Introduction
Diagnosis of MRONJ (medication-related osteonecrosis of the jaw) relies mainly on the patient’s history and clinical examination due to the following 3 criteria defined by the American Association of Oral and Maxillofacial Surgeons (AAOMS) which need to be present to consider the diagnosis of MRONJ[
1]:
current or previous treatment with antiresorptive or antiangiogenic agents;
no history of radiation therapy to the jaws or obvious metastatic disease to the jaws;
exposed bone or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region that has persisted for longer than 8 weeks.
History and clinical examination are supplemented with conventional panoramic radiographs to allow an assignment to the 4 stages 0-3 as defined by the AAOMS.[
1] On the basis of the assigned stage therapy concepts range from symptomatic treatment and conservative management of local risk factors in stage 0 to surgical debridement and extensive resection plus reconstruction in stage 3.[
2]
However, the above presented classification system does not seem to reflect the true severity of the disease as it focuses on the clinical finding of exposed bone to the mucosa or skin surfaces and does not consider the threedimensional (3D) extent of necrotic bone.[
3,
4] For example, stage 0 MRONJ being the nonexposed variant can be associated with widespread underlying necrosis of the jaw.[
5,
6,
7] Vice versa, clinical findings such as exposed bone and signs of infection with mucosal erythema, purulent discharge, and pain may not be associated with a widespread affection of the bone.[
8,
9] That’s why some authors discuss the addition of a 3D imaging to complement the diagnostics of MRONJ and to identify the full extension of compromised bone.[
10,
11]
For computer-assisted craniomaxillofacial surgery, virtual segmentation tools are available to fully estimate the 3D location and extent of tumors and cysts in data sets of 3D images.[
12,
13] These tools allow manual, semi-automatic, or automatic preoperative segmentation of pathological structures thereby leading to an improved diagnostic, the possibility of preoperative planning of resection and reconstruction, and a more secure surgery through intraoperative navigation. Such tools have not yet been used or validated for diagnostic and treatment of MRONJ in a clinically standardized manner.
This study investigated the sizes of defect volumes of MRONJ patients by segmentation of 3D data sets of spiral or cone beam computed tomographies. The calculated defect volumes were analyzed in regard to correlations to clinical parameters such as age, gender, drug agent, route of drug administration as well as invasiveness of the therapy, and number of performed operations. In a further step, the data sets of performed segmentations were used to develop a computer-assisted tool based on techniques of artificial intelligence (AI) to enhance the diagnostics of MRONJ.
Materials and Methods
This study was approved by the local ethics committee at the Hannover Medical School, Germany. All MRONJ cases treated between 2004 and 2017 in the Department for Oral and Maxillofacial Surgery of the Hannover Medical School for which a preoperative 3D imaging via spiral or cone beam computed tomography was available were analyzed.
Patients’ Data Acquisition
Thirty-one parameters for 164 patients were retrospectively collected from the clinical documentation using the patient management softwares “ivorisdent” (Computer konkret AG DentalSoftwarePower) and “SAP” (SAP Deutschland SE & Co. KG). Missing data were complemented by using the clinic’s analogue files and photographies. Data were organized using Microsoft Office Excel 2013 (Microsoft Corporation).
