Injuries of the Peripheral Mandibular Nerve, Evaluation of Interventions and Outcomes: A Systematic Review
Abstract
Introduction
Materials and Methods
Search Methods
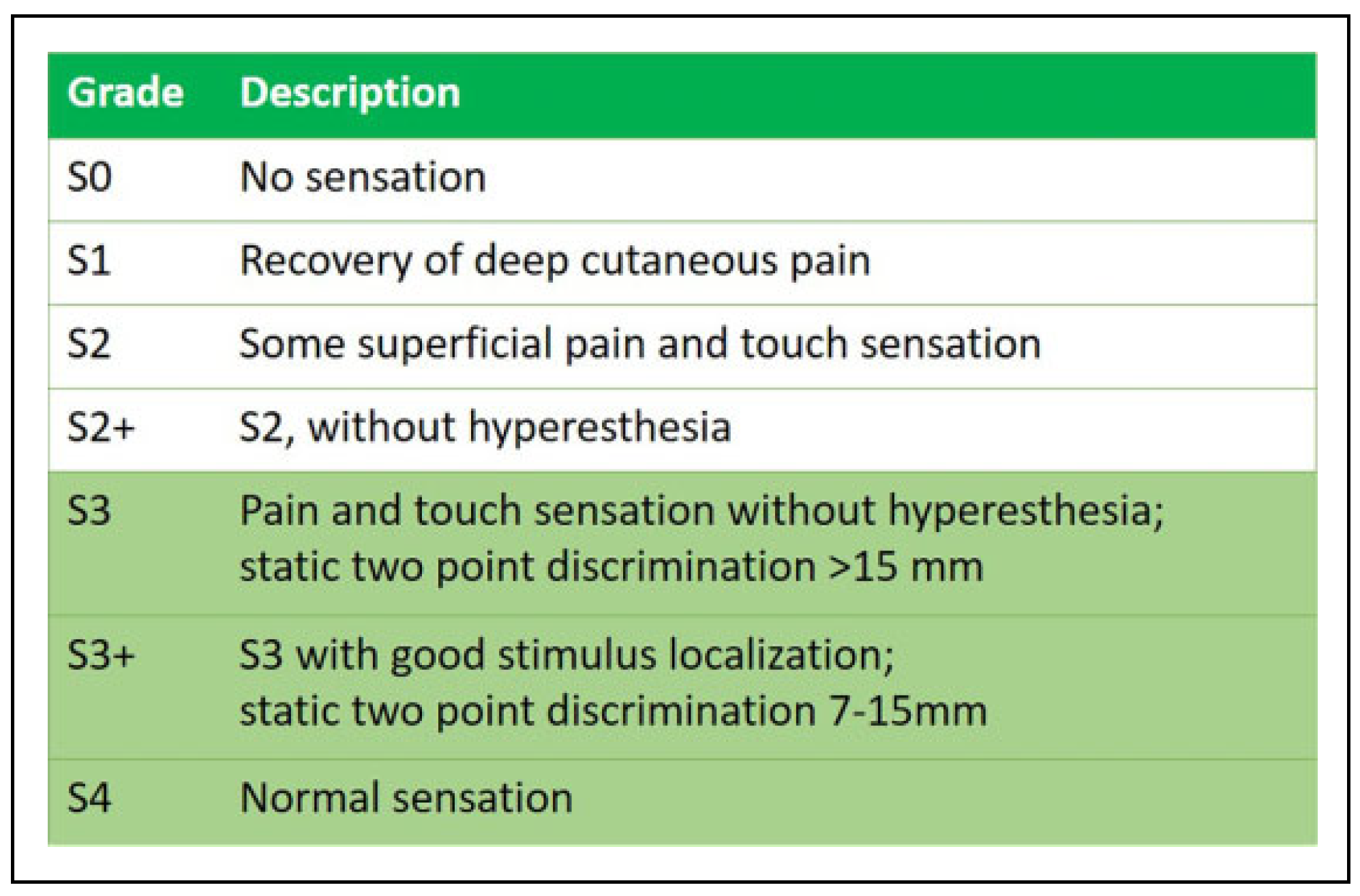
Quality Assessment
Statistical Analysis
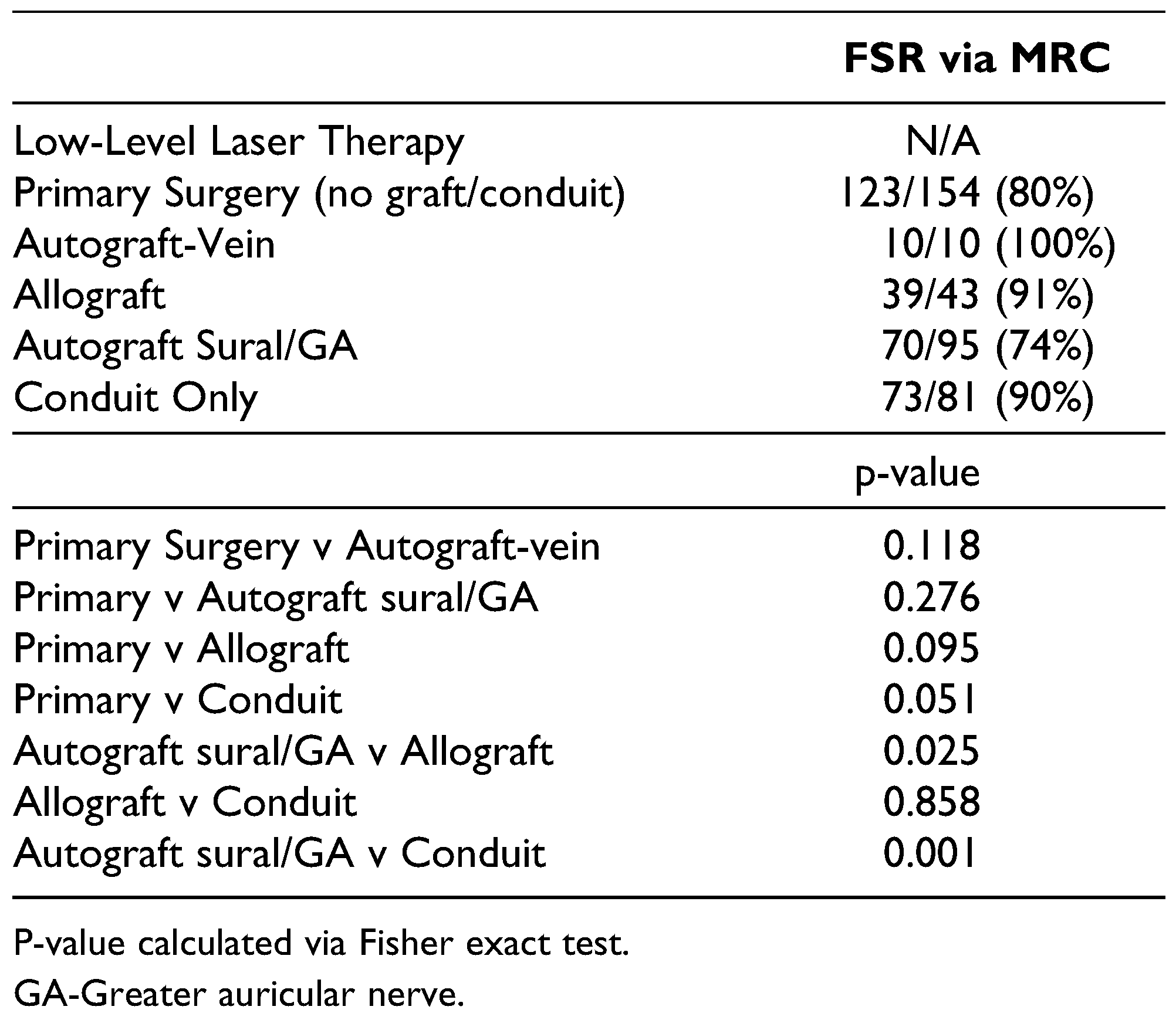
Results
Search Strategy
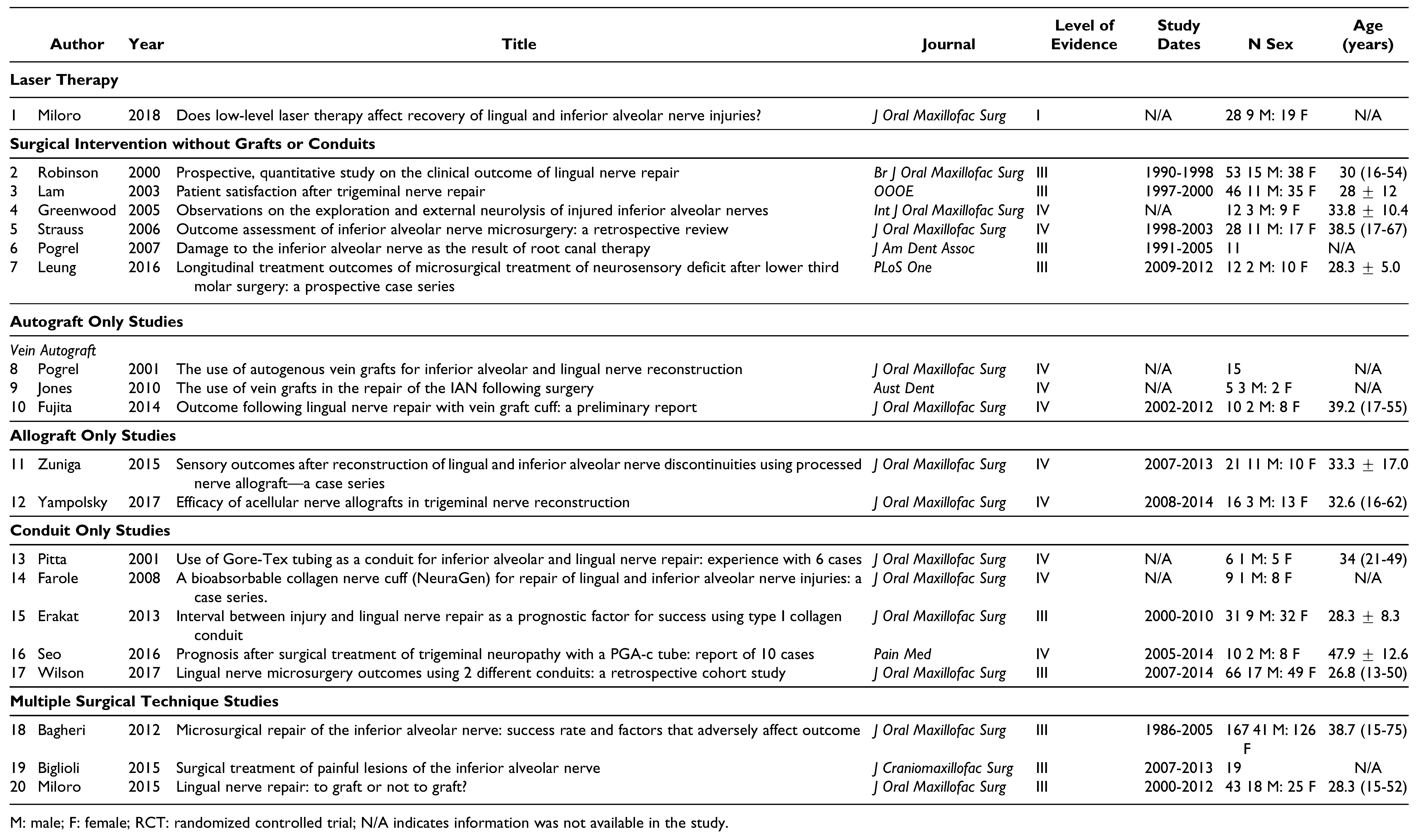
Eligibility of the Studies
Data Extraction
Timing of Repair
Lasers
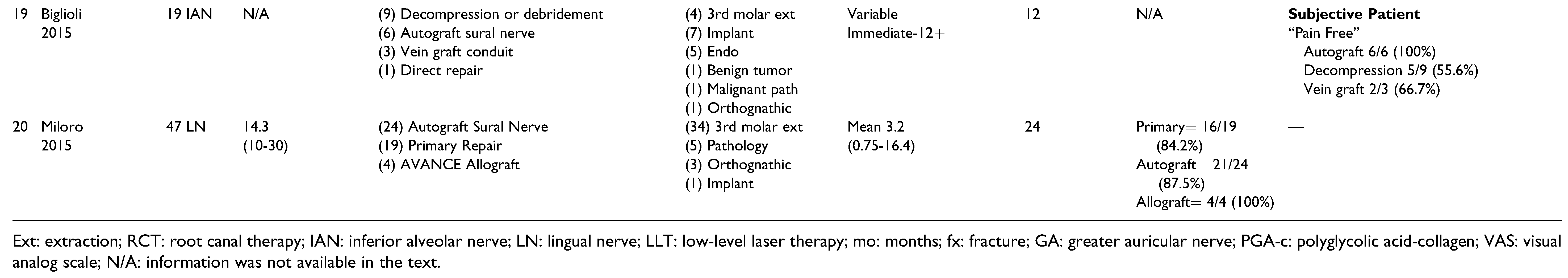
Decompression/Debridement/Neurolysis/Primary Repair
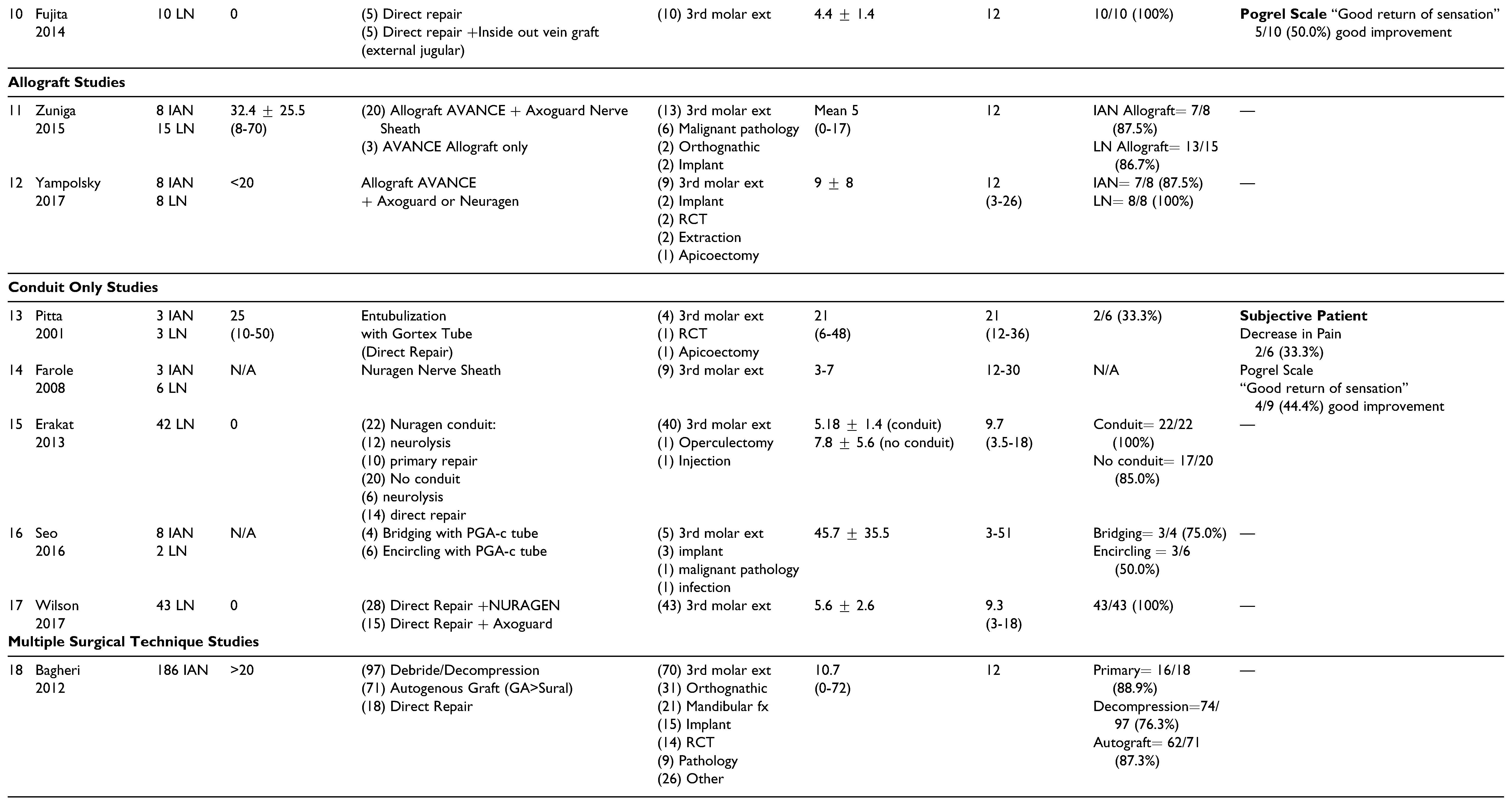
Autograft
Allografts
Conduits Without Grafting
Discussion
Conclusions
Funding
Ethical Approval
Patient Consent
Conflicts of Interest
References
- Tay, A.B.G.; Zuniga, J.R. Clinical characteristics of trigeminal nerve injury referrals to a university centre. Int J Oral Maxillofac Surg. 2007, 36, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wu, S.; Huang, H.; Lai, Y. Systematic review and meta-analysis on incidence of altered sensation of mandibular implant surgery. PLoS ONE. 2016, 11, e0154082. [Google Scholar] [CrossRef]
- Bagheri, S.C.; Meyer, R.A. When to refer a patient with a nerve injury to a specialist. J Am Dent Assoc. 2014, 145, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Juodzbalys, G.; Kubilius, M. Clinical and radiological classification of the jawbone anatomy in endosseous dental implant treatment. J Oral Maxillofac Res. 2013, 4, e2. [Google Scholar] [CrossRef]
