Abstract
Study Design: A cross-sectional study. Objective: Neurosensory disturbances (NSDs) of the infraorbital nerve (ION) are common following orbito-zygomaticomaxillary complex (ZMC) fractures. This study aimed to evaluate the effect of lag time between injury and treatment on recovery of NSDs of the ION following open reduction internal fixation. Methods: Subjects who had ZMC fracture and paresthesia were studied. The lag time between injury and treatment was considered as the predictive factor. The level of NSDs according to the brush test and two-point discrimination (TPD) test and self-reported NSD were the outcomes of this study. Self-reported NSD was quantified using a visual analog scale. Results: Forty patients were studied. The lag time between injury and treatment had a significant correlation with the result of the TPD test and the self-reported level of NSD. In 73.6% of patients who had NSD following ZMC fracture, every 1-day delay in treatment increased the incidence of self-reported paresthesia by 0.44. Conclusions: It seems, a delay in treatment of ZMC fractures increased the risk of NSD.
Introduction
Neurosensory disturbances (NSDs) of the infraorbital nerve (ION) are common following orbito-zygomaticomaxillary complex (ZMC) fractures. The ION is prone to injury due to its proximity to the orbito-ZMC as it passes through the infraorbital sulcus in the floor of the orbit to exit through the infraorbital foramen.[1] Compression, edema, ischemia, or laceration of the ION can lead to traumatic injury, which results in dysesthesia of the skin of the lower eyelid, nose, cheek, upper lip mucosa, teeth, and/or gingiva of the affected side. Complete anesthesia rarely occurs. Hypoesthesia is most frequently present, followed by paresthesia and hyperesthesia.[2] Fracture complexity and displacement of fracture segments can cause NSDs following ZMC fracture.[3] Patients with ZMC fracture usually complain of hypoesthesia, paresthesia, dysesthesia, and anesthesia of the upper lip, lower eyelid, cheek, skin of the nose, gingiva, and teeth at the fracture side [4]. The incidence of symptoms of the ION injury varies in the literature and ranges from 35% to 94% in ZMC fractures [2,5,6].
The effect of intervention time on the recovery of NSDs following ZMC fractures has not yet been clearly elucidated. In facial fractures, the treatment time plays an important role in reduction of complications [7].
The purpose of this study was to answer the following clinical question: Does the lag time between injury and treatment affect NSDs following open reduction internal fixation (ORIF) in patients with ZMC fractures? We hypothesized that surgical intervention shortly after trauma would decrease the recovery time of NSDs following ZMC fractures. Therefore, the aim of this study was to evaluate the effect of lag time between injury and treatment on recovery of NSDs of the ION following ORIF.
Materials and Methods
Authors designed a cross-sectional study. The study sample was derived from the population of patients who presented to the Department of Oral and Maxillofacial Surgery for management of mandibular fractures from 1 October 2016 to 30 September 2018. The committee of the medical ethics group of Shahid Beheshti University of Medical Sciences approved this study. Patients eligible for study inclusion had a unilateral ZMC fracture with displacement of less than 5 mm in the zygomatic maxillary buttress, the zygomatic frontal buttress, and the infraorbital rim with NSD of the infraorbital area and underwent ORIF, agreed to participate in this study, and showed up for the 6-month follow-up visit. Patients were excluded from the study enrollment if they did not have paresthesia, had a history of midface trauma, gunshot trauma, comminuted fractures, pathologic fractures, or severe displacement (more than 5 mm), required orbital floor reconstruction, refused study enrollment, or failed to show up for the follow-up. Patients with preoperative iatrogenic nerve injury were excluded as well. All patients underwent ORIF at the zygomaticomaxillary buttress with/without zygomaticofrontal buttress.
Neurosensory Evaluation
NSD was evaluated by the two-point discrimination (TPD) test and brush stroke test (semi-objective neurosensory testing [level A]) before and 6 months after the surgical procedure. The TPD test measures the minimal distance between 2 static points in vertical and horizontal directions perceived by an individual in the mental area with patient’s eyes closed. If patients reported different TPD values vertically and horizontally, the mean TPD value was used for statistical analysis. The brush test was conducted across the mental area in the anteroposterior direction using fine #2 sable brushes. Patient responses were recorded. Brush tests were performed in 2 sessions (on 2 different days) by 2 examiners. An inter-examiner reliability analysis was carried out to determine the level of agreement between the examiners. The self-reported NSD was documented for each patient before the surgical procedure and after 9 months using a visual analog scale.
