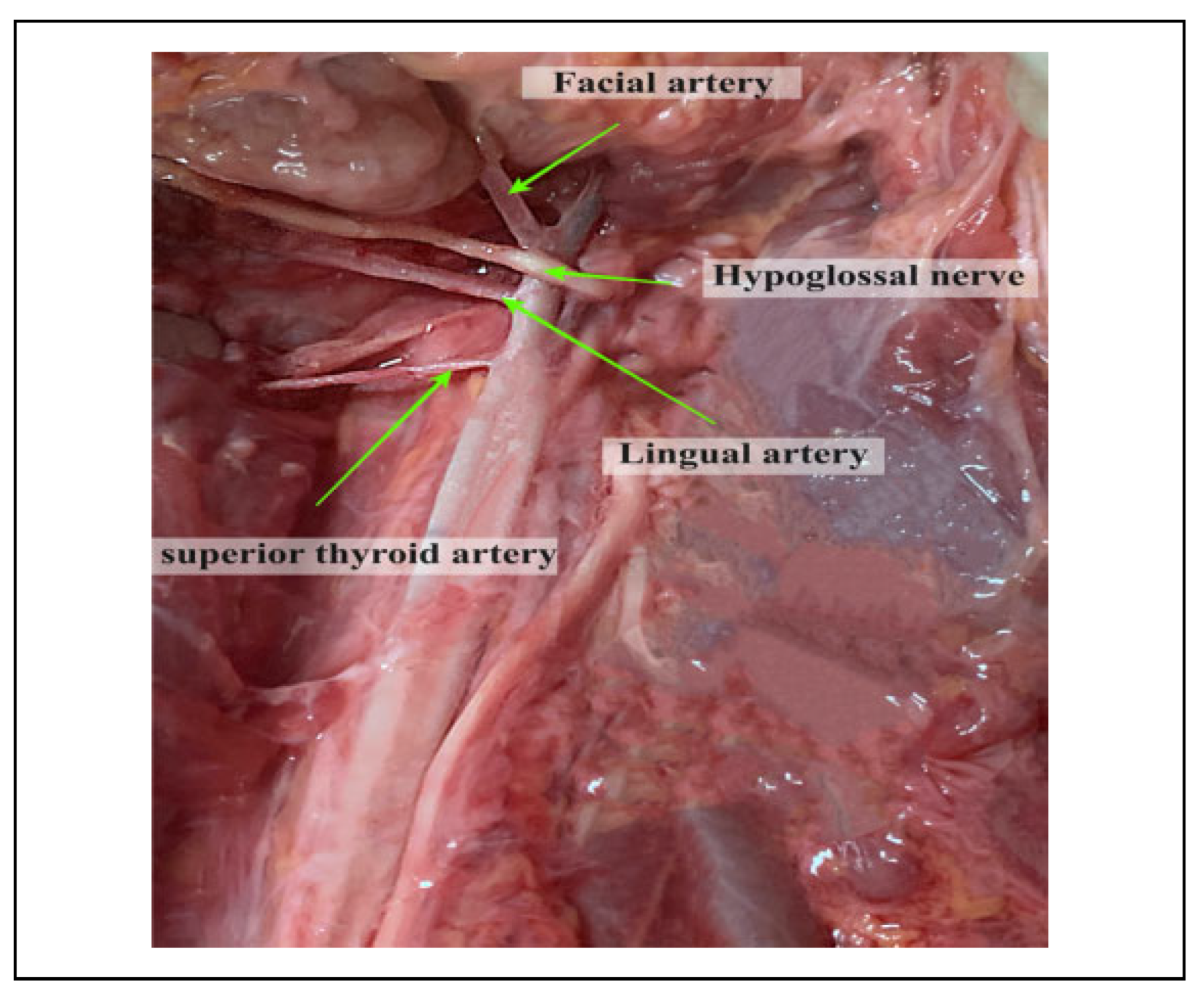
The lingual artery (LA) is one of the 3 anteromedial branches of the external carotid artery (ECA).[1] It arises opposite the tip of the greater cornu of the hyoid bone (GCHB), between the superior thyroid artery (STA) and the facial artery (FA). The artery passes between the hyoglossus and the middle pharyngeal constrictor, at which point it turns sharply upward to reach the floor of the mouth accompanied by the lingual veins and the glossopharyngeal nerve.[2] At this point it enters the inferior surface of the tongue and courses as far forward as its tip thus forming the chief source of blood to the structures located both in the floor of the mouth and the tongue.[3,4] However, this anatomy is subject to considerable variation and if not understood well, may pose difficulty in identifying the vessel during surgical, diagnostic or interventional procedures in the head and neck.
