Materials and Methods
The study was conducted for a period of 5 years between 2014 and 2018. A total of 60 patients were included in the study with facial scars and contour deformity, who opted for autologous fat grafting. All the patients were followed for a minimum period of 1 year from the date of surgery. Patients below 15 years and above 65 years and patients where fat grafting was employed as an adjunct procedure only were excluded from this study.
The patients were explained about the study and informed consent was taken. Patient’s information was collected and collated as per standard proforma prepared earlier. Preoperative photographs were taken as per standards described by American Association of Plastic Surgeons. Surgery was conducted by the same team for all patients in the study.
Selection of Donor Site
The commonly used donor areas were the lower abdomen, flanks, and thighs depending upon availability of fat, as determined by the skin pinch test. However, abdomen was the preferred choice due to the proven fact that lipoaspirate from abdomen is rich in stem cells.
Fat Harvesting
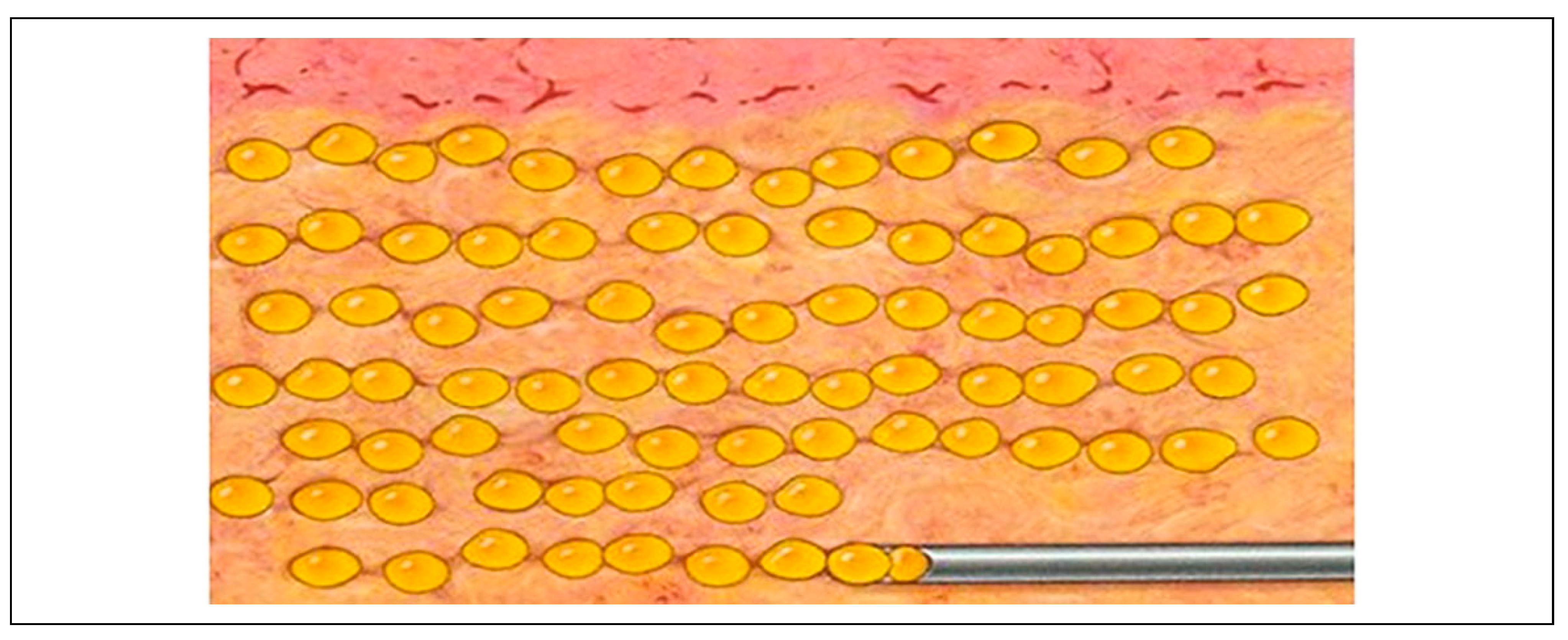
Fat grafting is typically done using a modified Coleman technique.[
1] A small stab incision is made in the anterior superior iliac spine (ASIS) region, and tumescent solution (1 ampule of adrenaline added to 1 liter of normal saline) is infiltrated with a blunt-tipped 3-mm cannula into the lower abdomen or thighs (
Figure 1). Fat is aspirated manually using 2-mm or 3-mm blunt-tipped cannula on a 50-ml syringe by withdrawing the plunger (
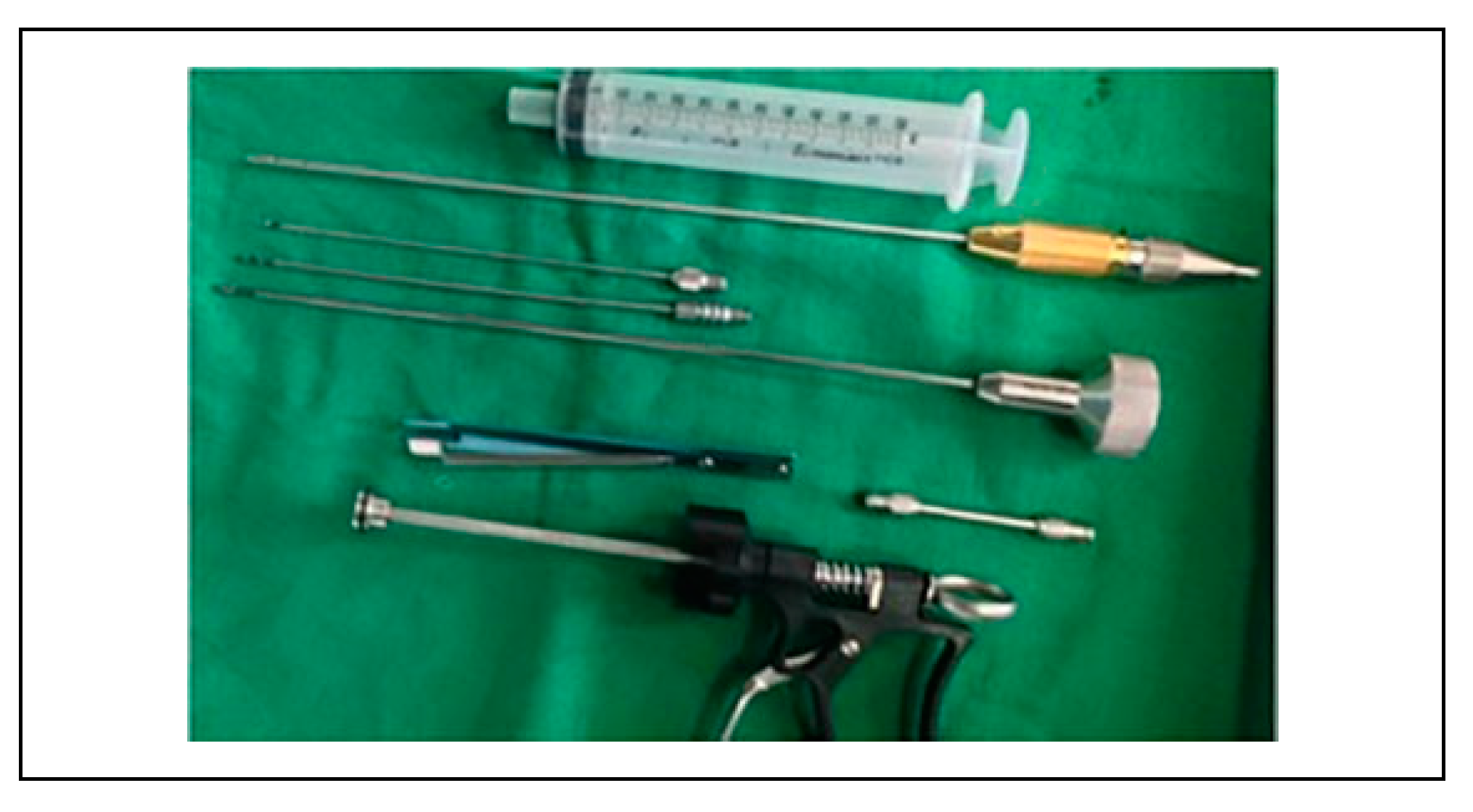
Figure 2). The power-assisted liposuction was not employed in our series because of the perceived damage to the harvested lipocytes. The aspirated fat is transferred into 20-ml and 10-ml syringes.