Paresthesia was defined. The severity of self-reported NSD was classified using the following scale: 0 to 3 mild NSD, 4 to 6 moderate NSD, and 7 to 10 severe NSD. If patients had an abnormal perception of a sensation in lack of any stimulus, it was considered as paresthesia. Hypoesthesia was referred to moderate or severe NSD as a decrease in sensation. If patients had spontaneous sensations in the absence of stimuli, it was documented as dysesthesia.
The results of patients’ neurosensory function tests were documented in their files. Neurosensory functions were measured by an oral and maxillofacial surgeon and a dentist (second and third authors).
The lag time between the incidence of trauma and surgical procedure was considered as the predictive factor of this study. Age and gender were variables, and recovery or level of NSD (based on the results of brush stroke tests and TPD test) were the outcomes of this study.
Statistical Analysis
The statistical analyses were performed using SPSS version 21 (SPSS Inc., Chicago, IL, USA).
The Pearson’s correlation test was used to evaluate any correlation between paresthesia recovery (TPD test and self-reported paresthesia) and the treatment time. Independent t-test was applied to compare paresthesia recovery between males and females. The к test was applied to assess inter-examiner reliability.
Results
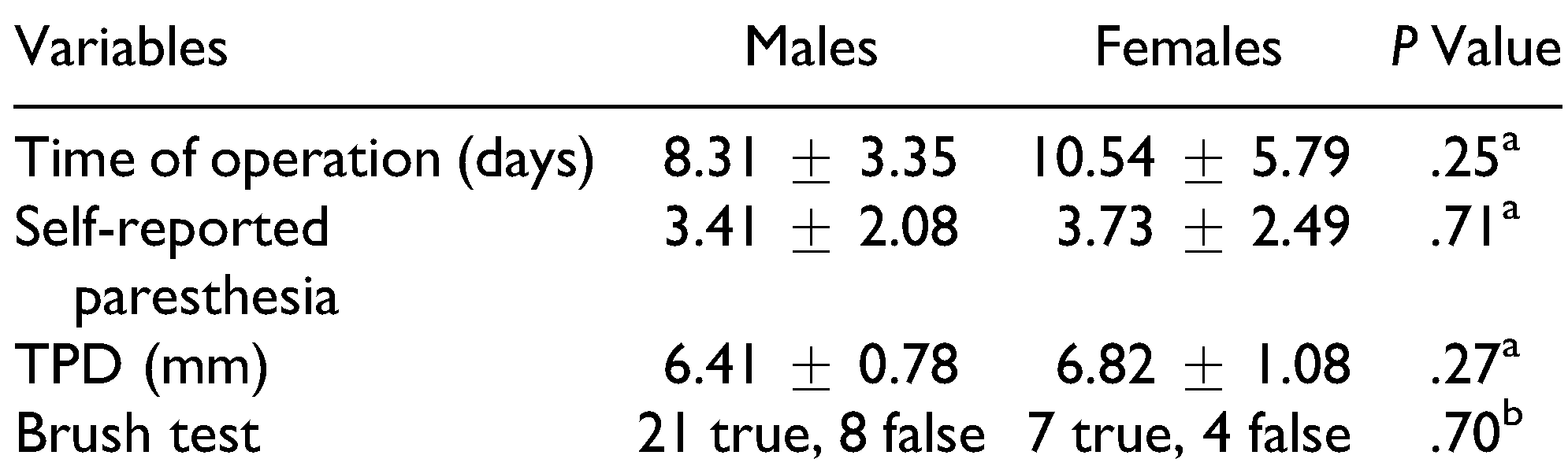
Forty patients (29 males and 11 females) with NSDs following ZMC fractures were studied. None of the patients had dysesthesia. The frequency of NSDs at 6 months after ZMC fracture according to the self-reported paresthesia score was as follows: 22.5% did not have NSDs, 37.5% had hypoesthesia, and 40% had moderate paresthesia (Table 1). Anesthesia was not reported by any patient. Twenty-eight patients (70%) reported the true direction of brush in brush stroke test while 12 patients (30%) did not detect it. The mean score of self-reported paresthesia at 6 months after surgery was 3.5 ± 2.17. The mean TPD value was 6.52 ± 0.88 mm. Patients underwent surgery averagely 8.92 ± 4.20 days after trauma. Analysis of the data did not demonstrate any difference between males and females for the time of surgery or the results of TPD test, brush stroke test, or self-reported paresthesia score (Table 2). The mean age of patients was 29.2 ± 8.24 years. Analysis of the data did not reveal any correlation between age and TPD test result or self-reported paresthesia score (Table 3).