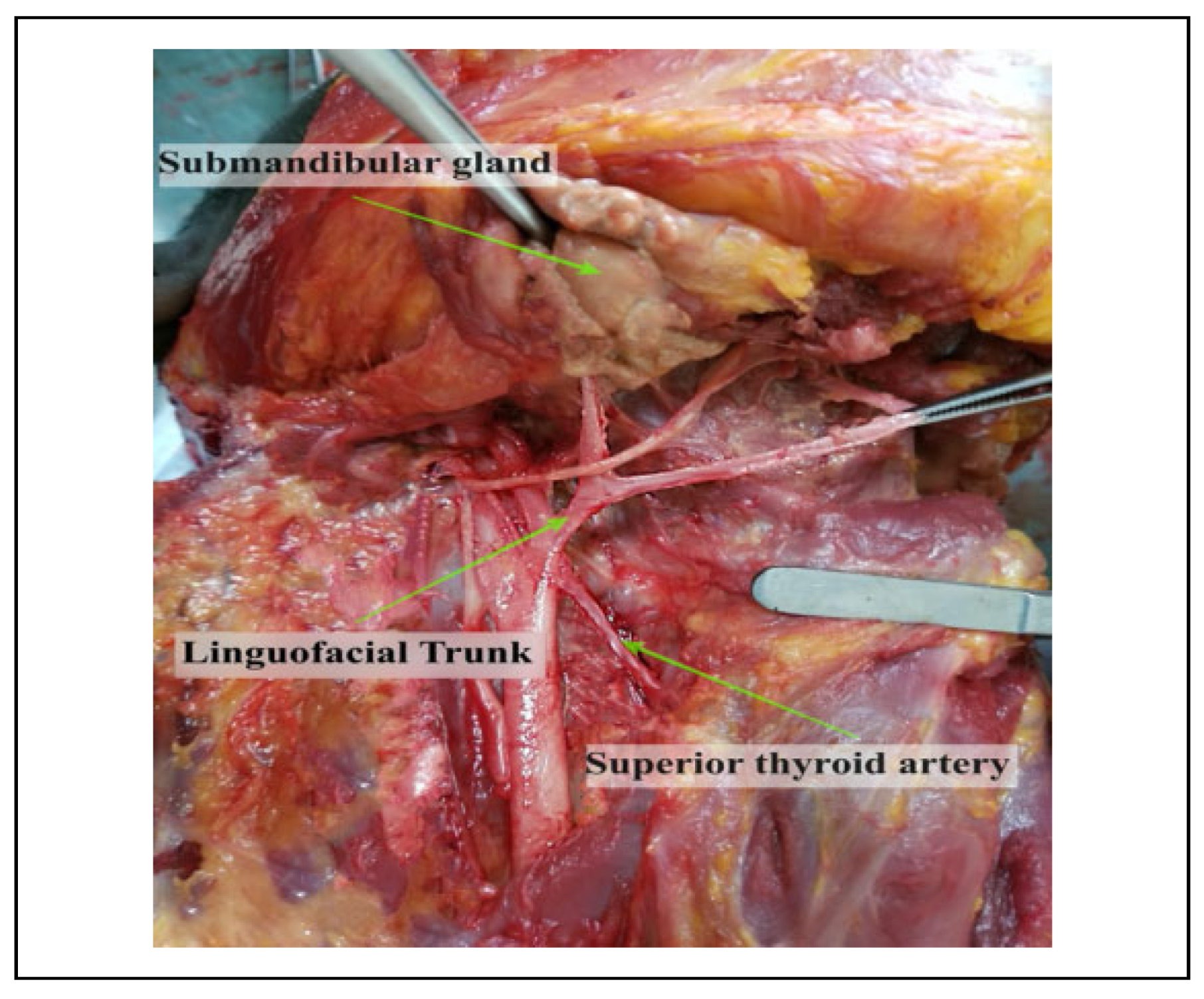
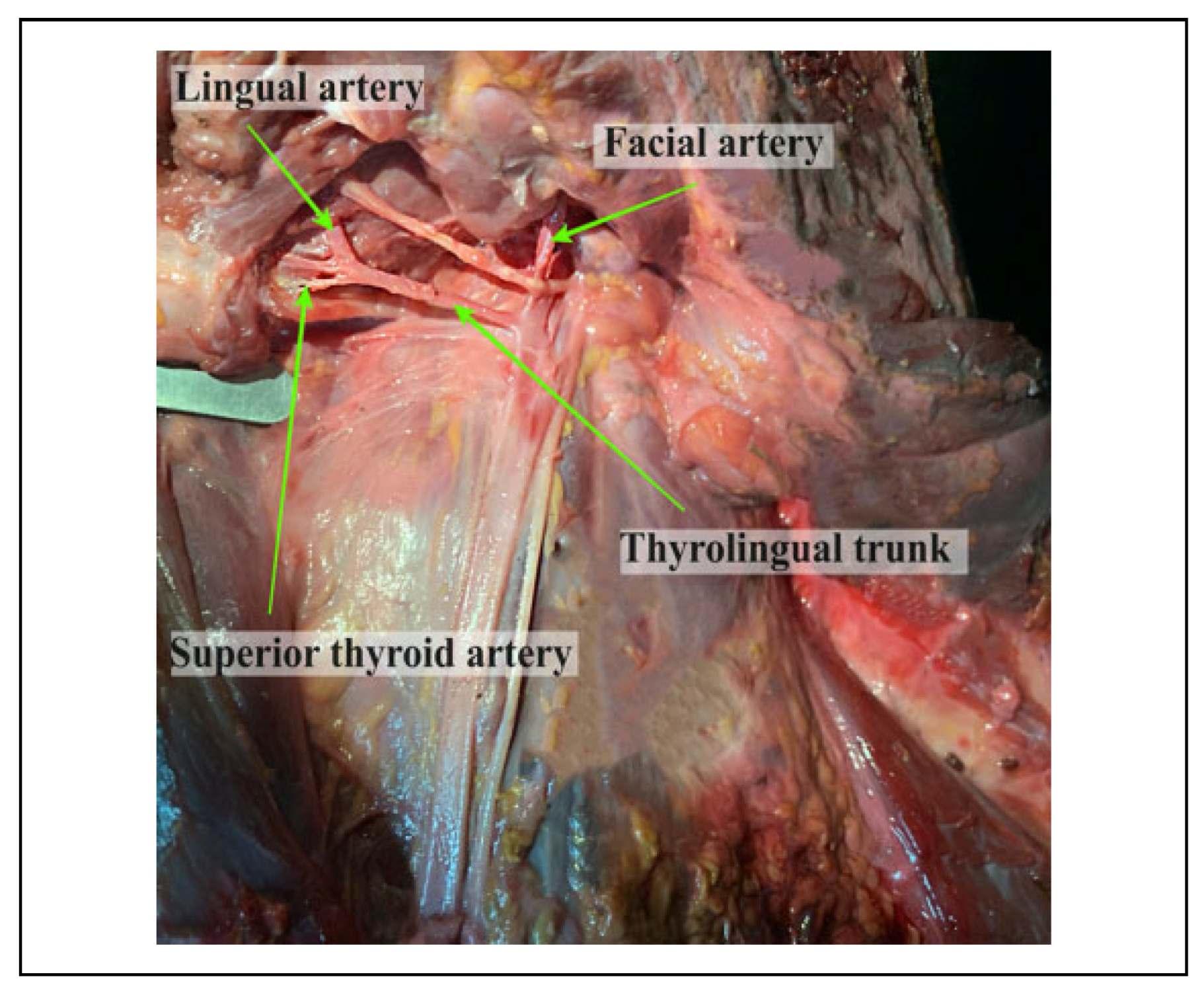
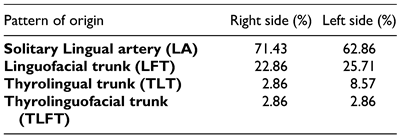
Population specific variations have been described regarding the patterns of origin of the LA. The linguofacial trunk (LFT) is reported to be most prevalent of the variants followed by the thyrolingual trunk (TLT) and the thyrolinguofacial trunk (TLFT) which is rarely observed.[5,6,7,8,9] These variants alter the relationship of the LA to landmarks such as the carotid bifurcation (CB), GCHB and the hypoglossal nerve (HN) which are used to locate the artery during surgery. This may predispose it to iatrogenic injuries resulting in rapid swelling of the submandibular area thus compromising the airway.[10,11] Additionally, procedures such as hemiglossectomy, total glossectomy and surgical resection of hemangiomas require ligation of the LA in order to avoid excessive hemorrhage.[3,12] However, these aberrations in the pattern of origin may result in inadvertent ligation of a common trunk thus resulting in severe complications.