Segmentation Protocol
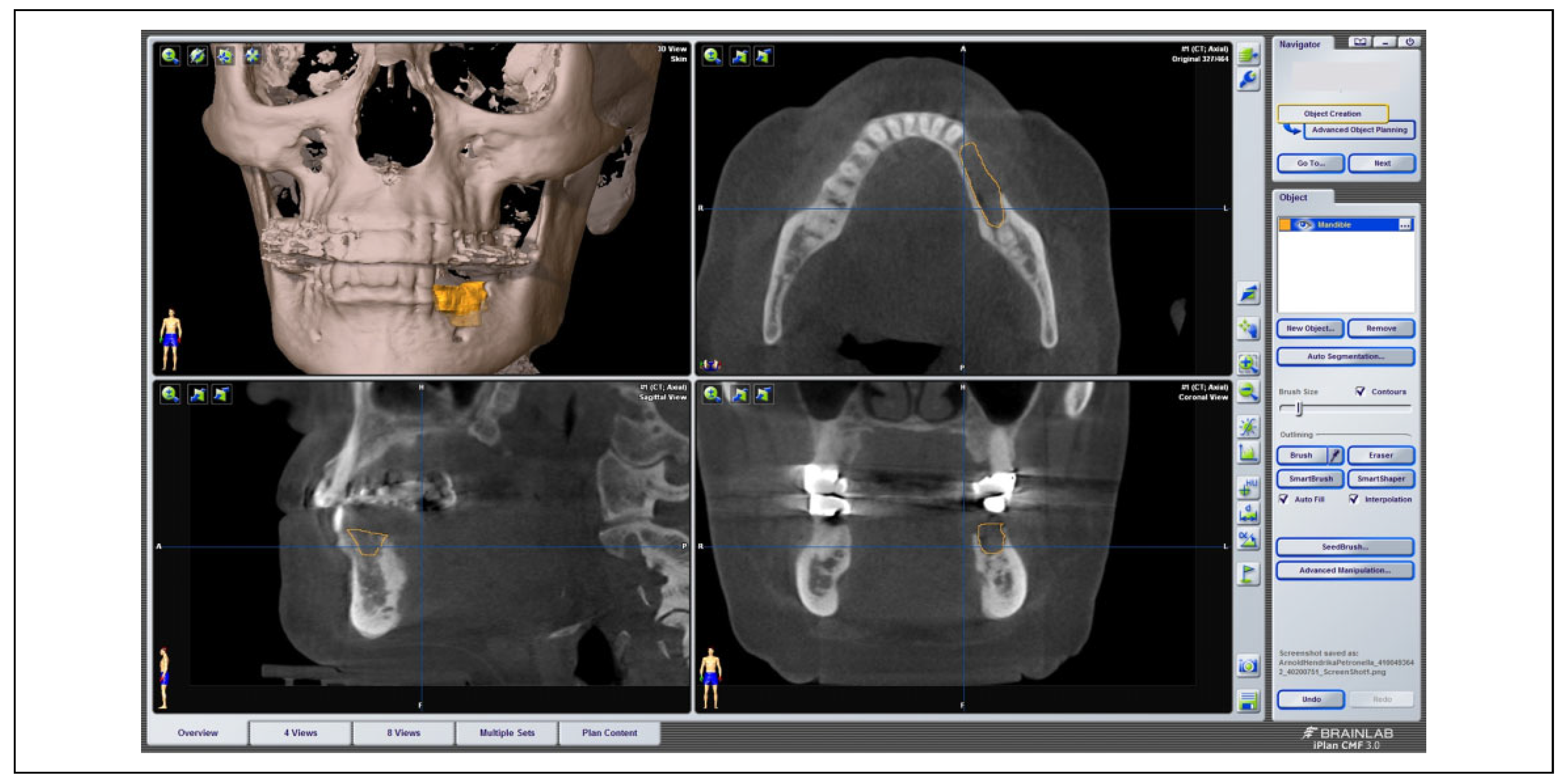
On the basis of 3D DICOM data sets of 175 patients suffering from MRONJ, the size of the necrosis was investigated using the software iPlan 3.0.5 (BrainLAB AG) (see
Figure 1). Via the “brush” tool, the outline of the necrosis was marked manually layer by layer in the axial view and thoroughly adjusted in the sagittal as well as coronal view if needed. Segmentation was performed for each patient 1 to 3 times for internal control. Exact cubic numbers of the virtually reconstructed 3D volume of necrosis was then determined via the “plan content” tool.
Statistical Analysis
The statistical analysis was conducted using SPSS for Windows (SPSS Inc.). The correlation between the 31 above mentioned parameters and the determined volume of the segmented necrosis was investigated involving the Kruskal-Wallis test as well as the Mann-Whitney test and the Spearman correlation. For all tests, P values <.05 were considered statistically significant.
Development of a Computer-Assisted Analysis Software for Necrosis Segmentation in MRONJ Cases
In a further step, the development of a computer-aided software for the improvement of the risk assessment, diagnosis and therapy decision in MRONJ patients was initiated in cooperation with the AO Research Institute Davos (Davos, Switzerland) of the AO foundation. The data from previously selected 175 MRONJ patients were used to construct a model based on machine learning. That model should anticipate and distinguish 2 types, the MRONJ patients and the healthy implant patients which served as a negative control with healthy bone without any defects. After data acquisition the preparation of data was started. This included the already described segmentation of necrosis volumes as well as the determination of the so-called regions of interest (ROIs). Afterward, the extracted properties were recorded in AMIRA (Amira version 6.5.0; FEI (Field Emission Inc.)) for image processing and then transferred to MATLAB (The MathWorks GmbH). Subsequently, all sets of data and images had been subjected to the AI working process. AI has been used to train the computer tool and ultimately to find the most suitable model to act as predictor tool. The MATLAB code was generated by the software to create a predictor code that can predict new features and responses based on a given set of properties of a known case. This procedure takes about 30 seconds. The working process is currently still in development.