Table 1.
Frequency of NSDs at 6 Months After Treatment.

Table 2.
Comparison of Variables and Outcomes Between Males and Females.

Table 3.
Correlation of Variables and Outcomes With Regard to Age of Patients.
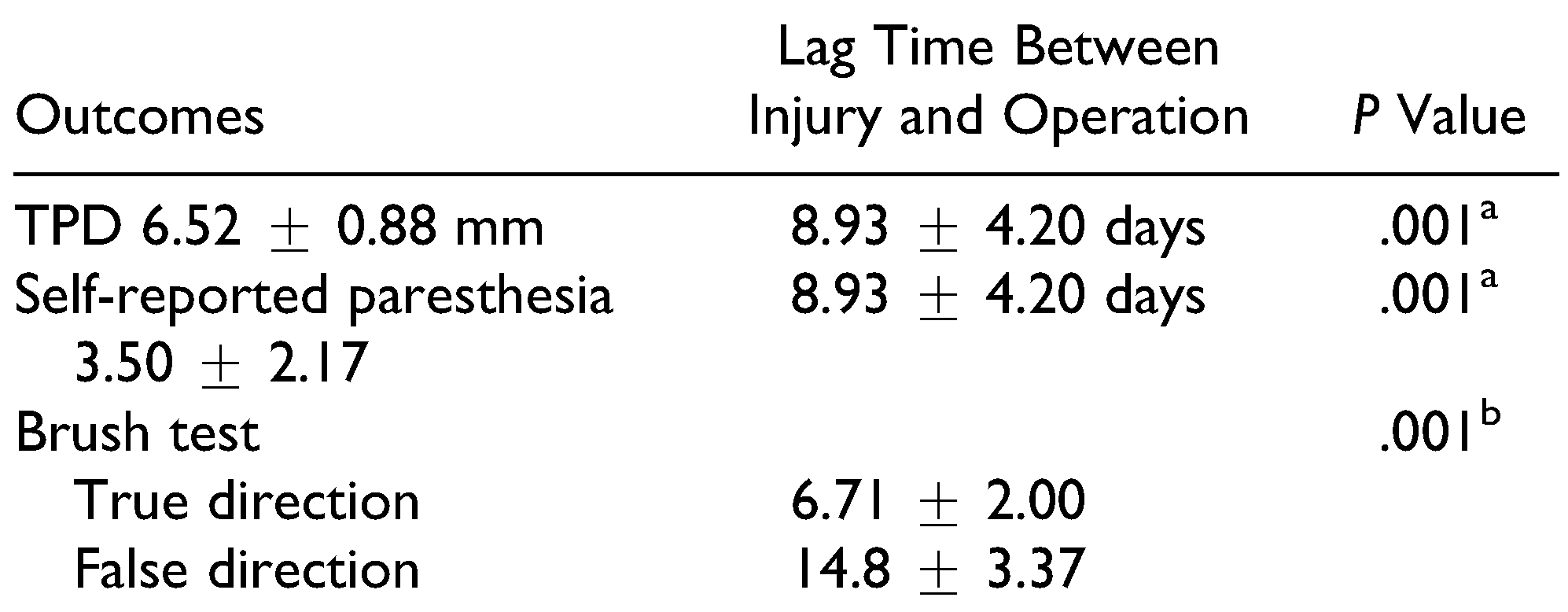
The results showed that the lag time between injury and treatment had a significant correlation with the TPD test result and severity of self-reported paresthesia (Table 4). There was a significant difference between patients who reported the true direction of brush and those who reported a false direction in the mean lag time between injury and treatment (Table 4).