Some of the anatomical variants of the LA place it in close proximity to the oral cavity hence making it more vulnerable to injury during intraoral surgeries.[13] They also present a significant challenge to radiologists in interpretation of angiograms during procedures such as embolization or intra-arterial injection of anti-cancer drugs into the LA.[14,15] Current literature is mainly focused on the variations of the ECA and comprehensive studies pertaining to the LA are scarce. Further, there is a dearth in regional data on the variations of the origin of the LA. Therefore, this study aims to determine the same.
Discussion
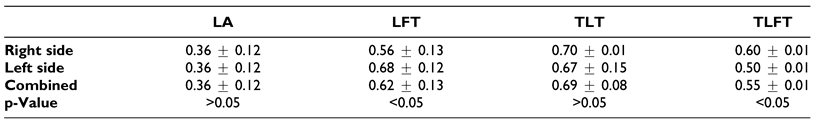
Findings from our study show that in all cases, the LA originated from the anteromedial surface of the ECA, similar to reports from text book literature.[16] Our findings however, differed from those by Herrera-Núñez et al (2020), where majority of the LA originated from the medial surface of the ECA (53%) while anteromedial origin was only noted in 30.9% of their population.[17]
The vessel was also noted to originate as a solitary branch in majority of the cases while in the least of cases, it originated as the TLFT. Our findings were contrasted that from other populations (
Table 5 and
Table 6) with some populations having higher values of the variations as compared to others.[13,18,19,20,21]
Several hypotheses have been suggested for the presence of the variant patterns of origin. For example, on the LFT existence, a review by Sirbu et al (2019) suggests that its occurrence could be determined by the remnants of the second aortic arch in the fetal period.[16] The existence of this variation may be critical in certain surgical procedures where uncontrolled bleeding may occur or during biopsies, tumor invasion or trauma in the oral and maxillofacial region. Similarly, during surgical procedures in the cervical region, one of the most common complication is the rupture of the ECA or one of its branches, which may occur due to the existence of these common vascular trunks. Under these conditions, angiography remains the ‘gold standard’ for the preoperative visualization of the vascularization of this region.[2]
As for the TLT variation, this type of variation may expose the LA to surgical risk during thyroid gland surgery. From the surgical and radiological point of view, knowing the variations in the origin of the LA in the cervical region is necessary in order to avoid errors in invasive procedures in this region, such as, for example, extraoral ligation of the LA. TLFT variants on the other hand, even though rare, might be susceptible to iatrogenic injury in procedures such as thyroidectomy or reconstruction of aneursyms.[25]
Owing to the possibility of the existence of any of these variants, we therefore recommend imaging procedures for detection prior to all surgical interventions in the cervical region.
Findings of our study revealed that the mean diameter of the vessels, regardless of patterns, were higher than that reported in standard text books Additionally, the TLT pattern had the largest diameter followed by the LFT and the TLFT. These findings differed to those of Fazan et al, 2009 where there were no statistically significant differences observed between the diameters of the different patterns[5] (
Table 7).
Arterial diameter is a good indicator of blood flow and is therefore relevant when ensuring good reperfusion of local structures during reconstructive surgery.[27] Therefore, large caliber vessels offer better healing options as compared to areas with small caliber vessels and as such the large vessel diameters in our case might be advantageous. Further, the differences observed among the vessel diameter might be due to racial differences which might influence the embryology of the vessels due to population heterogeneity in the genes governing their development.