Fat Processing
A usual harvest is a mixture of 3 main components: fat, blood, and tumescent fluid. Fat processing includes any procedure that may help to separate fat cells from the 2 other redundant components. This was done with decantation, centrifuge or manual centrifuge at low speed for 5 minutes (
Figure 3). After centrifuge, the topmost oil layer and bottom layer were discarded and the middle layer of purified fat was transferred to 2-cc syringes (
Figure 4).
During processing, we divided patients into two groups based on the emulsification of fat. Patients were randomly grouped into nanofat group and non-nanofat groups. Post-op results in these 2 groups were compared and analyzed (
Table 1).
Nanofat Grafting
Lipoaspirate was mechanically emulsified after rinsing. Emulsification of the fat was achieved by shifting the fat between one 10-cc syringe and 2-cc syringe connected to each other by a 3-way cannula (
Figure 5, Video 1). After multiple passes, the fat changed into an emulsion. At the end of the process, the fat became liquid and has a pale whitish appearance. The connective tissue remnants were removed. This effluent is called “nanofat.” Nanofat grafting was used to correct superficial rhytides, scars, and small contour deformities, especially in the face.[
2] It is helpful mainly to rejuvenate the skin. A 27-gauge needle was mounted on the 2-cc syringe for superficial intradermal and subdermal injection to improve the scar quality. Our method differs from the original technique in use of a 3-way cannula and the number of passes and hence a modification of the original nanofat method. The 3-way cannula attached to 2-ml syringes prevents any inadvertent spillage and offers a quick nanofat preparation almost ready for injection. Also, unlike the original technique of 30 passes, our method requires approximately 12–15 passes.
Injection Technique
Coleman 2-mm injection cannula or 18G needles were used for injection of fat. The cannula or needle fitted to a 2-cc Luer Lock Syringe containing the graft was introduced through the port. The cannula was gently advanced through the subcutaneous tissue, reaching the distal end of the area to be grafted. The graft was injected in small packets of 0.5 cc, as the cannula was withdrawn. This was then repeated in different directions and then in different planes (
Figure 6, Video 2).
In general, the plane of injection is just below the scars in the subcutaneous plane. In areas of severe scarring, a sharper tip is attached to the syringe for the final passes under the scar. This sharp tip is used to do subcision, that is, gently disrupt the scar tissue tethering the contour, and fat is then injected into the area.
It is a well-known fact that there is a resorption of fat with time. Hence, in our series, all contour deformities were overfilled. The filling was assessed clinically with overlying skin tightness and blanching. After infiltration the area should be firm, but not too tight.
Adjunct Procedures
Along with fat grafting, dermabrasion and collagen dressing were used to improve the aesthetic appearance of facial scars. This procedure is mainly used for the scars which are hyperpigmented and depressed in nature which are prevalent in our geographical location. In this procedure, first we inject fat below the scars followed by removal of scar especially epidermis and superficial layer of dermis using power-assisted diamond tipped dermabrader. A layer of collagen applied over the newly created abrasion (
Figure 7). We couldn’t study the need for adjunct procedure compared with the controls because most of the time in scars an adjunct procedure like dermabrasion and collagen dressing was needed in our study.
Multiple Sittings
Most of the scars required a single sitting of fat injection. Contour deformities like hemifacial atrophy and post-oncological excision with radiotherapy needed multiple sittings of fat injection. The reason behind multiple sittings of fat injection was resorption and requirement of a large volume of fat.
Post-Operative Care
Recipient area was left open with topical antibiotic applied at port sites. Cold compresses by ice packs were advised to reduce the postoperative edema and discomfort. Oral antibiotics were prescribed for 1 week and analgesics for 3 days following surgery.