Table 4.
Correlation of Lag Time Between Injury and Treatment With the Outcomes.
The linear regression model predicted that in 73.6% of patients who had paresthesia following ZMC fracture, every 1-day delay in treatment increased the incidence of self-reported paresthesia by 0.44 (R2 = 0.736 and B = 0.44 at a 95% confidence interval [CI], p = 0.001; Figure 1).
Figure 1.
Linear regression model presents the distribution of patients’ paresthesia level in various treatment times.
The inter-examiner reliability for the examiners was found to be к = 0.50 (p = 0.04) at a 95% CI, which showed a moderate agreement between the examiners.
Discussion
The ION is commonly injured in ZMC fractures as the fracture usually extends to the infraorbital foramen. The ION could also be affected in isolated orbital floor fractures. Spontaneous recovery of the nerve usually occurs but some patients complain of paresthesia or dysesthesia.[3] In this study, we assessed the effect of lag time between injury and treatment on recovery of NSDs following ZMC fracture.
Our study results demonstrated that the lag time between injury and treatment had a strong correlation with the result of TPD test and severity of self-reported paresthesia. Every 1-day delay in treatment of ZMC fracture fracture. This study evaluated patients at 6 months after the surgical intervention (only ORIF). A previous study reported that 18.8% to 78.8% of patients with ZMC fractures had some residual deficits at 6 months.[8] In our study, 37.5% of patients reported hypoesthesia while 40% reported moderate paresthesia.
Risk of permanent NSDs increases if no improvement is observed in NSDs 3 to 6 months after surgery or trauma, because bone healing is accomplished during this time period.[1] Lack of recovery during this period may suggest nerve injury. Some authors recommend longer time periods (6–12 months) for surgical interventions (decompression).[4,9] Complete loss of sensation may rarely occur. Hypoesthesia is most frequently present followed by paresthesia and dysesthesia [10].
Clinical neurosensory testing is generally divided into 2 basic types according to the specific receptors stimulated through nociceptive, mechanoceptive, and cutaneous contact. Mechanoceptive testing consists of static light touch, TPD test, and brush directional stroke test. The thermal discrimination and pinprick tests as well as localization, blunt/sharp discrimination, and dental vitality tests are categorized under the nociceptive testing group. TPD test is performed to assess large, myelinated, slowly adapting, A-α sensory nerve fibers. The sensation of static light touch and brush directional stroke test are applied to examine the discriminating ability of large, myelinated, quickly adapting, A-α sensory nerve fibers.[11] TPD, brush test, and self-reported NSD are subjective tests which were used in this study. In evaluation of NSD, TPD is very useful test as it can be done as quantitative sensory test (level A). It can reveal the recovery stage of NSDs following ION injury. For meticulous objective assessment of NSD, only electrodiagnostic test is truly objective. The distance between 2 examined points in TPD test is inversely related to the innervation density of the large myelinated group A-β fibers, so that a small distance (5 mm) means a high innervation density, less damage, and a larger number (10) means less nerve fibers, greater damage to the infraorbital nerve. It was suggested the pressure-specified sensory device is the best way to obtain the cutaneous sensory threshold for the trigeminal nerve.[12,13] Okochi et al. compared Weinstein monofilament test and perception threshold test for evaluation of NSDs following ZMC fractures.[14] They concluded that perception threshold test was more effective for detection of minor paresthesia, which could not be detected by Weinstein monofilament test. They also suggested that paresthesia in ZMC fractures is mainly due to the injury to A-d and C fibers.[14] The ION injury commonly occurs due to trauma. However, iatrogenic injury can also occur intraoperatively.[15] Manipulation of fracture segment during reduction and placement of rigid fixation devices (miniplates and screws) may lead to iatrogenic ION injury as well. In this study, we excluded cases of obvious iatrogenic ION injury.
Fogaça et al. studied NSD following ZMC fractures.[16] They reported 76% of patients had abnormal sensibility on the side of the zygomatic fracture when compared to the contralateral side. NSD was documented in 100% of the patients who needed orbital floor reconstruction. In 74% of patients with NSD, symptoms correlated with the fracture [16].