- Coulthard, P.; Kushnerev, E.; Yates, J.M.; et al. Interventions for iatrogenic inferior alveolar and lingual nerve injury. Cochrane Database Syst Rev. 2014, CD005293. [Google Scholar] [CrossRef]
- Jones, R.H.B. Repair of the trigeminal nerve: A review. Aust Dent J. 2010, 55, 112–119. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sunitha, M.; Chung, K.C. How to measure outcomes of peripheral nerve surgery. Hand Clin. 2013, 29, 349–361. [Google Scholar] [CrossRef]
- Wilson, M.T.; Chuang, S.; Ziccardi, V.B. Lingual nerve microsurgery outcomes using 2 different conduits: A retrospective cohort study. J Oral Maxillofac Surg. 2017, 75, 609–615. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G. Assessing risk of bias in included studies. In: Cochrane handbook for systematic reviews of interventions. John Wiley & Sons, Ltd; 2008:187-241. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470712184.ch8 (accessed on 10 April 2020).
- Zuniga, J.R. Sensory outcomes after reconstruction of lingual and inferior alveolar nerve discontinuities using processed nerve allograft—a case series. J Oral Maxillofac Surg. 2015, 73, 734–744. [Google Scholar] [CrossRef]
- Miloro, M.; Ruckman, P.; Kolokythas, A. Lingual nerve repair: To graft or not to graft? J Oral Maxillofac Surg. 2015, 73, 1844–1850. [Google Scholar] [CrossRef]
- Miloro, M.; Criddle, T. Does low-level laser therapy affect recovery of lingual and inferior alveolar nerve injuries? J Oral Maxillofac Surg. 2018, 76, 2669–2675. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.P.; Loescher, A.R.; Smith, K.G. A prospective, quantitative study on the clinical outcome of lingual nerve repair. Br J Oral Maxillofac Surg. 2000, 38, 255–263. [Google Scholar] [CrossRef]