Follow-Up
Patients were followed at 2 weeks, 6 weeks, 6 months, and 1 year postoperatively. First 2 follow-up’s were to document if any complications were seen. Third and fourth follow-ups were for aesthetic assessment. At the follow-up, photographs were taken and information was collected as per the prescribed proforma.
Assessment of postoperative aesthetic outcome in terms of satisfaction was done using the visual analog scale (VAS),[
3] which ranges from 1 to 10 by the patient and operative surgeon (
Figure 8;
Table 2).
Postoperative Complications
Postoperative complications were documented if any during the follow-up.
Results
The mean age was 30.8 + 9.8 years. Of 60 patients, 30 were facial scars and 30 were contour deformities. The most common donor site was lower abdomen. Among 30 patients for scar revision, 20 patients underwent dermabrasion and collagen dressing as adjunct procedures.
Recipient Site Complications
Among 60 patients, 29 patients had transient edema and 11 patients had bruising. There was no major complication like cellulitis, artery thrombosis, and pigmentation.
Donor Site Complications
Among 60 patients mild pain was noted in few, but there were no major donor site complications like hypertrophic scarring, infection, and damage to underlying structures.
Being a versatile and superficial procedure, the complications in fat grafting are very less. Even though there are reports of major complications like thrombosis and fat embolism, the incidence of these complications is very less and we did not encounter any.
Among 60 patients, 50 (83.3%) patients underwent procedure in a single sitting and 10 (16.7%) patients required multiple sittings (2 or more).
Comparison of Nanofat Grafting With Aesthetic Outcome
In our study, we classified fat grafted patients into 2 groups based on nanofat grafting of the harvested fat, into which patients were allocated randomly (every alternate patient into second group) (
Table 1). The preoperative and postoperative outcomes were compared and analyzed as follows.
Total number of patients in nanofat group—30
Total number of patients in the non-nanofat group—30
Fisher’s exact test .The 2-tailed P value equals 0.0011 (<0.05)
The association between rows (groups) and columns (outcomes) is considered to be very statistically significant. By the above statistical analysis, we observe that nanofatting improves the aesthetic outcome.
Comparison of Patient Satisfaction Scores by Different Observers Between Subjects With Different Diagnosis
Comparison of patient satisfaction scores by surgeons score between subjects with different diagnosis was performed using Student
t test (
Table 2). No significant difference was detected between patients and surgeons scores.
Discussion
Structural fat grafting is a commonly used procedure in plastic surgery. Fat can be considered as the ideal filler because
autologous fat is readily available and inexpensive;
fat is autologous and therefore lacks immunogenicity; and
it is safe and noncarcinogenic and it is easily acquired with a minimally invasive procedure.
Neuber did the first free fat graft in humans.[
4] The rationale for fat grafting hinges on the fact that the transplanted fat requires contact with living tissue. These grafts survive by diffusion until the process of neovascularization occurs. This tenuous process leads to one of the main drawbacks in this technique, which is an unpredictable long-term survival rate of the injected lipocytes. This problem has led to research and investigation into methods and techniques to increase fat viability. Fat injection has been used for facial rejuvenation to correct a variety of soft tissue defects in the face.
In our study, the age of patients ranged between 17 years to 64 years. The mean age was 30.8 + 9.8 years. Majority of the patients were between 21 and 30 years age-group. Fat grafting was done in 60 patients out of which 32 were females and 28 were males.
In 1950, Peer documented that autologous fat grafts lost approximately 45% of their weight and mass per year, or more, following transplantation in humans.[
5] Due to the subsequent decrease in graft size after implantation, many recommend serial injections to overcome the loss in filling volume. Gatti recommended a minimum of 3 fat grafting sessions for an appreciable augmentation.[
6] In our study among the 60 patients, 50 (83.3%) patients underwent single sitting and 10 (16.7%) patients required multiple sittings (2 or more).
The most common donor site in our study was lower abdomen in 53 (88.3%) patients. The other donor sites were thigh in 5 (8.3%) patients, abdomen and thighs in 1 (1.7%) patient and submental fat in 1 (1.7%) patient. In a survey of Plastic Surgeons in America, the most common donor site was abdomen (74%) followed by flanks and thighs.[
7] The abdomen was the first choice as it has ample amount of fat and the scar is well hidden. The harvest is easy, with the patient in supine position, where it is easy to prepare and infiltrate the tumescent solution.