Results
Age, Gender and Underlying Disease
Among the 164 patients suffering from MRONJ, there were 43.3% men and 56.7% women. The average age was 74.63 years with a standard deviation of 9.84 years and a median of 76 years. The youngest patient was 49 years old, the oldest patient was 96 years old. There were no significant differences in the age-related gender. Among men the average age was 74.70 years, for women it was 74.58 years.
In all, 9.1% of the MRONJ patients suffered from osteoporosis as the underlying disease justifying the indication for drug intake and 90.1% suffered from malignancies such as mamma carcinoma (35.97%), prostate carcinoma (28.6%), multiple myeloma (15.24%), renal cell carcinoma (4.26%), or non-Hodgkin lymphoma (2.44%).
The defect volumes calculated through segmentation (see the Radiological Analysis: Radiographic Signs and Defect Volume section) for the 4 most common underlying diseases were as follows: 1346.53 mm3 for osteoporosis, 1766.86 mm3 for multiple myeloma, 1875.76 mm3 for mamma carcinoma, and 2304.80 mm3 for prostate carcinoma. There was no significant correlation found between the underlying disease and the segmented defect volumes.
Antiresorptive Medication: Kind of Agent, Mode of Administration, and Duration of Medication
With regard to the antiresorptive medication, data about the kind of the drug agent, a change of the drug agent, the duration of the medication, and the mode of administration were collected. It showed that 76.21% of the patients had taken only one preparation. Among these patients, zoledronate was the most commonly used product at 42.68%, followed by denosumab at 12.19%. Among the 23.79% of patients who switched the drug at least once, ibandronate was the most prevalent drug with 23.78%, followed by zoledronate at 17.68%, and denosumab at 17.07%.
Depending on the drug agent, 89.0% of the patients received the antiresorptive medication exclusively intravenously and 6.7% per os. In 3.7% of cases, medication was applied both intravenous (i.v.) and per os (p.o.) at different times depending on the drug. For 1 patient, the mode of administration is unknown.
Among the patients taking antiresorptive medication, 9.1% took the medication for 0-11 months, 9.8% 12-24 months, 20.7% 25-36 months, and 12.2% 37-48 months. The largest group at 40.2% received antiresorptive medication over 4 years. As to the frequency of intake, by far the most frequent intake pattern at 61.6% was the 3-6 weeks intake with a monthly administration being the most common. Nearly 6.1% received the antiresorptive drugs every 1-3 weeks or every 6 months, 2.4% daily, and 1.2% annually.
The mean of the segmented volume (see the Radiological Analysis: Radiographic Signs and Defect Volume section) was 2004.81 mm3 for intravenous administration with a standard deviation of 1863.04 mm3, for peroral administration 1279.21 mm3 with a standard deviation of 1164.21 mm3, and for the use of both application forms 862.00 mm3 with a standard deviation of 876.71 mm3. Although in the descriptive statistics there was a higher mean volume for intravenous application compared to oral administration, there was no statistically significant correlation between the form of drug administration and segmented defect volume. Considering the duration of medication there was no correlation found to the segmented defect volume
Risk Factors
Among the patients, 30.5% were or have been active smokers and 64.0% have never smoked. For 5.5% there was no information available.
Concerning alcohol, 80.5% stated that they drink no or only rarely alcohol, 13.4% drink alcohol regularly and 1.2% were dry alcoholics. For 6.10% there was no information available.
About 42.1% of the patients have been under chemotherapy as a possible cofactor for the development of MRONJ.
Dental Status and Localization
In all, 70.1% of the patients had at least 1 tooth per jaw, 20.1% had teeth in only 1 jaw and 9.8% had no teeth at all. In 69.5% of all patients 1 or more tooth extractions were performed at the localization, at which necrosis later manifested. In 4.9% of the cases, an explanation of dental implants had been performed in their history. More than half of the patients (58.5%) were diagnosed with dental caries at at least 1 tooth.