- Lam, N.P.; Donoff, R.B.; Kaban, L.B.; Dodson, T.B. Patient satisfaction after trigeminal nerve repair. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003, 95, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, M.; Corbett, I.P. Observations on the exploration and external neurolysis of injured inferior alveolar nerves. Int J Oral Maxillofac Surg. 2005, 34, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Strauss, E.R.; Ziccardi, V.; Janal, M.N. Outcome assessment of inferior alveolar nerve microsurgery: a retrospective review. J Oral Maxillofac Surg. 2004, 62, 47–48. [Google Scholar] [CrossRef]
- Pogrel, M.A. Damage to the inferior alveolar nerve as the result of root canal therapy. J Am Dent Assoc. 2007, 138, 65–69. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Cheung, L.K. Longitudinal treatment outcomes of microsurgical treatment of neurosensory deficit after lower third molar surgery: A prospective case series. PLoS One. 2016, 11, e0150149. [Google Scholar] [CrossRef][Green Version]
- Chang, Y.; Rodriguez, E.D.; Chu, Y.; Tsai, C.; Wei, F. Inferior alveolar nerve reconstruction with interpositional sural nerve graft: A sensible addition to one-stage mandibular reconstruction. J Plast Reconstr Aesthet Surg. 2012, 65, 757–762. [Google Scholar] [CrossRef]