But, there are different studies like Rohrich et al[
8] and Pedoin et al which have investigated into the effects of harvested site and show no significant difference in aesthetic outcome of the fat obtained from the different donor sites.
Studies investigating the effect of the recipient site on fat grafting and retention have been inconclusive. Rieck and Schlaak demonstrated reduced fat retention following fat grafting to the muscle, which was attributed to increased mobilization. Mobile areas of the face, such as the glabella and lips, were also less amenable to correction, compared with less mobile areas, such as the malar and lateral cheek.[
9] Rohrich et al demonstrated that restoration of selective fat compartments with fat grafts to precisely control facial contouring generated a more natural and youthful appearance.
Xie et al in their study mentioned injecting volumes for facial rejuvenation ranging from 0.8 cc to 26 cc.[
10] In our study, we used 3 ml to 80 ml of fat for facial rejuvenation. Tonnard et al[
2] in his work on fat grafting, divided into 3 subunits, namely macrofat (lipoaspirate), microfat (fat harvested through multiple small hole cannula), and nanofat (lipoaspirate is centrifuged and emulsified) based on harvesting and processing. They have shown good clinical results with the use of nanofat.
Our study also has 2 groups namely nanofat and non-nanofat group. We were also able to statistically prove that nanofat has better cosmetic results (
Table 1). Ideally patients with radiotherapy should be placed in a separate group. As we had only 1 patient under this category, who was benefited with an excellent outcome, we decided to include this case in non-nanofat group. Further, since it is only a single case in our study, it was assumed that it will not cause any significant statistical difference. Also, it provides an opportunity for us to share this method for use in difficult clinical situations.
Even though there are no viable adipocytes in nanofat sample, studies of Tonnard et al showed that nanofat sample is a rich concentrate of adipocyte derived and substrate stem cells. The process of nanofat grafting will increase the density of stem cells in the recipient area. Similar results on nanofat grafting have been obtained by Uyulmaz et al.[
11]
An aesthetic procedure like fat grafting depends on patient satisfaction. Hence, the use of patient reported outcome scores like VAS scores will suit the research involving outcome in fat grafting. Moreover, VAS score being a visual score, it is easily understood by patients and application is very simple by both patient as well as surgeon (