The necroses manifested almost twice as often in the mandible as in the maxilla. Both in the upper and in the lower jaw, the premolar and molar region is significantly more often affected than the anterior region. In the maxilla, only 20.7% had necrosis in the front and 18.4% in the mandible. 15.2% of the patients developed osteonecrosis in both the upper and lower jaws.
Radiological Analysis: Radiographic Signs and Defect Volume
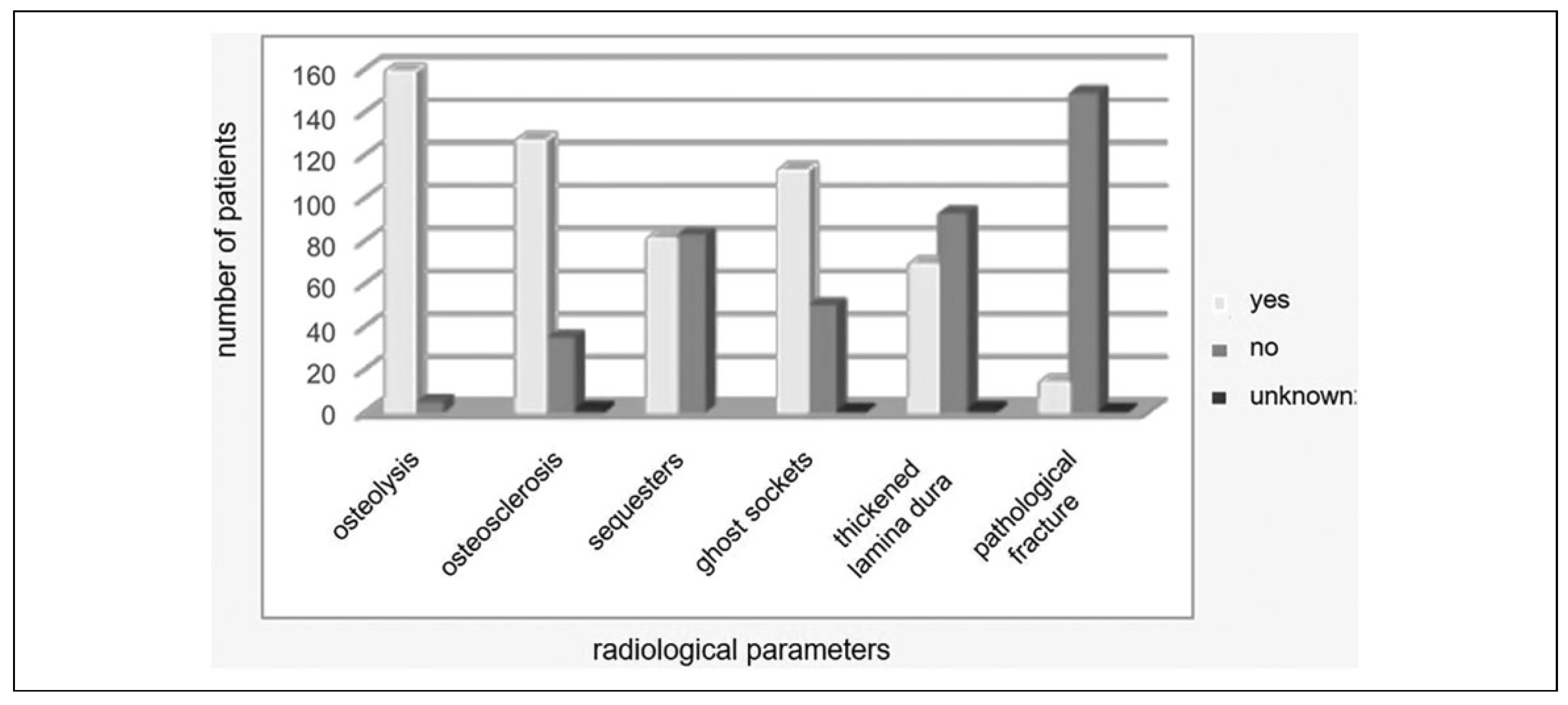
The available radiographs for every patient including the preoperative 3D imaging via spiral or cone beam computed tomography in all 164 cases as well as a panoramic radiograph for 114 patients were analyzed for radiologic signs of MRONJ such as osteolysis, osteosclerosis, sequester formation, ghost sockets, and pathologic fracture (see
Figure 2).
In 64% of the cases, signs of osteonecrosis were visible in the conventional 2D panoramic radiography, whereas in 36% osteonecrosis could only be seen in the 3D imaging. Especially, formation of sequesters was hardly visible in panoramic radiographs with only 2.6% of patients compared to 49.4% in 3D imaging.