- Schultes, G.; Gaggl, A.; Ka¨rcher, H. Vascularized transplantation of the long thoracic nerve for sensory reinnervation of the lower lip. Br J Oral Maxillofac Surg. 2000, 38, 138–141. [Google Scholar] [CrossRef]
- Shimizu, F.; Ooatari, M.; Uehara, M.; Takahashi, Y.; Kawano, K. Effect of concurrent mental nerve reconstruction at the same time as mandibular reconstruction using a fibula osteosepto-cutaneous flap. J Plast Reconstr Aesthet Surg. 2015, 68, 1228–1234. [Google Scholar] [CrossRef]
- Pogrel, M.A.; Maghen, A. The use of autogenous vein grafts for inferior alveolar and lingual nerve reconstruction. J Oral Maxillofac Surg. 2001, 59, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Tojyo, I.; Yamada, M.; Go, Y.; Matsumoto, T.; Kiga, N. Outcome following lingual nerve repair with vein graft cuff: A preliminary report. J Oral Maxillofac Surg. 2014, 72, 1433.e1–1433.e7. [Google Scholar] [CrossRef]
- Jones, R.H.B. The use of vein grafts in the repair of the inferior alveolar nerve following surgery. Aust Dent J. 2010, 55, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Yampolsky, A.; Ziccardi, V.; Chuang, S. Efficacy of acellular nerve allografts in trigeminal nerve reconstruction. J Oral Maxillofac Surg. 2017, 75, 2230–2234. [Google Scholar] [CrossRef] [PubMed]
- Erakat, M.S.; Chuang, S.; Shanti, R.M.; Ziccardi, V.B. Interval between injury and lingual nerve repair as a prognostic factor for success using type I collagen conduit. J Oral Maxillofac Surg. 2013, 71, 833–838. [Google Scholar] [CrossRef]