Table 2).
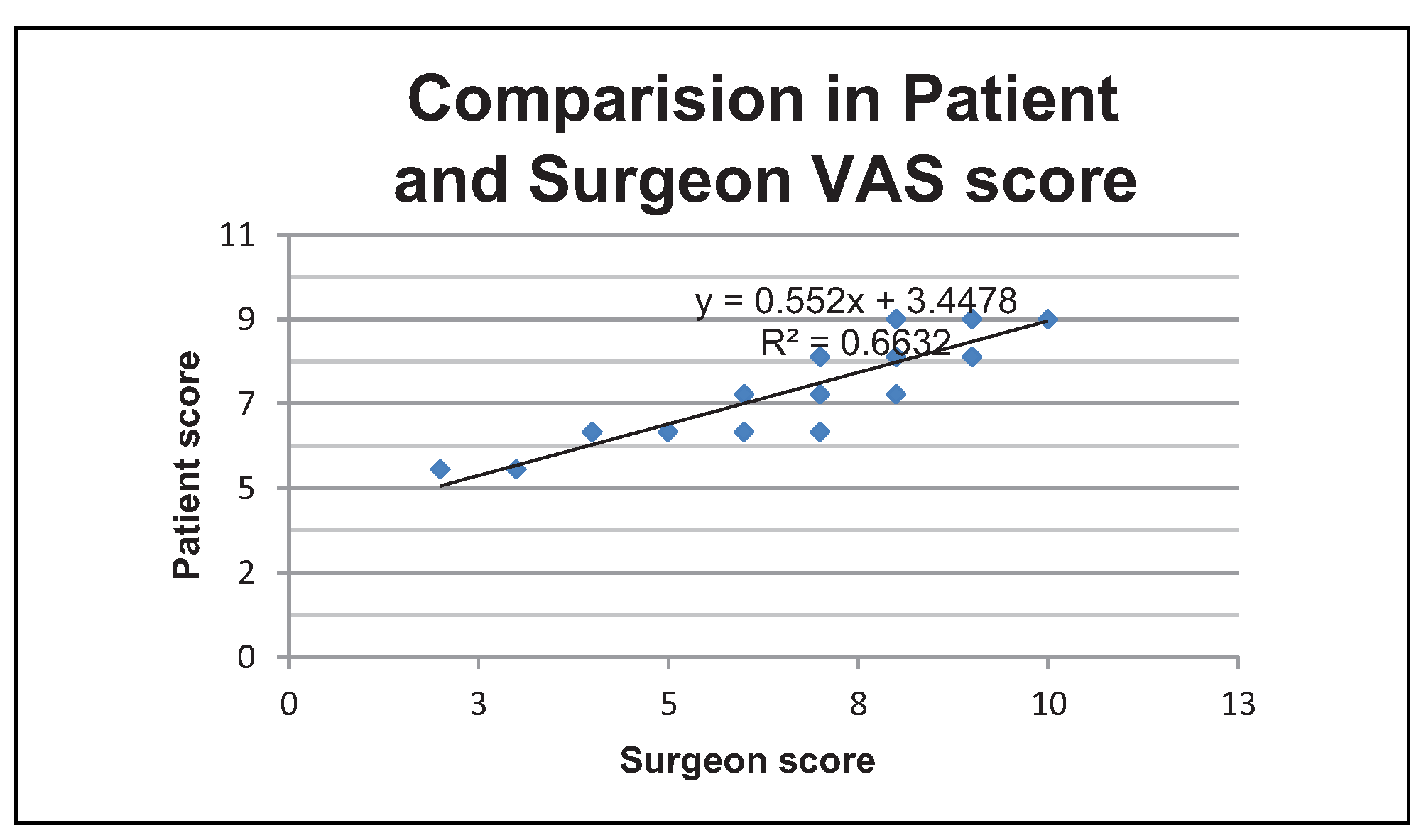
In our study, there is no significant difference in VAS scores of patient and surgeon following fat grafting (
P value 0.65). Our results coincided with other similar studies on structural fat grafting. The mean VAS scores in terms of patient satisfaction in the present study are 7 + 1.4 (
Figure 9).
Being a versatile and superficial procedure, the complications in fat grafting are very rare. Even though there are reports of major complications like thrombosis and fat embolism, the incidence of these complications are extremely rare. In our series, we did not find any major complications except edema and bruise, which were resolved within a week, by conservative measures.
Future Research
Adipose-derived stem cells (ASCs) have properties of self-renewal and multipotential differentiation. As compared to bone marrow-derived stem cells, ASCs isolation yields higher amount of stem cells. Therefore, the ASCs are of higher interest for stem cell-based therapies and skin tissue engineering. It may be defined as a multi-lineage stem cell population which is isolated from the stromal vascular fraction (SVF) of adipose tissue. Yoshimura et al have demonstrated that autologous fat grafting with lipoaspirate enriched with SVF, a rich source of ASCs, improves clinical outcome in breast augmentation and facial lipodystrophy patients.[
12] This observation has been termed cell-assisted lipotransfer. Novel strategies have thus been employed to enhance cytoprotection of the ASCs at the target site, which include ischemic preconditioning, pharmacological therapy, refinement of liposuction techniques to minimize trauma, as well as the addition of growth factors. Future experimental work will be centered on safe harvest of fat cells and to maximize the therapeutic efficacy at the target site. With the advent of ASC therapy, auto-logous fat transfer holds much promise for the future, especially in the realm of soft tissue reconstruction and aesthetic surgery. Future study should incorporate the use of quality of life scale index for patients comparison in different settings. There remains certain skepticism over the safety of the use of ASCs for post-oncological defects, which needs to be addressed in ethical and well-conducted human clinical trials. There has been a substantial increase in research interest to identify methodologies for optimizing fat graft survival by targeting both the adipocytes and stromal vascular fraction cells. Therefore, additional human studies are necessary to aid in the development of a universal protocol for a safe clinical practice.