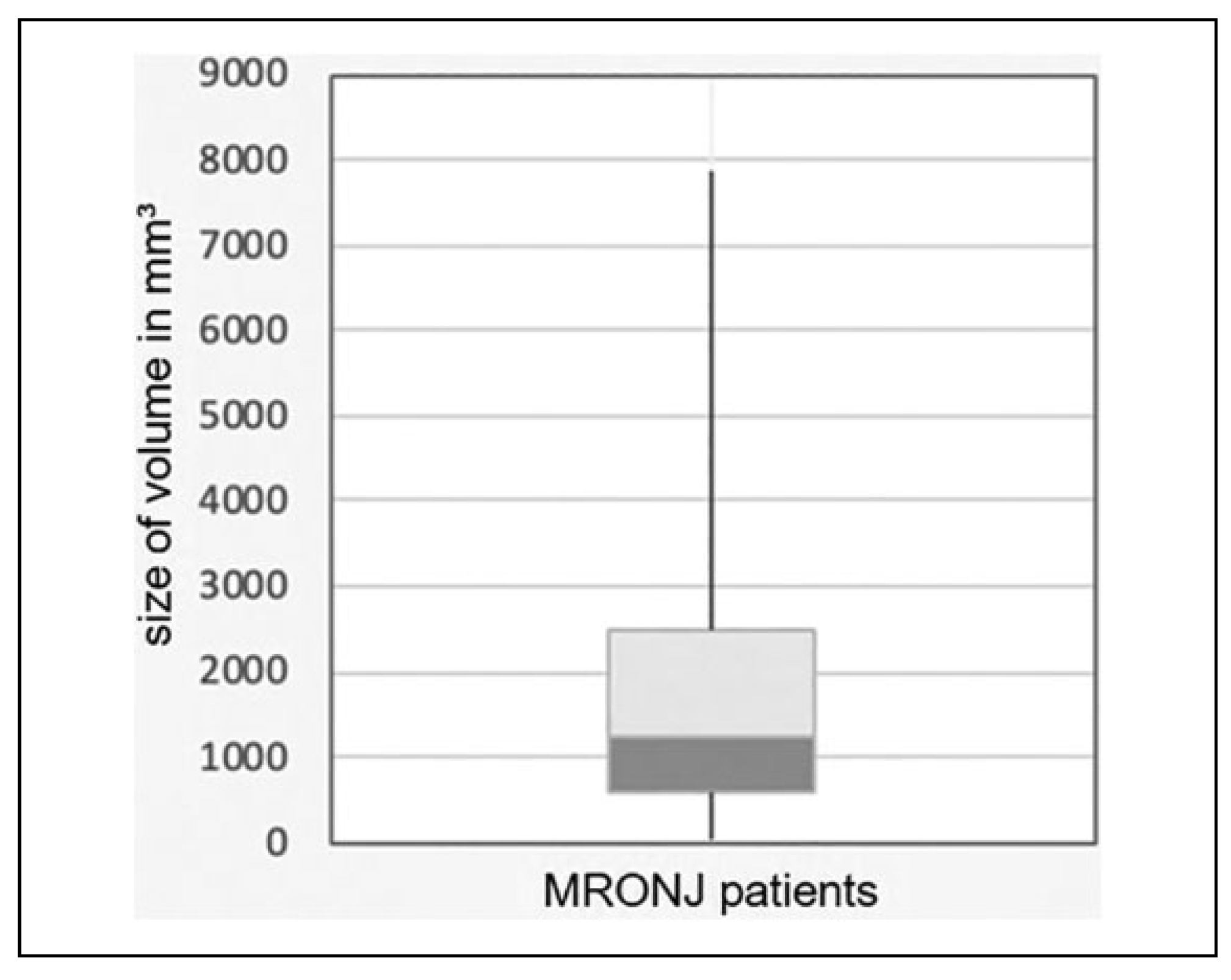
Analysis of the defect volume through segmentation of the 3D data set showed that the segmented volume was on average 1885.20 mm
3 with a standard deviation of 1793.87 mm
3. The smallest segmented volume was 42 mm
3 and the largest volume was 7882 mm
3. The median value was 1250 mm
3. Results are illustrated in
Figure 3.