- Pitta, M.C.; Wolford, L.M.; Mehra, P.; Hopkin, J. Use of Gore-Tex tubing as a conduit for inferior alveolar and lingual nerve repair: Experience with 6 cases. J Oral Maxillofac Surg. 2001, 59, 493–496. [Google Scholar] [CrossRef]
- Seo, K.; Terumitsu, M.; Inada, Y.; Nakamura, T.; Shigeno, K.; Tanaka, Y. Prognosis after surgical treatment of trigeminal neuropathy with a PGA-c tube: Report of 10 cases. Pain Med. 2016, 17, 2360–2368. [Google Scholar] [CrossRef]
- Farole, A.; Jamal, B.T. A bioabsorbable collagen nerve cuff (NeuraGen) for repair of lingual and inferior alveolar nerve injuries: A case series. J Oral Maxillofac Surg. 2008, 66, 2058–2062. [Google Scholar] [CrossRef]
- Bagheri, S.C.; Meyer, R.A.; Cho, S.H.; Thoppay, J.; Khan, H.A.; Steed, M.B. Microsurgical repair of the inferior alveolar nerve: Success rate and factors that adversely affect outcome. J Oral Maxillofac Surg. 2012, 70, 1978–1990. [Google Scholar] [CrossRef]
- Biglioli, F.; Allevi, F.; Lozza, A. Surgical treatment of painful lesions of the inferior alveolar nerve. J Craniomaxillofac Surg. 2015, 43, 1541–1545. [Google Scholar] [CrossRef]
- Pogrel, M.A. The results of microneurosurgery of the inferior alveolar and lingual nerve. J Oral Maxillofac Surg. 2002, 60, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, J.R.; Meyer, R.A.; Gregg, J.M.; Miloro, M.; Davis, L.F. The accuracy of clinical neurosensory testing for nerve injury diagnosis. J Oral Maxillofac Surg. 1998, 56, 2–8. [Google Scholar] [CrossRef]