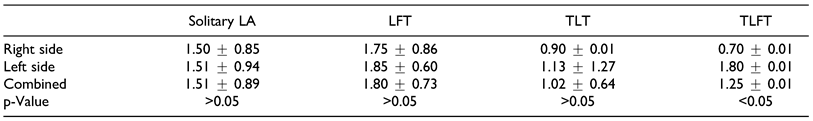
Our data showed that majority of the solitary LA originated at a point slightly higher to those reported by Fazan et al,[5] however, for the TLT trunks, our findings were similar to those reported from several case studies where values ranged from 1.45 cm to 3 cm (
Table 8).[8,9,28,29]
The knowledge these vascular variations might be very useful during intra-arterial chemotherapy for the treatment of tongue cancers, musculomucosal island flap for partial tongue reconstruction and superselective intra-arterial chemotherapy for head and neck carcinomas.[22,30] These variations should be taken into consideration, as they may increase the risk of hemorrhagic accidents during surgery.[31] Additionally, during procedures such as the ligation of ECA as a means of controlling hemorrhage, either traumatic or operative, surgical dictate for ligating the ECA is to demonstrate a branch and for ligating above the demonstrated branch which is usually the STA. However, in cases where there is an anomalous TLT trunk which originates at the bifurcation of common carotid artery, iatrogenic injury might occur following ligation of the LA as well.[28] Therefore in our setting, since most vessels originated a distance from the CB, patients might not be at a high risk of the aforementioned iatrogenic injury.
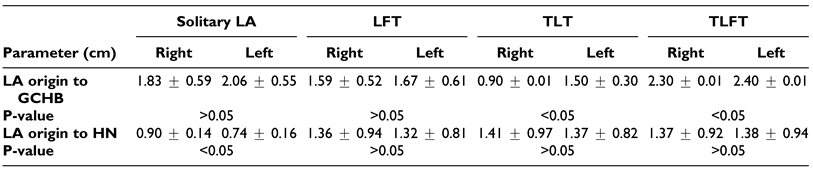
Considering the complicated anatomy of the cervical and submandibular region, the use of only 1 surgical landmark is not recommended.[32] In this case, we looked at 2 landmarks, the GCHB and the HN. A summary of the findings from our study showed that the LA was at different distances from the different landmarks based on the pattern of the origin of the LA.
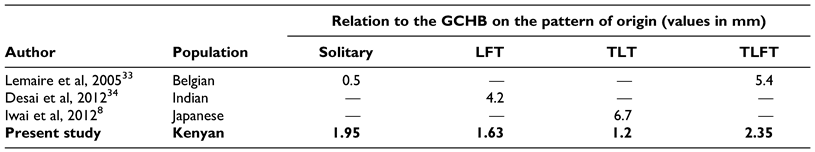
In the case of the solitary LA, the vessel was a greater distance to the GCHB as compared to values reported by Lemaire et al among the Belgian.[33] As for the LFT, TLT and TLFT, our mean values were lower than that reported in other populations (
Table 9).
Despite the population differences observed, it is important to note that the study by Lemaire et al and Desai et al were a case study and as such despite the comparison, the low sample size in these studies might not yield the best comparison.[9,34] The differences observed might be due to genetic heterogeneity that may govern vascular development. Both radiological diagnosis and surgical approach depend on anatomical knowledge of the region and the variability in the vasculature involved. A coherent understanding of the variations of the ECA and its branches are vital for procedures performed in the head and neck region.[8] Similarly, during trans-oral robotic surgery (TORS), especially for tongue cancers, several measures are usually taken to avoid injury to the deep vessels, such as skeletonization of the styloglossus and stylopharyngeus muscles. However in cases where deep resection of the tongue base is paramount, the lingual artery may be iatrogenically damaged, therefore laying emphasis on understanding its anatomy. The vessel, in most cases, arises around the hyoid bone. From the hyoid bone, it courses lateral to the middle constrictor muscle where it is crossed by the hypoglossal nerve, and then it passes deep to the hyoglossus muscle where it runs on the superior surface of the hyoid bone. It is in this location that it is vulnerable to injury. In our case, the vessel was 1.66 cm and 1.90 cm (on average) on the right and left sides respectively from the greater horn of the hyoid bone (regardless of the type of trunk). In the case where the dorsal lingual artery is encountered (instead of the main trunk) laterally in the tongue base, tracing it laterally will lead to the lingual artery trunk. Surgeons during TORS may therefore keep these in mind and when encountering the lingual artery, should clip it with several endoscopic hemoclips to prevent life threatening bleeding in the post-operative setting. In the case of hemostasis, compressing the neck at the level of the superior thyroid cornu to slow bleeding may be performed, especially, if the artery is inadvertently transected before clips are applied.[35]
The distance of the LA to the HN in our setting (1.18 cm) was higher than that reported by Souza et al (2017) among Indians (0.58 cm).[32] The nerve constitutes an important anatomic structure that is related to the LA.[36] The relation between the 2 structures might therefore help the surgeon in locating the HN in reference to the LA and vice versa.