Conclusion
It appears that treatment of ZMC fractures shortly after injury positively affects the recovery of NSDs following trauma. Patients who had a delayed treatment experienced higher level of self-reported paresthesia.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflicts of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Vriens, J.P.; Moos, K.F. Morbidity of the infraorbital nerve following orbitozygomatic complex fractures. J Craniomaxillofac Surg. 1995, 23, 363–368. [Google Scholar] [CrossRef] [PubMed]
- De Man, K.; Bax, W. The influence of the mode of treatment of zygomatic bone fractures on the healing process of the infraorbital nerve. Br J Oral Maxillofac Surg. 1988, 26, 419–425. [Google Scholar] [PubMed]
- Neovius, E.; Fransson, M.; Persson, C.; Clarliden, S.; Farnebo, F.; Lundgren, T.K. Long-term sensory disturbances after orbitozygomatic fractures. J Plast Reconstr Aesthet Surg. 2017, 70, 120–126. [Google Scholar] [PubMed]
- Pedemonte, T.C.; Basili, E.A. Predictive factors in infraorbital sensitivity disturbances following zygomaticomaxillary fractures. Int J Oral Maxillofac Surg. 2005, 34, 503–506. [Google Scholar] [PubMed]
- Ellis, E.; El-Attar, A.; Moos, K.F. An analysis of 2067 cases of zygomatico-orbital fracture. J Oral Maxillofac Surg. 1985, 43, 417–428. [Google Scholar] [PubMed]
- Schultze-Mosgau, S.; Erbe, M.; Rudolph, D.; Ott, R.; Neukam, F.W. Prospective study on post-traumatic and postoperative sensory disturbances of the inferior alveolar nerve and infraorbital nerve in mandibular and midfacial fractures. J Craniomaxillofac Surg. 1999, 27, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Maloney, P.L.; Lincoln, R.E.; Coyne, C.P. A protocol for the management of compound mandibular fractures based on the time from injury to treatment. J Oral Maxillofac Surg. 2001, 59, 879–884. [Google Scholar] [PubMed]
- Benoliel, R.; Birenboim, R.; Regev, E.; Eliav, E. Neurosensory changes in the infraorbital nerve following zygomatic fractures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005, 99, 657–665. [Google Scholar] [PubMed]
- Jungell, P.; Lindqvist, C. Paraesthesia of the infraorbital nerve following fracture of the zygomatic complex. Int J Oral Maxillofac Surg. 1987, 16, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Godhi, S.; Lall, A.B.; Ram, C.S. Evaluation of neurosensory changes in the infraorbital nerve following zygomatic fractures. J Maxillofac Oral Surg. 2012, 11, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Akal, Ü.K.; Sayan, N.B.; Aydogan, S.; Yaman, Z. Evaluation of the neurosensory deficiencies of oral and maxillofacial region following surgery. Int JOral Maxillofac Surg. 2000, 29, 331–336. [Google Scholar] [CrossRef]
- Karagoz, H.; Ozturk, S.; Siemionow, M. Comparison of neurosensory assessment methods in plastic surgery. Ann Plast Surg. 2016, 77, 206–212. [Google Scholar] [PubMed]
- Dellon, A.L.; Andonian, E.; DeJesus, R.A. Measuring sensibility of the trigeminal nerve. Plast Reconstr Surg. 2007, 120, 1546–1550. [Google Scholar] [CrossRef] [PubMed]
- Okochi, M.; Ueda, K.; Mochizuki, Y.; Okochi, H. How can paresthesia after zygomaticomaxillary complex fracture be determined after long-term follow-up? A new and quantitative evaluation method using current perception threshold testing. J Oral Maxillofac Surg. 2015, 73, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Hillerup, S. Iatrogenic injury to oral branches of the trigeminal nerve: Records of 449 cases. Clin Oral Investig. 2007, 11, 133–142. [Google Scholar] [PubMed]
- Fogaça, W.C.; Fereirra, M.C.; Dellon, A.L. Infraorbital nerve injury associated with zygoma fractures: Documentation with neurosensory testing. Plast Reconstr Surg. 2004, 113, 834–838. [Google Scholar] [PubMed]
© 2020 by the author. The Author(s) 2020.