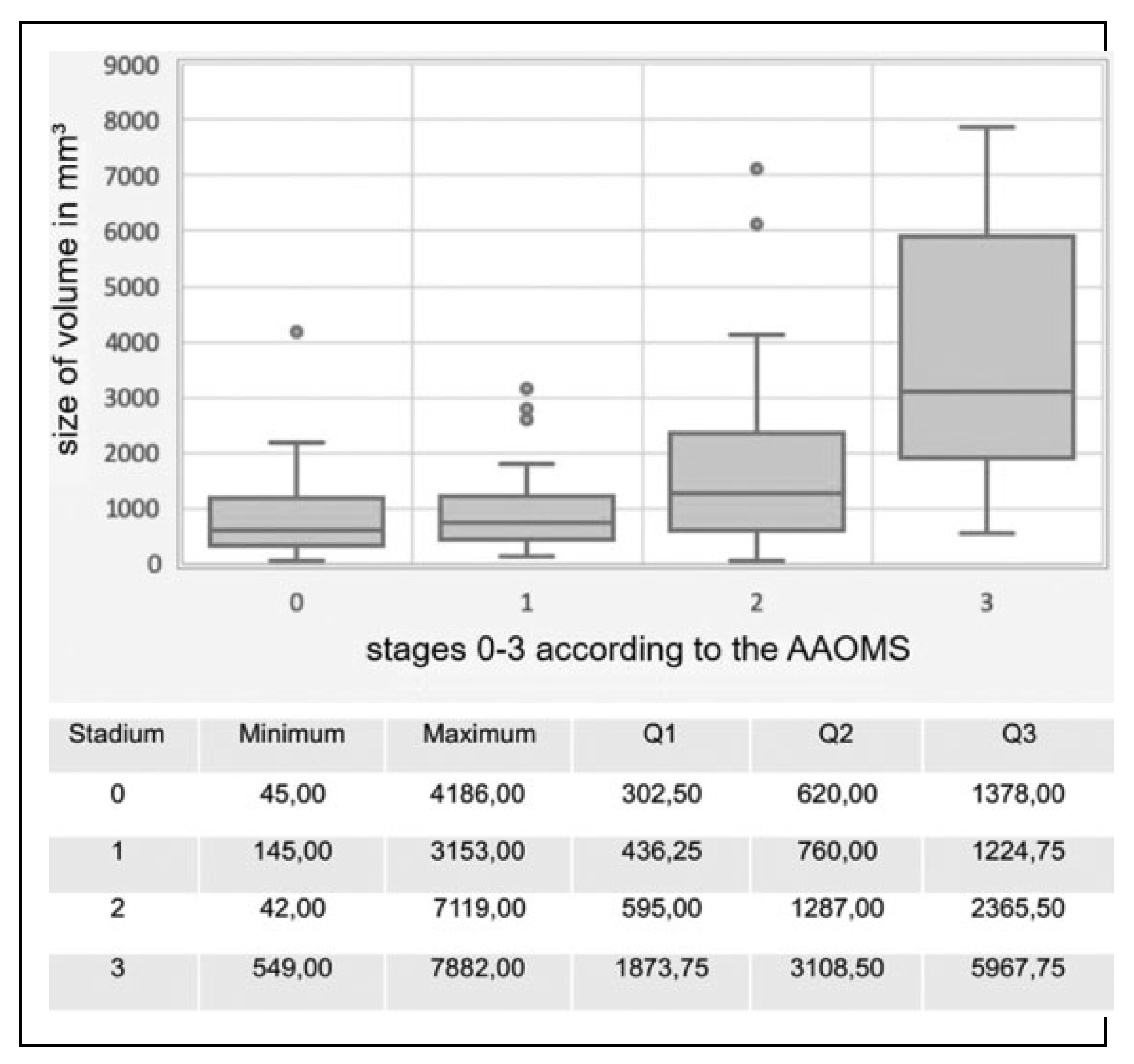
MRONJ Stages
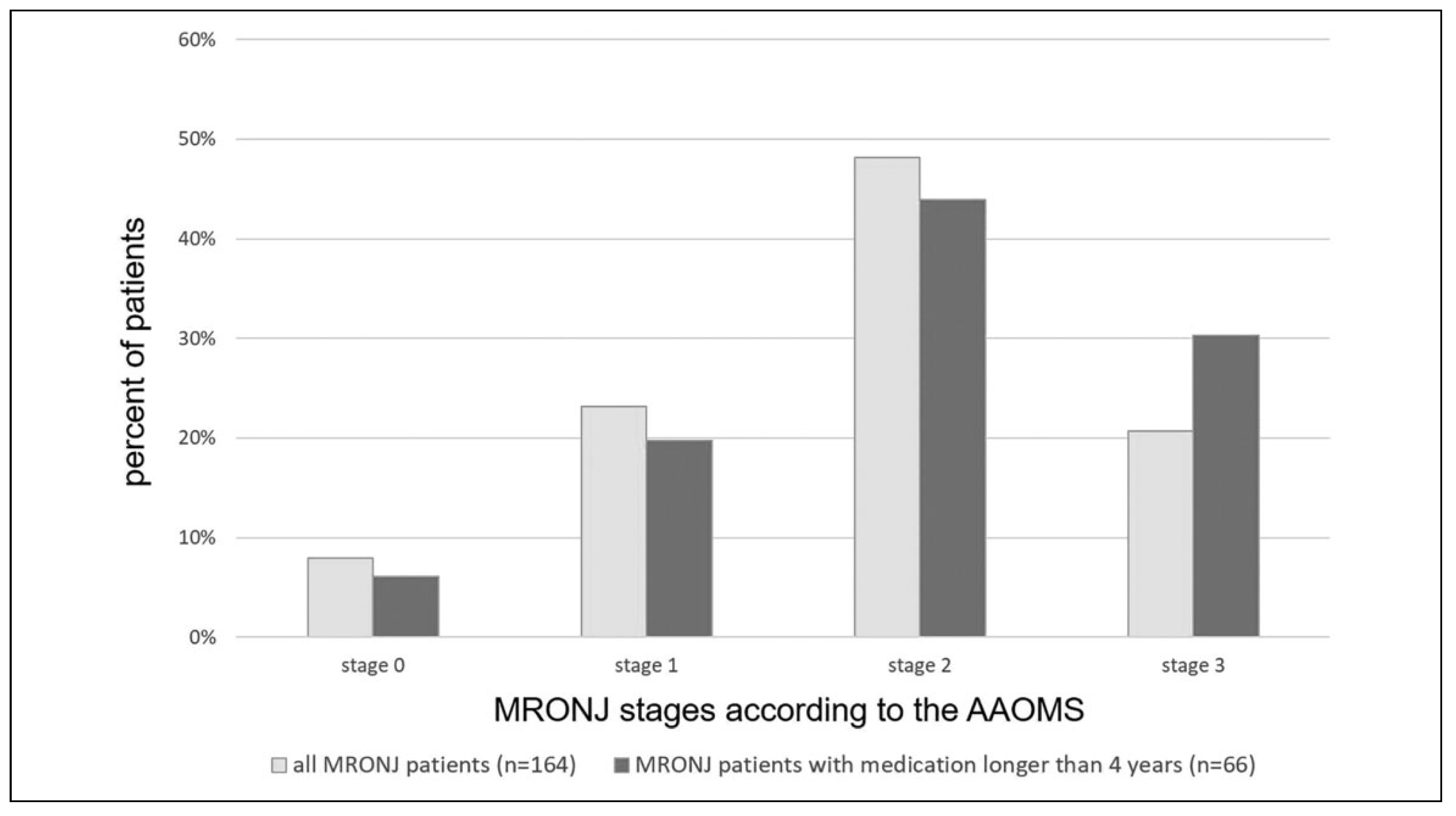
According to the stage classification of the AAOMS mentioned in the Introduction, 7.9% of the patients could be assigned to stage 0, 23.2% to stage 1, 48.2% to stage 2, and 20.7% to stage 3 as illustrated in
Figure 4. A closer look to patient under antiresorptive medication for longer than 4 years (
n = 66) showed that these patients shifted to the more severe stages with the amount of stage 3 patients rising to 1.5 times compared to all the 164 investigated patients (see
Figure 4).
At stage 0, the calculated volume was on average 1035.33 mm
3 with a standard deviation of 1170.78 mm
3, at stage 1 961.79 mm
3 with a standard deviation of 710.21 mm
3, at stage 2 1682.40 mm
3 with a standard deviation of 1421.99 mm
3, and at stage 3 3813.71 mm
3 with a standard deviation of 2233.78 mm
3. Further statistical quantities are shown in
Figure 5. For the relation between stage and volume, the correlation coefficient “r” according to Spearman was .52 and the
P value was .000. Thus, there was a strong, positive, significant correlation between MRONJ stage and calculated necrosis volume.