- Ricciardi, L.; Stifano, V.; Pucci, R.; et al. Comparison between VII-to-VII and XII-to-VII systematic review and pooled analysis of the functional outcomes. Neurosurg Rev. 2020, 44, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Nizam, S.A.; Ziccardi, V.B. Trigeminal nerve injuries: Avoidance and management of iatrogenic injury. Oral Maxillofac Surg Clin North Am. 2015, 27, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Kushnerev, E.; Yates, J.M. Evidence-based outcomes following inferior alveolar and lingual nerve injury and repair: A systematic review. J Oral Rehabil. 2015, 42, 786–802. [Google Scholar] [CrossRef]
- Miloro, M.; Stoner, J.A. Subjective outcomes following sural nerve harvest. J Oral Maxillofac Surg. 2005, 63, 1150–1154. [Google Scholar] [CrossRef]
- Wessberg, G.A.; Wolford, L.M.; Epker, B.N. Experiences with microsurgical reconstruction of the inferior alveolar nerve. J Oral Maxillofac Surg. 1982, 40, 651–655. [Google Scholar] [CrossRef]
- Kanaya, F.; Firrell, J.; Tsai, T.M.; Breidenbach, W.C. Functional results of vascularized versus nonvascularized nerve grafting. Plast Reconstr Surg. 1992, 89, 924–930. [Google Scholar] [CrossRef]
- Isaacs, J.; Safa, B.; Evans, P.J.; Greenberg, J. Technical assessment of connector-assisted nerve repair. J Hand Surg Am. 2016, 41, 760–766. [Google Scholar] [CrossRef]
- Ducic, I.; Yoon, J. Reconstructive options for inferior alveolar and lingual nerve injuries after dental and oral surgery: An evidence-based review. Ann Plast Surg. 2019, 82, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Safa, B.; Buncke, G. Autograft substitutes: Conduits and processed nerve allografts. Hand Clin. 2016, 32, 127–140. [Google Scholar] [CrossRef] [PubMed]

 |
 |
 |
 |
 |
 |
© 2021 by the author. The Author(s) 2021.
Share and Cite
Weyh, A.; Pucci, R.; Valentini, V.; Fernandes, R.; Salman, S. Injuries of the Peripheral Mandibular Nerve, Evaluation of Interventions and Outcomes: A Systematic Review. Craniomaxillofac. Trauma Reconstr. 2021, 14, 337-348. https://doi.org/10.1177/19433875211002049
Weyh A, Pucci R, Valentini V, Fernandes R, Salman S. Injuries of the Peripheral Mandibular Nerve, Evaluation of Interventions and Outcomes: A Systematic Review. Craniomaxillofacial Trauma & Reconstruction. 2021; 14(4):337-348. https://doi.org/10.1177/19433875211002049
Chicago/Turabian StyleWeyh, Ashleigh, Resi Pucci, Valentino Valentini, Rui Fernandes, and Salam Salman. 2021. "Injuries of the Peripheral Mandibular Nerve, Evaluation of Interventions and Outcomes: A Systematic Review" Craniomaxillofacial Trauma & Reconstruction 14, no. 4: 337-348. https://doi.org/10.1177/19433875211002049
APA StyleWeyh, A., Pucci, R., Valentini, V., Fernandes, R., & Salman, S. (2021). Injuries of the Peripheral Mandibular Nerve, Evaluation of Interventions and Outcomes: A Systematic Review. Craniomaxillofacial Trauma & Reconstruction, 14(4), 337-348. https://doi.org/10.1177/19433875211002049