Therapy Invasiveness and Number of Operations
Only the most invasive form of therapy that the patient has experienced was used for this evaluation. Of the total population of all patients, conservative therapy with irrigation and antibiotics was performed in 11.6% of the cases. In 1.2% of the cases, only decortication was performed, in most cases with 62.8% decortication was combined with plastic covering. Decortication with covering through microvascular flap graft was performed in 8.5% of patients. The most invasive form of therapy including continuity resection and microvascular flap graft was chosen for 15.8% of the cases. With a correlation coefficient of .22 and a P value of .004, there is a weak, monotonically increasing, significant correlation between the volume and the invasiveness of the treatment.
Nearly 11.6% of the patients were not operated but treated conservatively, 40.8% were operated once, 26.2% were operated twice, 9.75% were operated 3 times and 7.3% were operated 4 times. The remaining 4.2% had a highly recurring course of disease and had to be operated more than 4 times (2.4% 5-6 times, 1.8% 7-9 times). Every further operation means a relapse of disease for which there was a weak, positive correlation with the segmented defect volume (ρ = .21, P = .009).
Discussion
In case of MRONJ, currently the commonly used radiologic investigation supplementary performed is a panoramic radiograph.[
14] In our study, we investigated in how many cases radiological signs were visible in the panoramic radiograph compared to 3D data sets. It was shown that even with prior clinical knowledge of the disease and its localization only in two thirds of the cases osteolysis was evident on the 2D image compared to 3D data sets. The detection of sequester formation was even worse with 2.6% patients having sequesters in OPTG compared to 49.4% patients with sequesters found in 3D imaging. These differences are even more evident than found by Demir et al.[
15] That’s why we recommend performing a 3D imaging besides the clinical investigation in cases of suspected MRONJ. It allows conclusions to be drawn about the true extent of the osteonecrosis also beyond the exposed bone visual to the clinical eye. However, there is no established method for defect volume determination.
The present work describes for the first time a method for calculations of the defect volume of osteonecrosis of the jaws by segmentation based on 3D data sets. The utilized software iPlan provides accurate and reproducible results for head and neck tumor segmentation but has not been applied and validated in case of osteonecrosis of the jaws. Therefore, we determined the defect volume of 175 MRONJ patients treated in the Hannover Medical School between 2004 and 2017. The underlying patient population investigated in this study overall represents the patients’ entirety according to published epidemiological data regarding age, gender, and antiresorptive medication including drug agent, mode of administration, and medication duration as well as distribution to MRONJ stages as defined by the AAOMS.[
16,
17,
18] The classification of MRONJ by the AAOMS only assesses the severity of disease by the quality of the osteonecrosis (exposed bone, signs of infection) and not quantity (3D size). However, we found a strong, positive, correlation between MRONJ stages and the calculated size of the segmented necrosis volume with a high significance (
ρ = .52,
P = .000), although there were 2 single cases of stage 0 patients with a high-risk profile (patient no. 159 suffering from prostate carcinoma, 80 pack years, alcoholic, denosumab i.v., history of tooth extraction and patient no. 121 suffering from mamma carcinoma, zoledronate i.v.) showing a huge defect volume (4186 mm
3 and 2199 mm
3). As the defect volume also correlated with the invasiveness of the necessarily performed therapy (
ρ = .22,
P = .004) as well as the number of relapses (
ρ = .21,
P = .009), a classification system based on the actual defect volume would be more orientated toward the disease’s severity as well as the indicated therapy. Moreover, the routine usage of 3D imaging in cases of suspected MRONJ may help for preoperative therapy planning regarding defect extent for resection borders and mode of reconstruction as well as intraoperative certainty which is in particular useful for young surgeons.
Therefore, we put the acquired data sets of segmented volumes to a workflow based on AI in order to develop a computer-aided tool for ranking MRONJ patients. The developed tool at its current state is fast, cross-validated, and powerful in interactive training. With high predictive certainty, a distinction can be made between MRONJ patients and implant patients. But the tool was only able to achieve a low forecasting certainty regarding the different MRONJ stages 0-3. Moreover, automated segmentation by the tool is not possible probably due to the characteristic of the pathology, low resolution data sets, and artifacts. That’s why the work expended for the surgeon still involves segmentation. The project is under ongoing development. Patients with oral carcinoma infiltrating the jawbone are currently under investigation as another control group. A completion in the development of the workflow is not yet in sight.